Abstract
Micro- or nanoencapsulation has become one of the most attractive approaches to enhance the stability and bioavailability of bioactive compounds isolated from natural products. In addition, such encapsulation also provides an opportunity to modulate the release of bioactive compounds by using functional polymers that can be fine-tuned according to the need of the body. Over the last 10 years, increasing research works have been dedicated toward the encapsulation of bioactive compounds for various purposes. Numerous techniques, embracing micro- and nanoplatforms including spray drying, freeze drying, micro- and multiple emulsification, electrospinning, and coacervation, have been utilized to achieve the encapsulations. Such encapsulations have been found to improve the physicochemical properties of the bioactive compound, provide stability and enhanced bioavailability, controlled release of the compound, enhancing bioactivity, and masking of flavor or taste, along with several other benefits. Wide ranges of materials including lipids, synthetic, and natural polymers have been utilized, and the type and amount of the wall formers have been found to influence the performance and functionality of these preparations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
20.1 Introduction
Phytochemicals in plants that produce therapeutic activities are collectively known as bioactive compounds. These bioactive compounds are predominantly plant secondary metabolites and nutrients such as vitamins and minerals that may induce pharmacological activity at high doses are usually excluded from the definition of bioactive compounds (Bernhoft 2010). Abundant bioactive phytochemicals are found in vegetables, fruits, seeds, nuts, legumes, leaves, and other parts of plants (Al Juhaimi et al. 2018; Pachuau et al. 2019; Sagar et al. 2018; Septembre-Malaterre et al. 2018; Xiao and Bai 2019). Phenolic compounds such as flavonoids, courmarins, phenolic acids, xanthones, and ellagitannins along with alkaloids, phytosterols, carotenoids, anthocyanins, and tocopherols are some of the commonly found bioactive compounds from plant sources that are responsible for their bioactivities (Fig. 20.1) (Altemini et al. 2017; Da Silva et al. 2016; Xiao and Bai 2019). In addition to their bioactivities, phytochemicals such as phenolics in vegetables and fruits may also contribute to their stability against oxidation, taste, color, flavor, and odor (Naczk and Shahidi 2006).
Studies have reported myriads of pharmacological activities including antimicrobial, anti-hyperglycemic, anti-inflammatory, antioxidant, anti-proliferative, and anti-diabetic effects of bioactive phytochemicals isolated and characterized from various plant species (Martins et al. 2016; Ramesh Kumar et al. 2018; Rodriguez-Garcia et al. 2017). Diverse preparations made from these wide varieties of plant species have been used in the treatment of different diseases since ancient times as documented in Eber’s Papyrus, Traditional Chinese Medicines (TCM), and Ayurvedic formulations (Atanasov et al. 2015; Khan et al. 2019; Yang and Yue 2012). These traditional practices still remain valuable sources of information for modern drug discovery and development programs. In fact, data from Food and Drug Administration (FDA) revealed that about 40% of the approved molecules are either natural compounds or inspired by them and, from these, 74% are indicated for anticancer therapy (Seca and Pinto 2018). Still, it has been reported that about 95% of the world’s biodiversity are yet to be systemically investigated for their pharmacological activity and only 15% have been evaluated phytochemically (Atanasov et al. 2015; David et al. 2015). The current global scenario and the urgent need for effective therapy to treat chronic as well as infectious diseases including cancer and HIV implied that natural products will continue to play a pivotal role in drug discovery and development processes (Cragg and Newman 2013).
Although natural products are considered to be safe and their bioactive phytochemicals exhibit excellent pharmacological activities in vitro, they often failed to translate this into clinical or therapeutic effects in vivo. The major problems associated with bioactive phytochemicals include their low oral absorption and rapid systemic clearance which ultimately lowers their bioavailability and therapeutic efficacy in vivo (Kumar et al. 2010; Rein et al. 2012). For instance, oral administration of curcumin at 500 mg/kg in Sprague-Dawley rats produced maximum plasma concentration (Cmax) of 0.06 ± 0.01 µg/ml after 41.7 ± 5.4 min (Yang et al. 2007), while in healthy human volunteers, curcumin was detected in the serum only when it was administered orally at a dose higher than 8 g (Lao et al. 2006). Similarly, other potent bioactive compounds such as quercetin (Almeida et al. 2018; Kasikci and Bagdatlioglu 2016), paclitaxel (Malingre et al. 2001; Tiwari and Amiji 2006), and ellagic acid (Bala et al. 2006; Lei et al. 2003) also produced extremely low oral bioavailability eventually resulting in suboptimal oral therapy in vivo. Predominant factors that led to the poor oral absorption of these natural bioactive compounds are their eminently low aqueous solubility, poor dispersibility, and bioaccessibility which are the prerequisites for absorption through the gastrointestinal (GI) tract. For example, the maximum solubility of curcumin in aqueous buffer pH 5.0 is only 11 ng/ml (Ma et al. 2019) and the aqueous solubility of ellagic acid is about 9.7 µg/ml (Alfei et al. 2019). In addition, instability of various bioactive compounds on exposure to environmental factors such as pH, enzymes, light, heat, oxygen, and moisture may also contribute to their degradation in vitro and in vivo (Bohn et al. 2015; Islam Shishir et al. 2018). Moreover, biological factors including permeability of the intestinal epithelium, the presence of efflux transporters such as P-glycoprotein and the induction or inhibition of metabolizing enzymes may also serve as barriers to oral bioavailability of several bioactive compounds (Chu et al. 2008; Ma et al. 2019; Xie et al. 2011). Overcoming these challenges has become one of the most important focuses of the current research on improving the oral bioavailability of bioactive compounds. Techniques such as micronization (Aguiar et al. 2018) or nanonization (Aditya et al. 2019; Borhan et al. 2014) to reduce the particle size, co-administration of phytochemicals with bio-enhancers such as piperine (Gorgani et al. 2017), micro- and nanoencapsulation with different types of coating materials (Dias et al. 2017; Ezhilarasi et al. 2013) are some of the approaches designed to achieve the oral bioavailability enhancement of bioactive compounds.
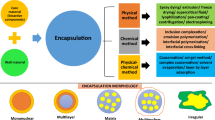
The process of applying relatively thin coatings around a substance which may be solids, liquids, or even gases is called encapsulation. Based on the size of the coated particles, the process may be microencapsulation or nanoencapsulation. Encapsulation separates the encapsulated materials also known as the core materials from the environment with the help of a coating material, usually a polymer. Thus, encapsulation provides protection of the core material and it may also add certain functionalities such as color for identification, masking of taste, or sustaining the release of the core materials. As a result, micro- or nanoencapsulation has become an indispensable technology in various industries including pharmaceuticals, cosmetics, nutraceuticals, and agriculture.
Encapsulation is one of the techniques that can be applied to enhance oral bioavailability, solubility, dispersibility, and stability of bioactive compounds (Beevers and Huang 2011; Ezhilarasi et al. 2013; Gomez-Estaca et al. 2015; Li et al. 2015; Shaikh et al. 2009). Numerous methods have been applied in the encapsulation of various natural bioactive compounds utilizing wide range of coating materials such as lipids, natural, and synthetic polymers (Anirudhan and Binusree 2016; Islam Shishir et al. 2018; Oehlke et al. 2017; Pinho et al. 2013; Shao et al. 2011; Sharma et al. 2007; Young et al. 2005). The selection of the coating materials is critical as the size, shape, and stability of the final encapsulated product as well as the release characteristics of the core material is largely determined by the type and amount of the coating materials used (Dias et al. 2017).
20.2 Rationale for Encapsulation of Bioactive Compounds
Encapsulation of natural bioactive compounds into various nano- or micro-platforms has the potential to resolve several drawbacks that are inherent to these phytochemicals. This may range from improving their physicochemical properties such as solubility and stability, to their pharmacokinetics such as absorption and the overall bioavailability. Some of the advantages offered by encapsulation of bioactive compounds can be summarized as follows:
(a) Enhanced bioavailability and therapeutic activity: Improvement in bioavailability of poorly absorbable phytochemicals such as polyphenols has been reported after their encapsulation into various systems such as solid lipid nanoparticles (SLN), micelles, liposomes, and others (Aqil et al. 2013; Murugan et al. 2009; Puligundla et al. 2017; Xie et al. 2011; Yang et al. 2008). This enhancement in bioavailability can be manyfold, as at least ninefold increase in oral bioavailability had been reported for poly(lactic-co-glycolic acid) (PLGA) nanoencapsulation of curcumin (Shaikh et al. 2009), while 942.53% enhancement in relative bioavailability was also reported for curcumin encapsulated in SLNs (Ji et al. 2014).
(b) Stability: Most of the bioactive compounds from plants are susceptible to light, heat, and pH changes developing unpleasant flavor or colors (Alborzi et al. 2012; Fang and Bhandari 2010; Prakash et al. 2018; Rezaei et al. 2019; Soukoulis and Bohn 2018). Encapsulation can prevent in vitro or in vivo degradation of bioactive compounds against these factors.
(c) Taste masking: Food or therapeutic applications of several phytochemicals have been limited by their unpleasant flavor, astringency, and bitter taste (De Souza et al. 2020). Encapsulation can eliminate this problem as the bioactive compound is not in contact with the taste buds located in the oral cavity. For instance, microencapsulation of quercetin with carnauba wax, shellac, or zein was able to reduce or mask the bitter taste of the compound (Khor et al. 2017), while the flavor of turmeric extract was effectively masked by brown rice flour and β-cyclodextrin-based microcapsules (Laokuldilok et al. 2016).
(d) Improvement of physicochemical properties: Micro- or nanoencapsulation offers the opportunity to improve the overall physicochemical properties such as morphology and wettability, presenting bioactive compounds into spherical, uniform size, and free-flowing powders which also facilitate their processing during manufacturing (Bertoni et al. 2019; Dima et al. 2016; Lu et al. 2011). Such improvement in powder flow property reduces the possibility of variation in the quality of the end products (Guajardo-Flores et al. 2015). Dissolution rate of poorly aqueous-soluble phytochemicals such as naringin and quercetin has also been reported to be enhanced by 20–55% (Pai et al. 2015) and 100 times (Barras et al. 2009), respectively, probably due to the dispersion of the bioactive compound in amorphous form within the matrix.
(e) Controlled or targeted release of bioactive compounds: Encapsulation enables controlled and targeted release of the encapsulated bioactive molecules, protecting against degradation and first-pass metabolism while enhancing their bioactivity following oral administration (Goiun 2004; Moreno et al. 2018; Sun et al. 2015; Yao et al. 2015) (Table 20.1).
20.3 Encapsulation of Bioactive Compounds
20.3.1 Microencapsulation
Microencapsulation is one of the most common methods employed to provide wide range of functionalities including protection of bioactive compounds against environmental factors and to enhance their bioavailability (Table 20.2). It is also possible to encapsulate bioactive oils such as essential oils and is a means of converting these oils into free-flowing powders which facilitate its handling, stability, and bioactivities. Due to their small and uniform particle size, microcapsules are distributed homogenously along the GI tract which promote absorption of their encapsulated compounds and enhance oral bioavailability (Lengyel et al. 2019). Various methods such as spray drying (Boonchu and Utama-ang 2015), spray congealing (Tomsik et al. 2019), coacervation phase separation (Silva et al. 2011; Jain et al. 2015), solvent evaporation (Paulo and Santos 2018; Sawale et al. 2017), and ionic gelation (Borgogna et al. 2010) have been utilized for encapsulating bioactive compounds. Wide range of wall-forming materials from natural and synthetic sources have been used in microencapsulation of bioactive compounds and the nature and quantity of these wall formers determined, to a large extent, the quality and functionality of the resultant microcapsules.
In an ideal delivery system, the bioactive compounds should not be allowed to affect the color or flavor or interact with the sensory organs and also the particles are presented small enough not to interfere with the texture (Champagne and Fustier 2007). Microencapsulation is a technology to achieve this objective in effective delivery of bioactive compounds. An extract of Gentiana lutea root containing bitter secoiridoids was coated with ethylcellulose–stearate system into microcapsules and was found to mask the bitterness in the mouth and the release of the bioactive compound in the GI effectively reduces the daily energy intake in human subjects (Mennella et al. 2016). Not only with the bitter taste alone, natural compounds often come with strong odor or flavor, leaving behind a sensation of astringency. Apart from enhancing its stability, the strong flavor and astringency of cinnamon extract, a rich source of proanthocyanidins, were successfully concealed by microcapsulation with gelatin/gum arabic and gelatin/κ-carrageenan systems (De Souza et al. 2020).
Spray drying is one of the most common methods employed for microencapsulation of bioactive compounds. Spray drying was employed to effectively encapsulate both the seed oils and peel extract of pomegranate to provide protection of the phenolics and fatty acid contents which are otherwise highly sensitive to environmental factors during their storage (Bustamante et al. 2017). Procyanidins extracted from grape seed oil was also encapsulated by spray-drying method using gum arabic and maltodextrin as coating materials and in this manner, the stability and shelf-life of the product were enhanced (Zhang et al. 2007). When freeze drying was compared against spray drying for microencapsulation of bioactive compounds extracted from Hibiscus Calyces using various encapsulating materials, encapsulation of anthocyanins was higher with freeze drying using gum arabic and yields higher antioxidant activity, while in terms of physical properties, better results were obtained with spray-drying method (Piovesana and Norena 2018).
Another improvement that microcapsules offered is the prospect of enhancing the solubility, and hence the oral bioavailability of bioactive compounds. Spray-congealing microencapsulation of wild garlic (Allium ursinum L.) extract was found to enhance the aqueous solubility more than 18 times of the pure extract, and in addition, there was only a minor decrease in the content of the bioactive compounds, allicin, and S-methyl methanethiosulfonate over 3-month period of storage (Tomsik et al. 2019). Microencapsulation also provides the opportunity for sustained or controlled release of bioactive compounds for prolonged therapeutic activity (Jain et al. 2015; Saifullah et al. 2019).
20.3.2 Nano-based Encapsulation Platforms for Bioactive Compounds
Various nanotechnology-based platforms have been developed to overcome the low oral bioavailability, systemic toxicity, or stability problems facing bioactive compounds. Enhancing oral bioavailability has been one of the major objectives of these nanomedicinal preparations. The main focus of such enhancement schemes encompasses the three oral bioavailability requirement steps like increasing the aqueous solubility of bioactive compounds, enhancing the lipid partitioning or permeability and reduction of first-pass metabolism (Pachuau 2019). Studies have shown that encapsulation of bioactive phytochemicals in various nanoparticulate systems was successful in achieving these objectives by controlling their release, facilitating their systemic absorption, bypassing pre-systemic metabolism, and enhancing the cellular uptake while also providing their stability (Ahmed et al. 2012; Aqil et al. 2013; Mukherjee et al. 2015).
20.3.2.1 Polymeric Nanocapsules
Nanocapsules differ from microcapsules only in their size; however, reduction of particle size to nanometer range leads to remarkable advancement in their physicochemical properties. Nanoencapsulation enhances dissolution of the drug, membrane permeability stabilization of labile drugs, and controlled release of the encapsulated drug (Pandey et al. 2005). As a result, polymeric nanocapsules facilitate both oral and parenteral administration of bioactive compounds. The type and amount of the wall formers in nanocapsules have been shown to influence the size, surface properties, stability, drug-loading capacity, aqueous solubility, and the release profile of the encapsulated compounds (Dos Santosa et al. 2016). Therefore, selection of appropriate wall-forming material is crucial to impart the desired functionality to the nanocapsules. Biocompatible and biodegradable poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymer poly(lactide-coglycolide) (PLGA) are among the most commonly used polymers for nanoencapsulation (Liu and Feng 2015). Encapsulation of ellagic acid in PLGA nanoparticles was found to increase the intestinal permeability from 66% in aqueous suspension of ellagic acid from to 87% in nanocapsules while also exhibiting its antioxidant effects (Bala et al. 2006). PLGA-based silymarin nanocapsule was also shown to sustain the release, improve bioavailability, and exhibit preferential toxicity toward prostate cancer cells, thus enhancing its overall therapeutic efficacy (Snima et al. 2014). Nanoencapsulation of curcumin with PLGA also delayed the progression of diabetic cataract in animal model (Grama et al. 2013) (Table 20.3).
Poorly aqueous-soluble anticancer phytochemical paclitaxel, isolated from Taxus brevifolia, which also suffers from P-glycoprotein-mediated efflux and metabolism by cytochrome P450 metabolic enzymes (Singla et al. 2002), had been alleviated through nanoencapsulation. The improvement in permeability of nanoencapsulated paclitaxel across Caco-2 monolayers was found to be 6.7–7.4 times of the lone paclitaxel and the plasma concentration time curve (AUC) and Cmax following oral administration also increased up to 5.7 times and 7.3 times, respectively (Iqbal et al. 2011).
Coating with mucoadhesive polymers is another means to improve oral bioavailability of bioactive compounds due to their ability to bring prolonged and intimate contact with GI tract surfaces resulting in better absorption. Chitosan has often been used to coat the polymeric nanocapsules due to its ability to adhere to the negatively charged mucosal surface, thus improving absorption and hence therapeutic activity (Chuah et al. 2014; Ensign et al. 2012; Mazzarino et al. 2012).
Capsaicin is a natural bioactive compound isolated from peppers with pungent odor and quick degradation and nanoencapsulation with natural wall formers gelatin and acacia was able to mask the pungency and instability of capsaicin (Jincheng et al. 2010; Wang et al. 2008). pH-dependent release and thermal stabilization of bioactive compounds have also been achieved using natural-based encapsulating materials (Akbari and Wu 2016; Pinheiro et al. 2015).
20.3.2.2 Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC)
Lipid-based nanocarriers like SLNs have become effective alternative delivery systems to conventional colloidal carriers like emulsions, liposomes, and polymeric micelles (Lin et al. 2017). In SLNs, the bioactive compounds are part of the solid lipid core matrix (Fig. 20.2) which is stabilized by a surfactant or a mixture of surfactants (Weiss et al. 2008). They provide a safe means of effective delivery system for poorly aqueous-soluble bioactive phytochemicals, enhancing their stability while also promoting their permeability and bioavailability. They originate from an O/W-type emulsion where the liquid oil (lipid) is replaced by solid oil that remains solid at body temperature (Akhavan et al. 2018). SLNs are biocompatible, considerably stable with good encapsulation efficiency, and can prolong the release of the encapsulated compounds (Akhavan et al. 2018; Weiss et al. 2008). The surface of SLN can also be functionalized with agents to facilitate their uptake and targeting the release of the drug to the desired site of action (Ganesan et al. 2018). In addition, SLN can also carry both lipophilic and hydrophilic drugs and can be sterilized and produced in large scale (Liu and Feng 2015). Nanostructured lipid carriers (NLC) are new generations of SLNs developed in the 1990s which incorporate a mixture of solid and liquid lipids to overcome the limitation of SLNs in effective drug delivery (Akhavan et al. 2018). NLCs improve the bioactive retention capacity of these nanostructures and facilitate controlled release of the bioactive compounds.

(Reproduced with permission from Lin et al. 2017, Copyright © Food and Drug Administration, Taiwan. Published by Elsevier Taiwan LLC)
Structures of solid lipid nanoparticles (SLNs)
Stabilization to oxidation offered by SLNs seemed to depend on the physicochemical properties of the encapsulated bioactives. Within the SLN, hydrophobic and high melting bioactive compounds like β-carotene are reported to be located within the inner matrix and kept at the α-subcell of the crystal structure thereby protecting it from oxidation, while less hydrophobic compounds like vitamin A arranged on the surfaces of the SLN leading to rapid oxidation (Salminen et al. 2016). However, between the SLNs and NLCs, NLCs were reported to provide better stability to β-carotene than the SLNs (Qian et al. 2013). The tendency of droplets aggregation and to coalescence still exists in SLNs which have the capacity to expel the encapsulated β-carotene to the particle exterior on storage, making it sensitive to degradation than when it is encapsulated in NLCs.
Flavonoids like quercetin and resveratrol suffer from low aqueous solubility, rapid metabolism, and photosensitivity which limited their oral bioavailability when taken orally (Li et al. 2009; Pandita et al. 2014). Formulation of quercetin into SLN remarkably enhances its gastrointestinal absorption and pharmacokinetic study in animal model demonstrated that the relative bioavailability of the SLN formulation against the quercetin suspension 571.4% indicating phenomenal increase in its oral bioavailability (Li et al. 2009). Stearic acid-based SLN of resveratrol was also reported to increase the oral bioavailability of the bioactive compound by eightfolds when compared to its suspension. Similarly, oral bioavailability of curcumin was also increased in a dose-dependent manner when formulated into soy lecithin-based SLN in animals. Improvement in oral bioavailability of curcumin-loaded SLN against solubilized curcumin rat plasma was reported to be 155 times at the dose of 1 mg/kg, 59 times at 12.5 mg/kg, 32 times at 25 mg/kg, and 39 times at the dose of 50 mg/kg (Kakkar et al. 2011). Bioavailability enhancement through SLN formulation has also been reported for other bioactive phytochemicals like an alkaloid vinpocetine which exhibit poor aqueous solubility and extensive first-pass metabolism (Luo et al. 2006; Medina 2010). Oral bioavailability from SLN vinpocetine formulation was found to be 4.16–4.17 times that of the suspension (Luo et al. 2006).
Recently, SLN-based sclareol was prepared following hot homogenization technique and evaluated its anti-proliferative activity in vitro against A549 human lung epithelial cancer cells which showed similar cytotoxicity to the plain sclareol but long-term stability and sustained release of sclareol were obtained with the SLN formulation (Hamishehkar et al. 2018).
NLC-based formulations also exhibit promising results in enhancing bioavailability of bioactive compounds. Silymarin was encapsulated in NLC-based formulation using glycerol distearates, oleic acids, lecithin, and Tween-80, which increases the oral bioavailability of silymarin against solid dispersion pellets and commercially available silymarin preparation, Legalon®, was 2.54- and 3.10-folds, respectively (Shangguan et al. 2014). However, when NLC was compared against microemulsion in its capacity to enhance oral bioavailability of luteolin in animal model, microemulsion fared better than the NLCs (Liu et al. 2014). The relative bioavailability of NLC-based and microemulsion-based luteolin formulations to the luteolin suspension were found to be 515.06% and 885.46%, respectively.
20.3.2.3 Liposomes and Phytosomes
Both liposomes and phytosomes are also lipid-based formulations suitable to facilitate the delivery of poorly aqueous-soluble phytochemicals. Liposomes are spherical vesicles (15–100 nm) consisting of phospholipid with a liquid core and variable layers, where the hydrophobic tails of the phospholipids face each other, while the hydrophilic heads are projected toward the inner aqueous core or the outside boundary of the liposome (Fig. 20.3) (Bonechi et al. 2019; Emami et al. 2018). They resemble biological membrane and can accommodate both hydrophilic and hydrophobic drugs within their layers which make them unique carrier for drug delivery. In spite of their advantages, successful delivery of liposomes through oral route has always been a challenge as the question remains whether they would remain stable along the GI tract conditions (Park et al. 2011). However, the effective delivery of phytochemicals through oral route has been demonstrated in animal models (Yi et al. 2013). Flammulina velutipes sterols was orally delivered through liposomes in Kunming mice and relative bioavailability of ergosterol and 22,23- dihydroergosterol at 162.9 and 244.2%, respectively, was obtained. The sterols, however, was rapidly eliminated within 4 h. A faster and better absorption of curcumin was also reported when it was encapsulated in leicithin-based liposome exhibiting improved oral bioavailability in animal model (Takahashi et al. 2009). Formulating the Curcuma longa (Ukon) extract (LUE) into liposomes was found to deliver better protection against carbon tetrachloride-induced liver injury as compared to the uncapsulated extract (Takahashi et al. 2008). Liposomes are also adaptable to functionalization with targeting ligands to improve their effectiveness in cancer therapy. Kappaphycus alvarezii extract containing multi-bioactive compounds was encapsulated into PEGylated-liposome and then functionalized with folic acid to target folate positive breast cancer cells (MCF-7) (Baskararaj et al. 2020). The constructed liposome was highly stable in the physiological buffers with steady drug release and also exhibit cytotoxicity toward MCF-7 in a concentration-dependent manner.

(Reproduced with permission from Selmaty et al. 2010, Copyright © Elsevier Science BV 2009)
Major difference between liposome and phytosome®
Liposomal formulation also contributes to the improvement in physicochemical properties of natural bioactive compounds such as solubility and stability. Chemical stability of resveratrol was enhanced by liposomes and along with 3-oxo-C12-homoserine lactone, about 70% inhibition of tumor growth in murine tumor model was reported when they are administered intravenously (Coimbra et al. 2011). Encapsulation of enriched-phenolic fraction of the extract from Pistacia vera L into nanoliposome exhibits multiple bioactivity including antioxidant, anti-inflammatory, and anti-melanogenic activities making it a promising cosmeceutical preparation for skin pigmentation disorders (Oskoueian et al. 2020). Promotion of stability and enhancement in bioactivity of quercetin (Hao et al. 2017) and bitter gourd (Momordica charantia) (Erami et al. 2019) were also reported with their nanoliposomal formulations.
Proliposome systems were also developed with an objective of enhancing the stability and oral bioavailability of the encapsulated drugs. Compared to liposomes, proliposomes are highly stable and obtained in dry, free-flowing particles which form liposomal suspension immediately on contact with water (Yan-yu et al. 2006). Presenting the whole system in the form of solid makes proliposome highly stable while the intrinsic pharmacological properties remain intact.
Phytosomes are complex of phospholipid with herbal extracts developed to enhance the oral bioavailability of natural bioactive compounds. It is a patented technology developed in Italy around 1989 and several phytosome products are already available in the market (Pachuau 2019). The complexation of phosphatidylcholine (phospholipid) with polyphenols in phytosomes led to the formation of intermolecular bond between these two and the amphiphilic nature of the phosphatidylcholine promotes the miscibility of the complex with aqueous and lipid solvents which guide the polyphenol to permeate the lipid-based GI tract lumen (Kidd 2009). Delivery of polyphenols in the form of phytosomes has the capacity to enhance the oral bioavailability of polyphenols at least by 2–6 times (Ajazuddin and Saraf 2010; Kidd 2009). The ratio of the bioactive compounds to the phosphatidylcholine has been considered to be important factor in phytosome formulations. This bioactive compound:phospholipid ratio may range from 0.5:1 to 3:1 and the optimum ratio may depend on the kind of phytochemicals being complexed (Pachuau 2019). Frequently, a combination of bioactive phytochemicals has been incorporated into phytosomes to attain their synergistic effects (Rathee and Kamboj 2018; Sharma and Sahu 2016).
20.3.2.4 Self-emulsifying Systems
Both micro- and nano-self-emulsifying systems are capable of enhancing oral bioavailability of poorly aqueous-soluble drugs by enhancing their solubility and dissolution rate. They are composed of a lipid, surfactant, bioactive compound, and a co-surfactant, and following oral administration, they swiftly form microemulsions with particle size less than 100 nm within the GI fluid bringing the drug into solution for enhanced absorption (Cui et al. 2009; Dwivedi et al. 2014). Micro- and nano-self-emulsifying systems have been demonstrated to enhance the oral bioavailability of various phytochemicals including curcumin (Cui et al. 2009), arteether (Dwivedi et al. 2014), silymarin (Li et al. 2010; Wu et al. 2006), curcumin, and thymoquinone (Alwadei et al. 2019), naringenin (Khan et al. 2015) as well as plant extracts (Arun and Maneesh 2016).
20.3.2.5 Niosomes
Niosomes are self-assembled non-ionic surfactant vesicles analogous to phospholipid vesicles of liposomes where hydrophilic drugs are encapsulated and hydrophobic drugs are partitioned into the hydrophobic tails of the non-ionic surfactant (Hu and Rhodes 1999; Uchegbu and Vyas 1998). Niosomes are more stable than liposomes and are more economical to prepare from a wide range of surfactants which make them an attractive alternative to liposomes (Song et al. 2015). Niosomal preparations are reportedly initiated from the cosmetic industry in the 1970s and gradually expanded toward drug delivery applications including phytochemicals (Muzzalupo and Mazzotta 2019). Niosomes of poorly soluble D-limonene were reported to prolong the release of D-limonene and its cytotoxicity toward HepG2, Macf-7, and A549 cancer cells was significantly enhanced (Hajizadeh et al. 2019). Niosome also facilitates the solubilization of Lawsone and compared to free Lawsone solution, the antitumor activity of Lawsone noisome against MCF-7 breast cancer cells was increased significantly (Barani et al. 2018).
Studies have shown that niosomes are also effective carrier for the delivery of bioactive phytochemicals across the skin. Compared to their solutions, more efficient delivery of ellagic acid across the human epidermis and dermis was observed when it was formulated as niosomes and this penetration of the skin was reported to be dependent on the vesicle size (Junyaprasert et al. 2012).
Niosomes may suffer from the issue of aggregation, fusion, and leakage of the encapsulated bioactive compounds. To alleviate this problem, proniosomes have been prepared which are dry and free-flowing type that spontaneously form niosomes on gentle agitation with hot water. Proniosomes were demonstrated to increase the oral bioavailability of poorly soluble alkaloid, vinpocetine by about 4- to 4.9-folds (Song et al. 2015).
20.3.2.6 Hydrogels
Hydrogels are hydrophilic three-dimensional polymer networks which can take up large amount of water. They are biodegradable and the three-dimensional network of the hydrogel disintegrates into non-toxic materials with excellent biocompatibility (Akhtar et al. 2016). Hydrogels are prepared from wide ranges of natural and synthetic polymers and can be presented in the various physical forms such as beads, microparticles, nanoparticles, and films and they have become one of the promising encapsulating systems to provide protection to sensitive phytochemicals and for their controlled delivery (Abaee et al. 2017; Gomez-Mascaraque et al. 2016a). The advantages hydrogels offer in encapsulation of bioactive compounds include the following:
-
(i)
Enhancing stability and bioacessibility of sensitive, poorly aqueous-soluble bioactive molecules (Gomez-Mascaraque et al. 2016a; Han et al. 2020; Zhang et al. 2016).
-
(ii)
pH-dependent and controlled release of bioactive compounds (Bourbon et al. 2016; Lopez Cordoba et al. 2013; Rutz et al. 2013).
-
(iii)
Efficient encapsulation of both hydrophilic and hydrophobic bioactive compounds (Bourbon et al. 2016).
-
(iv)
Enhancing bioactivity (Chan et al. 2010).
-
(v)
Improving bioavailability (McClements 2017).
-
(vi)
High drug-loading capacity due to their large spacing within the polymeric network (Teng et al. 2015).
Various hybrid hydrogel systems such as emulsion hydrogels, organogels and bigels have also been evaluated for encapsulation of bioactive compounds. Encapsulation of bioactive compounds within these networks can improve their stability, solubility and dispersibility thereby improving their overall bioavailability (Mao et al. 2019). Suitable functionalities such as responsiveness to pH, temperature, enzymes etc. can also be imparted to these hybrid hydrogels to provide the desired release characteristics of the formulation (McClements 2017). In emulsion (O/W) gel systems, the continuous aqueous phase gets solidified or gelled to provide structural integrity and flexibility to the whole system. As a result, it has wide application in functional food industry. Bioactive compounds such as curcumin (Geremias-Andrade et al. 2017), quercetin (Chen et al. 2018), α-tocopherol (Freire et al. 2018) have been delivered through emulsion gel systems with improved stability, bioaccessibility and controlled release. In addition, the structural state of emulsion hydrogels also allows them to be used as an efficient fat-replacement in various food products without affecting their sensory characteristics thereby promoting health benefits to consumers (Pintado et al. 2015, 2016).
20.3.2.7 Electrodynamic Processes
In recent years, electrodynamic encapsulation processes such as electrospraying and electrospinning have received increasing attention as an alternative to the more established and commonly employed techniques such as microencapsulation (Alehosseini et al. 2018; Jacobsen et al. 2018). During electrospraying, the high voltage electrostatic force converts the drug-loaded liquid solutions into fine droplets and gets evaporated during their flight toward the ground electrode (Alehosseini et al. 2018). A high degree of molecular interaction in the sprayed liquid produces electrospun fibers, while lower degree of interaction results in electrosprayed particles (Fig. 20.4). In conventional spray-drying process, drugs or bioactive compounds are exposed to high temperature in the range of 170–220 °C to dry and encapsulate the material and this condition of drying may degrade thermolabile drug substances (Jacobsen et al. 2018). However, in electrospraying, exposure to such high temperature has been eliminated which makes it more conducive for encapsulation of heat-sensitive compounds. Electrodynamic encapsulation of bioactive compounds also offers the following advantages (Drosou et al. 2017; Ghorani and Tucker 2015; Wen et al. 2017a; Wen et al. 2017b):

(Electrospinning) (Reproduced with permission from Ghorani and Tucker 2015, Copyright © Elsevier Science BV 2015)
Left: Schematic of physical representation at the molecular level of entanglement regimes for dilute and concentrated polymer concentration. Middle: Schematic diagram of a basic electrospinning (jet formation) and electrospraying (liquid-droplet atomization) processes. Right: Examples of SEM image of microspheres (electrospraying) and nanofibers
-
(i)
It is a relatively simple, cost-effective, and flexible method for fabrication of nanomaterials.
-
(ii)
It has the potential to scale up to large-scale manufacturing.
-
(iii)
The method is adaptable to wide range of encapsulating materials.
-
(iv)
Enhanced stability of bioactive compounds.
-
(v)
High surface-to-volume ratio of the nanoscale materials results in better bioavailability.
-
(vi)
Both hydrophilic and hydrophobic bioactive compounds can be encapsulated.
Electrospraying was employed to successfully encapsulate β-carotene in its solubilized form to enhance its stability and also to improve its bioavailability (Basar et al. 2020). When exposed under UV light, the free-β-carotene degrades within 180 min while only 20% degradation was found with the encapsulated β-carotene. Wide ranges of polymers are adaptable to electrodynamic processing including polysaccharides cellulose and its derivatives, guar gum, pectin, starch, chitosan, alginate, etc. (Wen et al. 2017b). Viscosity, surface tension, and electrical conductivity of the polymeric solution were found to influence the morphology of the electrosprayed capsules or fibers (Gomez-Mascaraque et al. 2016b).
20.3.2.8 Solid Dispersion and Micelles
Solid dispersion technique is one of the most commonly employed methods to improve the solubility and bioavailability of poorly aqueous-soluble drugs. Crystalline drugs often exhibit poor solubility in aqueous-based solvents as the lattice energy must be overcome to bring them into solution (Li et al. 2013). Formulation into amorphous solid dispersion helps solubilization of such crystalline drugs as the encapsulating polymers in the solid dispersions trapped them in the amorphous state which facilitates their dissolution once they come in contact with the aqueous solvents. Various cellulose derivatives have been reported to exhibit strong enhancement of curcumin dissolution while also protecting from chemical degradation and affecting pH-dependent release (Li et al. 2013). Various formulations of curcumin such as nanocrystal solid dispersion prepared with hydroxypropyl cellulose SL (CSD-Cur), amorphous solid dispersion with hydroxypropyl methylcellulose acetate succinate (ASD-Cur), and nanoemulsion (NE-Cur) were prepared and the increase in oral bioavailability compared to the unformulated curcumin was reported to be 12-folds for ASD-Cur, 16-folds for CSD-Cur and, 9-folds for NE-Cur (Onoue et al. 2010).
Self-assembled colloidal dispersion of amphiphilic surfactants, also known as micelles, has been another important technique to enhance the solubility of hydrophobic drugs. Certain amphiphilic polymers are also known to form micelles and are known as polymeric micelles (Husseini and Pitt 2008). Polymeric micelles have been shown to enhance the solubility and bioactivity of bioactive compounds. When Pluronic P123- and Solutol HS15-based polymeric micelles were prepared loaded with naringenin, sustained release of naringerin and significant enhancement in its cytotoxicity against cancer cell line were observed (Zhai et al. 2013). Oral bioavailability of paclitaxel in glycyrrhizic acid micelle was also found to enhance sixfolds as compared to Taxol (Yang et al. 2015).
20.4 Conclusion
Enhancement in stability and bioavailability of phytochemicals has been one of the most important challenges in their effective utilization. Thousands of bioactive compounds have been isolated and characterized over the years; however, their therapeutic effectiveness in vivo is often limited by their poor physicochemical properties and modest oral absorption. Several studies have demonstrated that encapsulation into various micro-and nanoplatforms is capable of alleviating such obstacle. Microencapsulation techniques have been proven and commonly employed method for such enhancement. Novel techniques like hydrogels and their variants such as emulsion gels, organo gels, and bigels along with electrodynamic processes like electrospraying and electrospinning techniques are certainly promising approaches to facilitate the effective delivery of these bioactive compounds.
References
Abaee A, Mohammadian M, Jafari SM (2017) Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trend Food Sci Technol 70:69–81
Aditya NP, Hamilton IE, Noon J, Norton IT (2019) Microwave assisted nanonization of poorly water-soluble curcumin. ACS Sustain Chem Eng 7:9771–9781
Aguiar GPS, Arcari BD, Chaves LMPC, Magro CD, Boschetto DL, Piato AL et al (2018) Micronization of trans-resveratrol by supercritical fluid: dissolution, solubility and in vitro antioxidant activity. Ind Crop Prod 112:1–5
Ahmed K, Li Y, McClements DJ, Xiao H (2012) Nanoemulsion-and emulsion-based delivery systems for curcumin: encapsulation and release properties. Food Chem 132:799–807
Ajazuddin and Saraf S (2010) Applications of novel drug delivery system for herbal formulations, Fitoterapia 81: 680–689
Akbari A, Wu J (2016) Cruciferin nanoparticles: Preparation, characterization and their potential application in delivery of bioactive compounds. Food Hydrocolloid 54:107–118
Akhavan S, Assadpour E, Katouzian I, Jafari SM (2018) Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trend Food Sci Technol 74:132–146
Akhtar MF, Hanif M, Ranjha NM (2016) Methods of synthesis of hydrogels. A review, Saudi Pharm J 24:554–559
Al Juhaimi F, Ozcan MM, Ghafoor K, Babiker EE, Hussain S (2018) Comparison of cold-pressing and soxhlet extraction systems for bioactive compounds, antioxidant properties, polyphenols, fatty acids and tocopherols in eight nut oils. J Food Sci Technol 55:3163–3173
Alborzi S, Lim LT, Kakuda Y (2012) Encapsulation of folic acid and its stability in sodium alginate-pectin-poly(ethylene oxide) electrospun fibres. J Microencapsul 30:64–71
Alehosseini A, Ghorani B, Sarabi-Jamab M, Tucker N (2018) Principles of electrospraying: a new approach in protection of bioactive compounds in foods. Crit Rev Food Sci Nutr 58:2346–2363
Alfei S, Turrini F, Catena S, Zunin P, Parodi B, Zuccari G et al (2019) Preparation of ellagic acid micro and nano formulations with amazingly increased water solubility by its entrapment in pectin or non-PAMAM dendrimers suitable for clinical applications. New J Chem 43:2438–2448
Almeida AF, Borge GIA, Piskula M, Tudose A, Tudoreanu L, Valentová K et al (2018) Bioavailability of quercetin in humans with a focus on interindividual variation. Compr Rev Food Sci F 17:714–731
Altemini A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA (2017) Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6:42
Alwadei M, Kazi M, Alanazi FK (2019) Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals—curcumin and thymoquinone. Saudi Pharm J 27:866–876
Anirudhan TS, Binusree J (2016) Dextran based nanosized carrier for the controlled and targeted delivery of curcumin to liver cancer cells. Int J Biol Macromol 88:222–235
Aqil F, Munagala R, Jeyabalan J, Vadhanam MV (2013) Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett 334:133–141
Arun K, Maneesh J (2016) Effect of Self Nanoemulsifying Drug Delivery System (SNEDDS) on Intestinal Permeation and Anti-Diabetic Activity of Berberis aristata Extract: In-Vitro and Ex-Vivo Studies. J Nanopharm Drug Deliv 3:51–62
Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P et al (2015) Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv 33:1582–1614
Bala I, Bhardwaj V, Hariharan S, Kharade SV, Roy N, Ravi Kumar MNV (2006) Sustained release nanoparticulate formulation containing antioxidant ellagic acid as potential prophylaxis system for oral administration. J Drug Target 14:27–34
Barani M, Mirzaei M, Torkzadeh-Mahani M, Nematollahi MH (2018) Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast cancer cell line: a nano-herbal treatment for cancer. Daru 26:11–17
Barras A, Mezzetti A, Richard A, Lazzaroni S, Roux S, Melnyk P et al (2009) Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int J Pharm 379:270–277
Basar AO, Prieto C, Durand E, Villeneuve P, Sasmazel HT, Lagaron J (2020) Encapsulation of β-carotene by emulsion electrospraying using deep eutectic solvents. Molecules 25:981
Baskararaj S, Panneerselvam T, Govindaraj S, Arunachalam S, Parasuraman P, Pandian SRK et al (2020) Formulation and characterization of folate receptor-targeted PEGylated liposome encapsulating bioactive compounds from Kappaphycus alvarezii for cancer therapy. Biotech 10:136
Beevers CS, Huang S (2011) Pharmacological and clinical properties of curcumin. Botanics 1:5–18
Bernhoft A (2010) A brief review on bioactive compounds in plants. Bernhoft A(ed) Bioactive compounds in plants -benefits and risks for man and animals. Norwegian Acad Sci Lett, Oslo, pp 11–17
Bertoni S, Albertini B, Passerini N (2019) Spray Congealing: an emerging technology to prepare solid dispersions with enhanced oral bioavailability of poorly water soluble drugs. Molecules 24:3471
Bohn T, McDougall GJ, Alegría A, Alminger M, Arrigoni E, Aura AM et al (2015) Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites–a position paper focusing on carotenoids and polyphenols. Mol Nutr Food Res 59:1307–1323
Bonechi C, Donati A, Tamasi G, Pardini A, Rostom H, Leone G (2019) Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys Chem 246:25–34
Boonchu T, Utama-ang N (2015) Optimization of extraction and microencapsulation of bioactive compounds from red grape (Vitis vinifera L.) pomace. J Food Sci Technol 52:783–792
Borgogna M, Bellich B, Zorzin L, Lapasin R, Cesaro A (2010) Food microencapsulation of bioactive compounds: Rheological and thermal characterisation of non-conventional gelling system. Food Chem 122:416–423
Borhan MZ, Ahmad R, Rusop M, Abdullah S (2014) Effect of nanonization on physicochemical properties of Centella asiatica powders. Adv Mat Res 917:106–112
Bourbon AI, Cerqueira MA, Vicente AA (2016) Encapsulation and controlled release of bioactive compounds in lactoferrin-glycomacropeptide nanohydrogels: Curcumin and caffeine as model compounds. J Food Eng 180:110–119
Bustamante A, Hinojosa A, Robert P, Escalona V (2017) Extraction and microencapsulation of bioactive compounds from pomegranate (Punica granatum var. Wonderful) residues. Int J Food Sci Technol 52:1452–1462
Champagne CP, Fustier P (2007) Microencapsulation for the improved delivery of bioactive compounds into foods. Curr Opin Biotechnol 18:184–190
Chan E, Yim Z, Phan S, Mansa RF, Ravindra P (2010) Encapsulation of herbal aqueous extract through absorption with ca-alginate hydrogel beads. Food Bioprod Process 88:195–201
Chen X, McClements DJ, Zhu Y, Zou L, Li Z, Liu W et al (2018) Gastrointestinal fate of fluid and gelled nutraceutical emulsions: impact on proteolysis, lipolysis, and quercetin bioaccessibility. J Agric Food Chem 66:9087–9096
Choi AY, Kim CT, Park HY, Kim HO, Lee NR, Lee KE et al (2013) Pharmacokinetic characteristics of capsaicin-loaded nanoemulsions fabricated with alginate and chitosan. J Agric Food Chem 61:2096–2102
Chu Z, Chen JS, Liau CT, Wang HM, Lin YC, Yang MH et al (2008) Oral bioavailability of a novel paclitaxel formulation (Genetaxyl) administered with cyclosporin a in cancer patients. Anticancer Drugs 19:275–281
Chuah LH, Roberts CJ, Billa N, Abdullah S, Rosli R (2014) Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloid Surface B 116:228–236
Coimbra M, Isacchi B, van Bloois L, Torano JS, Ket A, Wu X et al (2011) Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int J Pharm 416:433–442
Cragg GM, Newman DJ (2013) Natural products: a continuing source of novel drug leads. Biochim Biophys Acta - General Subjects 1830:3670–3695
Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H et al (2009) Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm 371:148–155
Da Silva BV, Barreira JCM, Oliveira MBPP (2016) Natural phytochemicals and probiotics as bioactive ingredients for functional foods: extraction, biochemistry and protected-delivery technologies. Trends Food Sci Technol 50:144–158
David B, Wolfender J, Dias DA (2015) The pharmaceutical industry and natural products: historical status and new trends. Phytochem Rev 14:299–315
De Souza VB, Thomazini M, Chaves IE, Ferro-Furtado R, Favaro-Trindade CS (2020) Microencapsulation by complex coacervation as a tool to protect bioactive compounds and to reduce astringency and strong flavor of vegetable extracts. Food Hydrocoll 98:105244
Dias DR, Botrel DA, De Barros Fernandes VR, Borges SV (2017) Encapsulation as a tool for bioprocessing of functional foods. Curr Opin Food Sci 13:31–37
Dima C, Patraşcu L, Cantaragiu A, Alexe P, Dima S (2016) The kinetics of the swelling process and the release mechanisms of Coriandrum sativum L. essential oil from chitosan/alginate/inulin microcapsules. Food Chem 195:39–48
Donsi F, Annunziata M, Sessa M, Ferrari G (2011) Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT-Food Sci Technol 44:1908–1914
Dos Santosa PP, Flores SH, Rios AO, Chiste RC (2016) Biodegradable polymers as wall materials to the synthesis of bioactive compound nanocapsules. Trend Food Sci Technol 53:23–33
Drosou CG, Krokida MK, Biliaderis CG (2017) Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: a comparative assessment of food-related applications. Drying Technol 35:139–162
Dutta RS, Hauzel L, Roy PK, Kalita P, Devi TB, Deka D et al (2018) Nanoprecipitated ethylcellulose-curcumin particles for controlled release and enhanced antioxidant activity. Curr Nanosci 14:298–306
Dwivedi P, Khatik R, Khandelwal K, Srivastava R, Taneja I, Rama Raju KS et al (2014) Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of arteether: pharmacokinetics, toxicity and antimalarial activity in mice. RSC Adv 4:64905–64918
Emami S, Azadmard-Damirchi S, Peighambardoust SH, Valizadeh H, Hesariet J (2018) Liposomes as carrier vehicles for functional compounds in food sector. J Experiment Nanosci 11:737–759
Engel JB, Heckler C, Tondo EC, Daroit DJ, Da Silva Malheiros P (2017) Antimicrobial activity of free and liposome-encapsulated thymol and carvacrol against Salmonella and Staphylococcus aureus adhered to stainless steel. Int J Food Microbiol 252:18–23
Ensign LM, Cone R, Hanes J (2012) Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv Drug Deliv Rev 64:557–570
Erami SR, Amiri ZR, Jafari SM (2019) Nanoliposomal encapsulation of Bitter Gourd (Momordica charantia) fruit extract as a rich source of health-promoting bioactive compounds. LWT - Food Sci Technol 116:108581
Ezhilarasi PN, Karthik P, Chhanwal N, Anandharamakrishnan C (2013) Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Technol 6:628–647
Ezpeleta I, Irache JM, Stainmesse S, Chabenat C, Gueguen J, Popineau Y et al (1996) Gliadin nanoparticles for the controlled release of all-trans-retinoic acid. Int J Pharm 131:191–200
Fang Z, Bhandari B (2010) Encapsulation of polyphenols: a review. Trends Food Sci Technol 21:510–523
Feng Y, Sun C, Yuan Y, Zhu Y, Wan J, Firempong CK et al (2016) Enhanced oral bioavailability and in vivo antioxidant activity of chlorogenic acid via liposomal formulation. Int J Pharm 501:342–349
Freire M, Cofrades S, Perez-Jimenez J, Gomez-Estaca J, Jimenez-Colmenero F, Bou R (2018) Emulsion gels containing n-3 fatty acids and condensed tannins designed as functional fat replacers. Food Res Int 113:465–473
Ganesan P, Ramalingam P, Karthivashan G, Ko YT, Choi DK (2018) Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int J Nanomed 13:1569–1583
Geremias-Andrade IM, Souki NPDBG, Moraes ICF, Pinho SC (2017) Rheological and mechanical characterization of curcumin-loaded emulsion-filled gels produced with whey protein isolate and xanthan gum. LWT Food Sci Technol 86:166–173
Ghorani B, Tucker N (2015) Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll 51:227–240
Goiun S (2004) Microencapsulation: industrial appraisal of existing technologies and trends. Trend Food Sci Technol 15:330–347
Gomez-Estaca J, Gavara R, Hernandez-Munoz P (2015) Encapsulation of curcumin in electrosprayed gelatin microspheres enhances its bioaccessibility and widens its uses in food applications. Innov Food Sci Emerg Technol 29:302–307
Gomez-Mascaraque LG, Soler C, Lopez-Rubio A (2016a) Stability and bioaccessibility of EGCG within edible micro-hydrogels. Chitosan vs. gelatin, a comparative study. Food Hydrocoll 61:128–138
Gomez-Mascaraque LG, Sanchez G, Lopez-Rubio A (2016b) Impact of molecular weight on the formation of electrosprayed chitosan microcapsules as delivery vehicles for bioactive compounds. Carbohydr Polym 150:121–130
Gorgani L, Mohammadi M, Najafpour GD, Nikzad M (2017) Piperine—The bioactive compound of black pepper: From isolation to medicinal formulations. Compr Rev Food Sci F 16:124–140
Grama CN, Suryanarayana P, Patil MA, Raghu G, Balakrishna N, Ravi Kumar MNV et al (2013) Efficacy of biodegradable curcumin nanoparticles in delaying cataract in diabetic rat model. PLoS ONE 8:e78217
Guajardo-Flores D, Rempel C, Gutierrez-Uribe JA, Serna-Saldivar SO (2015) Influence of excipients and spray drying on the physical and chemical properties of nutraceutical capsules containing phytochemicals from black bean extract. Molecules 20:21626–21635
Hajizadeh MR, Maleki H, Barani M, Fahmidehkar MA, Mahmoodi M, Torkzadeh-Mahani M (2019) In vitro cytotoxicity assay of D-limonene niosomes: an efficient nano-carrier for enhancing solubility of plant-extracted agents. Res Pharm Sci 14:448–458
Hamishehkar H, Bahadori MB, Vandghanooni S, Eskandani M, Nakhlband A, Eskandani M (2018) Preparation, characterization and anti-proliferative effects of sclareol loaded solid lipid nanoparticles on A549 human lung epithelial cancer cells. J Drug Deliv Sci Technol 45:272–280
Han J, Zhang Z, Shang W, Yan J, McClements DJ, Xiao H et al (2020) Modulation of physicochemical stability and bioaccessibility of β-carotene using alginate beads and emulsion stabilized by scallop (Patinopecten yessoensis) gonad protein isolates. Food Res Int 129:108875
Hao J, Guo B, Yu S, Zhang W, Zhang D, Wang J (2017) Encapsulation of the flavonoid quercetin with chitosan-coated nano-liposomes. LWT - Food Sci Technol 85:37–44
Hu C, Rhodes DG (1999) Proniosomes: a novel drug carrier preparation. Int J Pharm 185:23–35
Huang H, Belwal T, Liu S, Duan Z, Luo Z (2019) Novel multi-phase nano-emulsion preparation for co-loading hydrophilic arbutin and hydrophobic coumaric acid using hydrocolloids. Food hydrocolloid 93:92–101
Husseini GA, Pitt WG (2008) Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv Drug Deliv Rev 60:1137–1152
Iqbal J, Sarti F, Perera G, Bernkop-Schnurch A (2011) Development and in vivo evaluation of an oral drug delivery system for paclitaxel. Biomaterials 32:170–175
Islam Shishir MR, Xie L, Sun C, Zheng X, Chen W (2018) Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci Technol 78:34–60
Jacobsen C, Garcia-Moreno PJ, Mendes AC, Mateiu RV, Chronakis IS (2018) Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Annu Rev Food Sci Technol 9:525–549
Jain A, Thakur D, Ghoshal G, Katare OP, Shivhare US (2015) Microencapsulation by complex coacervation using whey protein isolates and gum acacia: an approach to preserve the functionality and controlled release of β-carotene. Food Bioprocess Technol 8:1635–1644
Ji H, Tang J, Li M, Ren J, Zheng N, Wu L (2014) Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Deliv 23:459–470
Jincheng W, Xiaoyu Z, Sihao C (2010) Preparation and properties of nanocapsulated capsaicin by complex coacervation method. Chem Eng Comm 197:919–933
Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D (2012) Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int J Pharm 423:303–311
Kakkar V, Singh S, Singla D, Kaur IP (2011) Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res 55:495–503
Kasikci MB, Bagdatlioglu N (2016) Bioavailability of quercetin. Curr Nutr Food Sci 4:146–151
Katuwavila NP, Perera AD, Karunaratne V, Amaratunga GA, Karunaratne D (2016) Improved delivery of caffeic acid through liposomal encapsulation. J Nanomat 9701870:1–7
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J (2015) Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv 22:552–561
Khan H, Marya Belwal T, Tariq M, Atanasov AG, Devkota HP (2019) Genus Vanda: A review on traditional uses, bioactive chemical constituents and pharmacological activities. J Ethnopharmacol 229:46–53
Khor CM, Ng WK, Kanaujia P, Chan KP, Dong Y (2017) Hot-melt extrusion microencapsulation of quercetin for taste-masking. J Microencapsul 34:29–37
Kidd PM (2009) Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev 14:226–246
Kumar A, Ahuja A, Ali J, Baboota S (2010) Conundrum and therapeutic potential of curcumin in drug delivery. Crit Rev Ther Drug 27:279–312
Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM et al (2006) Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 6:10
Laokuldilok N, Thakeow P, Kopermsub P, Utama-ang N (2016) Optimisation of microencapsulation of turmeric extract for masking flavor. Food Chem 194:695–704
Lei F, Xing D, Xiang L, Zhao Y, Wang W, Zhang L (2003) Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J Chromatogr B 796:189–194
Lengyel M, Kallai-Szabo N, Antal V, Laki AJ, Antal I (2019) Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci Pharm 87:20
Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H (2009) Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release 133:238–244
Li X, Yuan Q, Huang Y, Zhou Y, Liu Y (2010) Development of silymarin self-microemulsifying drug delivery system with enhanced oral bioavailability. AAPS PharmSciTech 11:672–678
Li B, Konecke S, Wegiel LA, Taylor LS, Edgar KJ (2013) Both solubility and chemical stability of curcumin are enhanced by solid dispersion in cellulose derivative matrices. Carbohydr Polym 98:1108–1116
Li Z, Jiang H, Xu C, Gu L (2015) A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll 43:153–164
Lin C, Chen C, Lin Z, Fang J (2017) Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal 25:219–234
Liu Y, Feng N (2015) Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv Colloid Interfac 221:60–76
Liu Y, Wang L, Zhao Y, He M, Zhang X, Niu M et al (2014) Nanostructured lipid carriers versus microemulsions for delivery of the poorly water-soluble drug luteolin. Int J Pharm 476:169–177
Lopez Cordoba A, Deladino L, Martino M (2013) Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohydr Polym 95:315–323
Lu Q, Li D, Jiang J (2011) Preparation of a tea polyphenol nanoliposome system and its physicochemical properties. J Agric Food Chem 59:13004–13011
Luo Y, Chen D, Ren L, Zhao X, Qin J (2006) Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J Control Release 114:53–59
Ma Z, Na W, He H, Tang X (2019) Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J Control Release 316:359–380
Mady FM, Shaker MA (2017) Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int J Nanomed 12:7405
Malingre MM, Beijnen JH, Schellens JHM (2001) Oral delivery of taxanes. Invest New Drug 19:155–162
Mao L, Lu Y, Cui M, Miao S, Gao Y (2019) Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit Rev Food Sci Nutr 20:1–16
Martins N, Barros L, Ferreira ICFR (2016) In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci Technol 48:1–12
Mazzarino L, Travelet C, Murillo SO et al (2012) Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J Colloid Interfac Sci 370:58–66
McClements DJ (2017) Recent progress in hydrogel delivery systems for improving nutraceutical bioavailability. Food Hydrocoll 68:238–245
Medina AE (2010) Vinpocetine as a potent antiinflammatory agent. P Natl Acad Sci USA 107:9921–9922
Mennella I, Fogliano V, Ferracane R, Arlorio M, Pattarino F, Vitaglione P (2016) Microencapsulated bitter compounds (from Gentiana lutea) reduce daily energy intakes in humans. Br J Nutr 116:1841–1850
Moreno T, Cocero MJ, Rodriguez-Rojo S (2018) Storage stability and simulated gastrointestinal release of spray dried grape marc phenolics. Food Bioprod Process 112:96–107
Mukherjee PK, Harwansh RK, Bhattacharyya S (2015) Bioavailability of herbal products: approach towards improved pharmacokinetics, In: Mukherkee PK (ed) Evidence-based validation of herbal medicines. Elsevier, Amsterdam
Murugan V, Mukherjee K, Maiti K, Mukherjee PK (2009) Enhanced oral bioavailability and antioxidant profile of ellagic acid by phospholipids. J Agric Food Chem 57:4559–4565
Muzzalupo R, Mazzotta E (2019) Do niosomes have a place in the field of drug delivery? Expert Opin Drug Deliv 16:1145–1147
Naczk M, Shahidi F (2006) Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal 41:1523–1542
Nallamuthu I, Devi A, Khanum F (2015) Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J Pharm Sci 10:203–211
Oehlke K, Behsnilian D, Mayer-Miebach E, Weidler PG, Greiner R (2017) Edible solid lipid nanoparticles (SLN) as carrier system for antioxidants of different lipophilicity. PLoS ONE 12:e0171662
Onoue S, Takahashi H, Kawabata Y, Seto Y, Hatanaka J, Timmermann B, Yamada S (2010) Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J Pharm Sci 99:1871–1881
Oskoueian E, Karimi E, Noura R, Ebrahimi M, Shafaei N, Karimi E (2020) Nanoliposomes encapsulation of enriched phenolic fraction from pistachio hulls and its antioxidant, anti-inflammatory, and anti-melanogenic activities. J Microencapsul 37:1–13
Pachuau L (2019) Nanostructures for improving oral bioavailability of herbal medicines. In: Mazumder B et al (eds) Nanotechnology: Therapeutic, Nutraceutical and Cosmetic Advances. CRC Press, Boca Raton, FL
Pachuau L, Devi CM, Goswami A, Sahu S, Dutta RS (2019) Seed oils as a source of natural bioactive compounds, In: Akhtar MS, Swamy MK, Sinniah UR(eds.) Natural Bio-active Compounds. Springer Nature, Singapore, pp 209–236
Pai DA, Vangala VR, Ng JW, Ng WK, Tan RBH (2015) Resistant maltodextrin as a shell material for encapsulation of naringin: production and physicochemical characterization. J Food Eng 161:68–74
Pandey R, Ahmad Z, Sharma S, Khuller GK (2005) Nano-encapsulation of azole antifungals: potential applications to improve oral drug delivery. Int J Pharm 301:268–276
Pandita D, Kumar S, Poonia N, Lather V (2014) Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res Int 62:1165–1174
Park K, Kwon IC, Park K (2011) Oral protein delivery: current status and future prospect. React Funct Polym 71:280–287
Paulo F, Santos L (2018) Microencapsulation of caffeic acid and its release using a w/o/w double emulsion method: assessment of formulation parameters. Drying Technol 37:950–961
Pinheiro AC, Bourbon AI, Cerqueira MA, Maricato E, Nunes C, Coimbra MA et al (2015) Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr Polym 115:1–9
Pinho E, Grootveld M, Soares G, Henriques M (2013) Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr Polym 101:121–135
Pinho E, Soares G, Henriques M (2015) Evaluation of antibacterial activity of caffeic acid encapsulated by β-cyclodextrins. J Microencapsul 32:804–810
Pintado T, Herrero A, Ruiz-Capillas C, Triki M, Carmona P, Jimenez-Colmenero F (2015) Effects of emulsion gels containing bioactive compounds on sensorial, technological, and structural properties of frankfurters. Food Sci Technol Int 22:132–145
Pintado T, Herrero AM, Jimenez-Colmenero F, Ruiz-Capillas C (2016) Emulsion gels as potential fat replacers delivering β-glucan and healthy lipid content for food applications. J Food Sci Technol 53:4336–4347
Piovesana A, Norena CPZ (2018) Microencapsulation of bioactive compounds from Hibiscus calyces using different encapsulating materials. Int J Food Eng 14:20170170
Prakash B, Kujur A, Yadav A, Kumar A, Singh PP, Dubey NK (2018) Nanoencapsulation: an efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 89:1–11
Puligundla P, Mok C, Ko S, Liang J, Recharla N (2017) Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J Funct Food 34:139–151
Qian C, Decker EA, Xiao H, McClements DJ (2013) Impact of lipid nanoparticle physical state on particle aggregation and β-carotene degradation: Potential limitations of solid lipid nanoparticles. Food Res Int 52:342–349
Ramesh Kumar B, Anupam A, Manchikanti P, Rameshbabu AP, Dasgupta S, Dhara S (2018) Identification and characterization of bioactive phenolic constituents, anti-proliferative, and anti-angiogenic activity of stem extracts of Basella alba and rubra. J Food Sci Technol 55:1675–1684
Rathee S, Kamboj A (2018) Optimization and development of antidiabetic phytosomes by the Box-Behnken design. J Liposome Res 28:161–172
Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, Pinto MS (2012) Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol 75:588–602
Rezaei A, Fathi M, Jafari SM (2019) Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocolloid 88:146–162
Rodriguez EB, Almeda RA, Vidallon ML, Reyes CT (2019) Enhanced bioactivity and efficient delivery of quercetin through nanoliposomal encapsulation using rice bran phospholipids. J Sci Food Agric 99:1980–1989
Rodriguez-Garcia A, Hosseini S, Martinez-Chapa SO, Cordell GA (2017) Multi-target activities of selected alkaloids and terpenoids. Mini-Rev Org Chem 14:272–279
Romo-Hualde A, Yetano-Cunchillos AI, Gonzalez-Ferrero C, Saiz-Abajo MJ, Gonzalez-Navarro CJ (2012) Supercritical fluid extraction and microencapsulation of bioactive compounds from red pepper (Capsicum annum L.) by-products. Food Chem 133:1045–1049
Rutz JK, Zambiazi RC, Borges CD, Krumreich FD, da Luz SR, Hartwig N et al (2013) Microencapsulation of purple Brazilian cherry juice in xanthan, tara gums and xanthan-tara hydrogel matrixes. Carbohydr Polym 98:1256–1265
Saenz C, Tapia S, Chavez J, Robert P (2009) Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem 114:616–622
Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG (2018) Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci F 17:512–531
Saifullah M, Islam Shishir MR, Ferdowsi R, Rahman MRT, Vuong QV (2019) Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: a critical review. Trend Food Sci Technol 86:230–251
Salminen H, Gommel C, Leuenberger BH, Weiss J (2016) Influence of encapsulated functional lipids on crystal structure and chemical stability in solid lipid nanoparticles: towards bioactive-based design of delivery systems. Food Chem 190:928–937
Sawale PD, Patil GR, Abdul Hussain S, Singh AK, Bijoy Singh RR (2017) Release characteristics of polyphenols from microencapsulated Terminalia arjuna extract: Effects of simulated gastric fluid, Int J Food Prop 20:3170–3178
Seca AML, Pinto DCGA (2018) Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int J Mol Sci 19:263
Selmaty A, Selmaty M, Rawat MSM, Francheschi F (2010) Supramolecular phospholipids–polyphenolics interactions: The PHYTOSOME® strategy to improve the bioavailability of phytochemicals. Fitoterapia 81:306–314
Septembre-Malaterre A, Remize F, Poucheret P (2018) Fruits and vegetables, as a source of nutritional compounds and phytochemicals: changes in bioactive compounds during lactic fermentation. Food Res Int 104:86–99
Sessa M, Balestrieri ML, Ferrari G, Servillo L, Castaldo D, D’Onofrio N et al (2014) Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem 147:42–50
Shaikh J, Ankola DD, Beniwal V, Singh D, Ravi Kumar MNV (2009) Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci 37:223–230
Shangguan M, Lu Y, Qi J, Han J, Tian Z, Xie Y et al (2014) Binary lipids-based nanostructured lipid carriers for improved oral bioavailability of silymarin. J Biomater Appl 28: 887–896
Shao J, Zheng D, Jiang Z, Xu H, Hu Y, Li X et al (2011) Curcumin delivery by methoxy polyethylene glycol–poly(caprolactone) nanoparticles inhibits the growth of C6 glioma cells. Acta Biochim Biophys Sin 43:267–274
Sharma S, Sahu AN (2016) Development, characterization, and evaluation of hepatoprotective effect of Abutilon indicum and Piper longum phytosomes. Pharmacogn Res 8:29–36
Sharma G, Italia JL, Sonaje K, Tikoo K, Ravi Kumar MNV (2007) Biodegradable in situ gelling system for subcutaneous administration of ellagic acid and ellagic acid loaded nanoparticles: evaluation of their antioxidant potential against cyclosporine induced nephrotoxicity in rats. J Control Release 118:27–37
Silva DF, Favaro-Trinade CS, Rocha GA, Thomazini M (2011) Microencapsulation of lycopene by gelatin-pectin complex coacervation. J Food Process Pres 36:185–190
Singla AK, Garg A, Aggarwal D (2002) Paclitaxel and its formulations. Int J Pharm 235:179–192
Snima KS, Arunkumar P, Jayakumar R, Lakshmanan VK (2014) Silymarin encapsulated Poly(D, L-lactic-co-glycolic acid) nanoparticles: A prospective candidate for prostate cancer therapy. J Biomed Nanotechnol 10:559–570
Song S, Tian B, Chen F, Zhang W, Pan Y, Zhang Q et al (2015) Potentials of proniosomes for improving the oral bioavailability of poorly water-soluble drugs. Drug Develop Ind Pharm 41:51–62
Soukoulis C, Bohn T (2018) A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit Rev Food Sci Nutr 58:1–36
Sun M, Gao Y, Pei Y, Guo C, Li H, Cao F et al (2010) Development of nanosuspension formulation for oral delivery of quercetin. J Biomed Nanotechnol 6:325–332
Sun Y, Xia Z, Zheng J, Qiu P, Zhang L, McClements DJ, Xiao H (2015) Nanoemulsion-based delivery systems for nutraceuticals: Influence of carrier oil type on bioavailability of pterostilbene. J Funct Food 13:61–70
Takahashi M, Kitamoto D, Imura T, Oku H, Takara K, Wada K (2008) Characterization and bioavailability of liposomes containing a Ukon extract. Biosci Biotechnol Biochem 72:1199–1205
Takahashi M, Uechi S, Takara K, Asikin Y, Wada K (2009) Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J Agric Food Chem 57:9141–9146
Teng Z, Xu R, Wang Q (2015) Beta-lactoglobulin-based encapsulating systems as emerging bioavailability enhancers for nutraceuticals: a review. RSC Adv 5:35138–35154
Tiwari SB, Amiji MM (2006) Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J Nanosci Nanotechnol 6:3215–3221
Tomsik A, Saric L, Bertoni S, Protti M, Albertini B, Mercolini L et al (2019) Encapsulations of wild garlic (Allium ursinum L.) extract using spray congealing technology. Food Res Int 119:941–950
Uchegbu IF, Vyas SP (1998) Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm 172:33–70
Wang JC, Chen SH, Xu ZC (2008) Synthesis and properties research on the nanocapsulated capsaicin by simple coacervation method. J Disper Sci Technol 29:687–695
Wang Y, Wang S, Firempong CK, Zhang H, Wang M, Zhang Y et al (2017) Enhanced solubility and bioavailability of naringenin via liposomal nanoformulation: preparation and in vitro and in vivo evaluations. AAPS PharmSciTech 18:586–594
Weiss J, Decker EA, McClements DJ, Kristbergsson K, Helgason T, Awad T (2008) Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophys 3:146–154
Wen P, Zong M, Linhardt RJ, Feng K, Wu H (2017a) Electrospinning: a novel nano-encapsulation approach for bioactive compounds. Trend Food Sci Technol 70:56–68
Wen P, Wen Y, Zong MH, Linhardt RJ, Wu H (2017b) Encapsulation of bioactive compound in electrospun fibers and its potential application. J Agric Food Chem 65:9161–9179
Woranuch S, Yoksan R (2013) Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr Polymer 96:578–585
Wu W, Wang Y, Que L (2006) Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharmaceut Biopharm 63:288–294
Xiao J, Bai W (2019) Bioactive phytochemicals. Crit Rev Food Sc 59:827–829
Xie X, Tao Q, Zou Y, Zhang F, Guo M, Wang Y et al (2011) PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem 59:9280–9289
Yang S, Yue J (2012) Discovery of structurally diverse and bioactive compounds from plant resources in China. Acta Pharmacol Sin 33:1147–1158
Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH (2007) Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 853:183–189
Yang CS, Sang S, Lambert JD, Lee MJ (2008) Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res 52:S139–S151
Yang FH, Zhang Q, Liang QY, Wang SQ, Zhao BX, Wang YT et al (2015) Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: paclitaxel-loaded glycyrrhizic acid micelles. Molecules 20:4337–4356
Yan-yu X, Yunmei S, Zhipeng C, Qineng P (2006) Preparation of silymarin proliposome: a new way to increase oral bioavailability of silymarin in beagle dogs. Int J Pharm 319:162–168
Yao M, McClements DJ, Xiao H (2015) Improving oral bioavailability of nutraceuticals by engineered nanoparticle-based delivery systems. Curr Opin Food Sci 2:14–19
Yashaswini PS, Kurrey NK, Singh SA (2017) Encapsulation of sesamol in phosphatidyl choline micelles: Enhanced bioavailability and anti-inflammatory activity. Food Chem 228:330–337
Yi C, Fu M, Cao X, Tong S, Zheng Q, Firempong CK (2013) Enhanced oral bioavailability and tissue distribution of a new potential anticancer agent, Flammulina velutipes sterols, through liposomal encapsulation. J Agric Food Chem 61:5961–5971
Young S, Wong M, Tabata Y, Mikos AG (2005) Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release 109:256–274
Zhai Y, Guo S, Liu C, Yang C, Dou J, Li L et al (2013) Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloid Surface A 429:24–30
Zhang L, Mou D, Du Y (2007) Procyanidins: extraction and microencapsulation. J Sci Food Agric 87:2192–2197
Zhang Z, Zhang R, McClements DJ (2016) Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll 61:1–10
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pachuau, L., Laldinchhana, Roy, P.K., Zothantluanga, J.H., Ray, S., Das, S. (2021). Encapsulation of Bioactive Compound and Its Therapeutic Potential. In: Pal, D., Nayak, A.K. (eds) Bioactive Natural Products for Pharmaceutical Applications. Advanced Structured Materials, vol 140. Springer, Cham. https://doi.org/10.1007/978-3-030-54027-2_20
Download citation
DOI: https://doi.org/10.1007/978-3-030-54027-2_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54026-5
Online ISBN: 978-3-030-54027-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)