Abstract
Response of culturable microbes on the surface of apples treated with slightly alkaline electrolyzed water (SAIEW) is largely unexplored. Thus, the aim of this study was to characterize culturable microbes on the surface of SAIEW treated ‘Granny Smith’ apples using conventional and molecular approach. Results showed that SAIEW treatments and storage duration influenced culturable microbes isolated from the surface of ‘Granny Smith’ apples stored at 5 °C for 21 days. Enterobacterial repetitive intergenic consensus (ERIC-PCR) analysis distinctively identified 27 groups of bacteria from 56 plate isolates. Using random amplified polymorphic DNA (RAPD-PCR) typing and RAPD1283 primers, 10 distinct band patterns were identified from 30 fungal isolates. Sequencing of 16S rRNA and intergenic spacer (ITS1 and ITS4) region, identified eight bacteria and four fungi, respectively, to species level. Study showed that SAIEW treatment inhibited growth of Staphylococcus epidermidis, S. capitis, Ochrobactrum soli, and Aspergillus inuii on the surface apples during storage.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postharvest diseases destroy 10–30% of the total yield of fruit and vegetable crops during handling, transportation, storage, and marketing. Therefore, preventing the proliferation and development of post-harvest microbial spoilage and pathogens during storage is an important challenge for maintaining apple fruit quality and safety. Domesticated apple (Malus × domestica Borkh) belongs to the Rosacea family, and Pyreae tribe. Apples are rich source of dietary fibre, polyphenols, flavonoids and antioxidant activity (Wang et al., 2015). Postharvest apple fruit surface is colonized by several different microorganisms (Abdelfattah et al., 2016; 2021), and during long-term storage it is susceptible to spoilage and infections by several fungal pathogens.
Chlorine-based solution prepared commercially from sodium hypochlorite (NaOCl) with concentrations ranging from 5 to 200 mg/L is the most used sanitizing agent for washing fresh produce including apples and has been authorized for use by the South African Foodstuffs, Cosmetics and Disinfectants Act. At effective concentration treatment with NaOCl results in a reduction of pathogens on fresh produce (Issa-Zacharia et al., 2011). At higher concentration for increased effectiveness may cause product tainting, and result in the deposition of chemical residues on the product. The risks of chemical residues on fresh ready-to-eat product have raised public health concerns regarding the use of chlorinated water treatments, leading to additional regulatory barriers, limitations, safety, and regulations for the usage (Nan et al., 2019). This heightened the need for effective, environmentally friendly, and low-cost preservation alternatives for apple fruit.
Electrolyzed water (EW) has been shown to possess excellent antimicrobial properties against several microbial pathogens, and it is gaining popularity in the food industry (Yang et al., 2020; Youssef and Hussien, 2020). It is produced through the electrolysis of salt solution in a chamber with an anode and a cathode separated by a diaphragm membrane (Iram et al., 2021). Through the electrolysis process, two different characteristics streams of active water are produced: (a) alkaline electrolyzed water (AIEW) side and (b) acidic electrolyzed water (AEW) from on the cathode and anode side, respectively (Huang et al., 2006; 2008; Iram et al., 2021). On the other hands, systems without a membrane generate a neutral electrolysed water (Han et al., 2018). Forghani et al. (2015) and Lee et al. (2022) reported on the efficacy of slightly-AEW for the microbial decontamination of Kashk and virucidal effect on human norovirus. Recently, Nyamende et al. (2022) showed that slightly alkaline electrolyzed water (SAIEW) was effective in reducing the natural microbial load on ‘Granny Smith’ apples. However, the authors did not characterize the microbial colonies found on the plates to understand the shift in the microbial community on the apple surface.
According to Wassermann et al. (2019a), the apple fruit surface is teeming with a wide variety of spoilage or pathogenic microorganisms, which are closely associated with fruit postharvest deterioration. Recent studies have shown that different apple fruit harbour distinctly different nature fungal and bacterial communities that vary in diversity and abundance (Abdelfattah et al., 2016; 2021). Many techniques have been developed for the identification of microorganisms. This includes the use of conventional morphology and molecular methods. Molecular tools allow for rapid and accurate identification of microbial populations. Polymerase chain reaction (PCR) is a molecular based technique that provides researchers with a simple, economic and fast approach for microbial characterization to genus and species level (Yao et al., 1996). PCR-based deoxyribonucleic acid (DNA) fingerprinting techniques are rapid, having moderate-to-high sensitivity and are cost-effective compared to DNA sequencing and metagenomics. In addition, this fingerprinting approach allows for multiple samples to be analysed at the same time (Cellier et al., 2020). Molecular fingerprinting via DNA have been demonstrated to distinguish between microbial communities, however, this approach may not provide phylogenetic information in some instances (Singh et al., 2019). Furthermore, PCR techniques based on the amplification of ribosomal RNA genes (16S rRNA), enterobacterial repetitive intergenic consensus (ERIC), random amplified polymorphic DNA (RAPD) and the non-coding internal transcribed spacers (ITS) region have been successfully used to characterize microbiota on diverse substrate (Farzi et al., 2019; Sadeghian et al., 2016; Wassermann et al., 2019b).
Based on previous findings on the efficacy of SAIEW treatment to reduce microbial load on ‘Granny Smith’ apples reported by Nyamende et al. (2022). Thus, this is study tested the hypothesis that SAIEW treatment will result in a shift in microbial community on the surface of ‘Granny Smith’ apples. Therefore, the aim of this study was to use a combination of conventional and molecular approach to characterize the microbial community on the surface of ‘Granny Smith’ apples. Set objectives were: (a) to compare the shift in microbial profile on the surface of the treated and untreated apple fruit during cold storage; and (b) to identify predominant microbial community on the apple fruit surface via 16S rRNA and ITS region sequencing.
Materials and methods
Plant materials and postharvest treatment
Fresh ‘Granny Smith’ apples (Malus domestica) were harvested at commercial maturity from the Agricultural Research Council (ARC) Elgin Research Farm, Grabouw, South Africa. Maturity index was based on total soluble solids (TSS) = 10.66° Brix. Harvested fruits were transported in cool trucks from the farm to the Post-Harvest iQ Laboratory, ARC Infruitec-Nietvoorbij, Stellenbosch, where they were sorted upon arrival. Only mature, healthy, and unblemished fruits were selected and stored at 0.5 °C for 3 months, prior to pre-treatments and quality analysis.
The SAIEW was generated by electrolysis of a combined solution of dilute hydrochloric acid in the range 0.001–0.9% and sodium chloride using ELA-12 000ANW system (ECA Technologies, Envirolyte, South Africa). The solution flows through an electrolytic cell at 2 mL/min; 3.8–3.9 V, and amperage 10 A. The electrolyte was diluted with micro-filtered tap water at a flow rate of 4 L/min until available chlorine concentration (ACC) of 500 mg/L, oxidation-reduction potential (ORP) > − 800 mV, and pH = 10–11.5 was attained. ACC and ORP, and pH were measured immediately after production using an ORP meter (HM-60 V, TOA Electronics Ltd., Tokyo, Japan) and pH meter (D-22, Horiba, Kyoto, Japan), respectively. The AIEW was collected at low temperature (4 °C) and used immediately in the study.
All treatment solutions were diluted to desired concentrations prior to dipping the apples and untreated samples were considered as controls. For standard commercial treatment food grade sodium hypochlorite (NaOCl, 11.5% M/V, Protea Chemicals, Sandton, South Africa) solution was used. Selected dipping duration for this study was based on the average processing time between receiving to sorting apples in chlorinated water in the pack-house. Description of treatments and abbreviations as used further in this study are presented in Table 1. All samples were stored at 5 °C with 90 ± 2% RH for 21 days and samples were taken for analyses on days 0, 7, 14, and 21. The storage conditions selected for this experiment was to mimic the packhouse holding rooms or facility for fruit dispatch or bulk distribution.
Microbial analysis and morphological characterization
Each whole apple fruit was submerged in 0.85% physiological saline (PS) solution in a 600 mL beaker and kept on an orbital shaker at 120 rotations per min (rpm) for an hour at 28 °C. Three-fold serial dilutions were prepared using 1.0 mL of the inoculum solution transferred into 9.0 mL of sterile PS. Surface area (95.7 cm2 ± 4.53) of the ‘Granny Smith’ apples was estimated based on the approach reported by Clayton et al. (1995). In this study, different selective growth media were not used, because this would result in over amplification of specific microorganisms and surpress or eliminate others. Therefore, for total aerobic bacteria, plate count agar (PCA, NutriSelect®Plus, Merck (Pty) Ltd., Johannesburg, South Africa) was used, while for the yeasts and molds potato dextrose agar (PDA) supplemented with streptomycin (NutriSelect®Plus, Merck (Pty) Ltd., Johannesburg, South Africa) was used. Plates were incubated for 3–5 days at 25 °C for yeasts and molds, and 2 days at 30 °C for aerobic-mesophilic bacteria.
After plate count was completed, each typical colonies were differentiated based on their morphological and microscopic features on solid media, colonies were assessed on criteria based on Christopher and Bruno (2003), Sousa et al. (2013), and Salo and Novero (2020). After preliminary identification, pure colonies were sub-cultured and freeze cultures prepared. Freeze cultures were prepared in a 1:1 ratio, i.e., 900 µL of 60% glycerol (Merck (Pty) Ltd., Johannesburg, South Africa) and 900 µL of the isolated culture, under sterile conditions and thereafter stored at -80 °C at the ARC Infruitec-Nietvoorbij Gene Bank, Stellenbosch until further analysis.
Isolation and inoculum preparation
Freeze cultures were removed from the Biobank, and bacteria samples were spotted onto both PCA and De Man, Rogosa and Sharpe (MRS agar, Merck (Pty) Ltd., Johannesburg, South Africa). MRS agar was included to confirm presence of lactic acid bacteria. For the yeasts and molds, Yeast Mold (YM) agar (Merck (Pty) Ltd., Johannesburg, South Africa) was used as the general media for cryo-preservation (universally used by biobanks). All spotted plates were incubated for 3–5 days at 25 °C for yeasts and molds, and 2 days at 30 °C for bacteria. Single culture colonies from the spotted plates were inoculated into YM broth (for the cultivation of yeast and mold), and into PCA broth (for bacteria). Both broths were incubated for 3–5 days at 25 °C for yeasts and molds, and PCA broths for 2 days at 30 °C for aerobic-mesophilic bacteria.
Genomic extraction
For genomic extraction, lithium acetate (LiOAc) and sodium dodecyl sulfate (SDS) (Separation Scientific, Honeydew, South Africa). were used. Total genomic deoxyribonucleic acid (DNA) from yeast and bacteria were isolated using the LiOAc-SDS lysis extraction method as described by Pulpipat et al. (2013). Bacterial cell pellet was suspended in 200 µL of 200 mM LiOAc 1% SDS solution and incubated for 15 min at 70 °C. A volume of 500 µL of 96% ethanol was added for DNA precipitation. Samples were mixed briefly, and DNA was collected by centrifugation (Eppendorf microcentrifuges 5425 R, Stevenage, UK) at 13,800×g for 5 min. The supernatant was removed, and the DNA pellet was washed with 500 µL of 70% ethanol to remove some salts from the pellet and centrifuged again at 13,800×g for 5 min. Thereafter, the DNA pellets were dried at 26 °C and then suspended in 30 µL Tris-EDTA (TE) buffer (Separation Scientific, Honeydew, South Africa). The cell debris was removed by centrifugation at 13,800×g for 15 s and the supernatant was used for PCR template.
Molecular characterisation of the isolates
PCR—ERIC and internal transcribed spacers (ITS)
Total bacteria genomic materials were amplified using ERIC1 and ERIC2 (Supplementary, S-Table 1) to identify the bacterial population. The ERIC-PCRs were carried out in a 25 µL reaction volume that contained 2 µL DNA templates according to Sallman et al. (2018). Fungal DNA were amplified by PCR according to a method described by Siavoshi et al. (2018) with primers ITS1 and ITS4 (S-Table 1). ITS-PCR were carried out in volumes of 50 µL comprising: 1 µL of the DNA template, 10× amplification buffer with Mg (Separation Scientific, Honeydew, South Africa); a 2.5 mM dNTP (Separation Scientific, Honeydew, South Africa); 25 mL MgCl2; ITS1 and ITS4 primer (IDT, Whitehead Scientific, South Africa); 20 mg/mL bovine serum albumin (MilliporeSigma Chemical Co., St Louis, MO, USA); 5 u/µL super-therm polymerase (Separation Scientific, Honeydew, South Africa) and addition of distilled water.
Both PCR was performed using a programmable thermal cycler with heated lid (Techni, 3Prime, LASEC, South Africa). Amplification conditions were as follows: initial denaturation at 95 °C for 7 min; samples held at 75 °C for 1 min; followed by 35 cycles of denaturation at of 94 °C for 5 min and 35 cycles of 94 °C for 1 min, 54 °C for 1 min, and 72 °C for 5 min followed by a final extension of 72 °C for 10 min. The samples were maintained at 4 °C until analysis by gel electrophoresis.
Amplification of 16s ribosomal DNA
Bacterial rRNAs were amplified by PCR according to a method by Konecka and Olszanowski (2019) with primers 16SD and 16SR (S-Table 1). The 16s rDNA PCRs were carried out in a 50 µL reaction volume that contained 2 µL of the cDNA template, a 10× amplification buffer with Mg; a 2.5 mM dNTP; 16sd and 16sr primers (IDT, Whitehead Scientific, South Africa); 25 mL MgCl2; 5 u/µL super-therm polymerase. Reactions were performed using the programmable thermal cycler with heated lid (PTC-100, Techni, 3Prime, Lasec, South Africa), with the following conditions: initial denaturation at 94 °C for 30 s followed by 30 cycles at 94 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 2 min and a final extension of 72 °C for 8 min. Thereafter, samples were maintained at 4 °C until analysed by gel electrophoresis.
RAPD analysis
RAPD-PCRs were carried out in a 25 µL reaction volume that contained 2 µL rDNA templates according to a method by Farzi et al. (2019). RAPD-PCRs were amplified with RAPD1283 primer (S-Table 1). The following was added to each 25 µL reaction mixture: a 10× amplification buffer with Mg2++ (Separation Scientific, Honeydew, South Africa); a 2.5 mM dNTP (Separation Scientific, Honeydew, South Africa); 25 mL MgCl2; primer RAPD1283 (IDT, Whitehead Scientific, South Africa); 20 mg/mL bovine serum albumin (Sigma Chemical Co., St Louis, MO); and 5 u/µL Super-Therm polymerase (Separation Scientific, Honeydew, South Africa) and addition of distilled water. The PCR was performed using a Programmable Thermal Cycler with heated lid (Techni, 3Prime, Lasec, South Africa). The thermal conditions were initial denaturation step of 95 °C for 4 min, followed by 30 cycles (denaturation at 94 °C for 30 s, annealing at 38 °C, extension at 72 °C for 2 min with a final extension of 72 °C for 8 min) and a final hold at 10 °C for 10 s. Samples were maintained at 4 °C until analysis by gel electrophoresis.
Electrophoresis
The presence and quality of the extracted genomic DNA and all PCR amplicon samples was analysed using a 1.5% agarose gel containing 0.01% (v/v) − 1 ethidium bromide (Separation Scientific, Honeydew, South Africa) at 4 °C. Loading dye (5 µL) was added to each genomic DNA extract and all PCR amplicons, thereafter 10 µL of each sample was inoculated into separate wells of the gel and electrophoresed for 90 min at 90 V (VACUTEC Thermocycler, South Africa). Thereafter, the gel images were captured using a Gel Doc image analyser (UVTEC Image Analyser, United Kingdom) to visualise chromosomal banding patterns.
Sequencing and sequence information
All amplicons of the internal transcribed spacer (ITS) and 16S regions for post-PCR cleanup and sequencing reactions were conducted at the Central Analytical Facility (CAF) at Stellenbosch University (South Africa). Post-PCR cleanup was perfomed using Nucleofast 96 well cleanup plate from Macherey Nagel according to the manufacturer’s protocol on a Tecan EVO150 Robotic Workstation. One directional DNA sequencing was done using the manufacturers protocol with slight modifications on the BigDye Terminator V3.1 sequencing kit (Applied Biosystems, Thermo Fisher Scientific Inc., Waltham, MA, USA). After sequencing the products were treated with SDS and then transferred onto Sephadex columns (Princeton Scientific Corp., Bethlehem, PA, USA). Protocols supplied by the manufacturer was followed on the Tecan EVO150 Robotic workstation. Received sequences were analysed using FinchTV™. Nucleotide homology searches were performed using the BLASTn search function (Altschul et al. 1990) on the NCBI website (https://blast.ncbi.nlm.nih.gov). Sequences were cross referenced with those in the GenBank NCBI database.
Statistical analysis
Factorial analysis of variance (ANOVA) was used to elucidate the impacts of treatments, dipping time and storage duration on microbial load on the surface of ‘Granny Smith’ apples at 95% confident interval using Statistica Software vr. 13 (TIBCO, StatSoft Inc., Tulsa, OK, USA). Duncan multiple range test was used to determine the difference between mean values. All analyses were conducted in triplicate and results were presented as mean (n = 3) ± standard deviation.
Results and discussion
Morphological characterization
Gram staining showed a total of four Gram-positive and four Gram-negative bacteria were confirmed (Supplementary, S-Fig. 1 A, B). In addition, the microscopic assessments showed that the bacteria shapes were bacilli (rod shaped), coccobacilli intermediate between cocci (round shaped) and bacilli, as well as cocci (round shaped). Colonies were differentiated based on their morphological features on solid media such as surface colour, colony elevation, margin, and form on the plate, as well as appearance (S-Table 2). For instance, colonies shared circular form but were different in colour, texture, margin, and appearance (S-Fig. 1 C).
Throughout the entire storage period, a total of 56 and 30 morphologically distinct bacterial and fungal colonies were isolated, respectively. Colony morphology plays a crucial role in the identification of bacterial and fungal species, because of their unique growth patterns on solid agar plates. Notably in this study, the undiluted and 10− 1 serial dilution plates had tiny colonies with limited characterization attributes, however, sub-culturing unique colonies gave better outcome. Colony density on agar plate (> 20 colonies) could result in restricted and non-well-defined colony formation due to other surrounding colonies (Sousa et al., 2013). Furthermore, the choice and composition of solid agar media have been shown to influence bacterial and fungal colony morphogenesis (Bonachela et al., 2011; Fries et al., 2002; Flemming et al., 2007). Therefore, proper separation or distance between colonies should be maintained and consistency for best characterization outcomes.
Based on the plate count and isolates the predominant microbial population after treatment with electrolyzed water on the apple fruit surface were bacteria. Similar results indicating that the microbes on the surface of apple fruit is predominately bacterial as compared to fungal was reported by Wassermann et al. (2019a). The lower fungal diversity on the apple fruit surface could be attributed to: the poor soil nutrient content, which could limit the growth and airborne of fungal spore (Gu et al., 2020); low water activity on the fruit surface (Maffei et al., 2016); and orchard rural or urban production strategies, such as organic or conventional practices (the frequency of use and concentrations of fungicides and pesticides applied to apple fruit trees (Shen et al., 2018b). In contrast, to this study, Abdelfattah et al. (2021) focused on the global analysis of the apple fruit microbiome after harvest which indicated that the predominant microbial population was that of fungi. The difference between the authors report and the current study is primarily the characterisation approach considered.
Molecular bacterial characterization
ERIC-PCR
Amplicon patterns obtained via ERIC PCR are shown in (S-Fig. 2 A–D). Amplicon of varying base pair (bp) sizes ranging from 100 to 3000 bp were observed across the selected bacteria genomic DNA. Out of fifty-six (56) bacterial genomic DNA extracts that were selected across all the treatments during the storage period, 19 of them were characterized as similar based on ERIC PRC analysis. This includes bacterial genomic DNA extracts with 100–3000 bps on (L: 8, 12, 15, 20, 21, 24 and 26); (L: 9, 8 and 23); (L: 3, 1 3, 22 and 30); (L: 4, 15, and 20) and (L:5 and 14) as shown in S-Fig. 2. The similarities could have meant that the bacterial genomic DNA extracts were that of closely related species. Based on the similar bacterial genomic DNA band pattern samples were grouped according to their similarities and differences. It has been proven that ERIC-PCR has the ability to discriminate between strains of the same or closely related species (Aljindan et al., 2018). This study clearly showed that ERIC-PCR was able to discriminate between members of the same or closely related species. Therefore, using ERIC-PCR resulted in the generation of 27 distinct ERIC-PCR genotypes out of the 56 isolates. This shows the accuracy of molecular characterization of microorganisms as compared to the traditional plate isolation methods.
16s rRNA typing
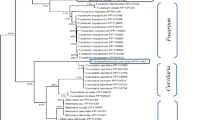
Following amplification of the 16s rRNA gene fragment, an analysis of the amplified products on agarose gel revealed that the amplification took place in 16 bacterial isolates to generate 1500 bp amplicons [Fig. 1(A)]. Sequence analysis of the amplicons showed a high level of similarity between the test sequences and the reference sequences in the GenBank (Table 2). On day 0, and before storage, on the freshly harvested untreated apples, a total of seven different bacteria were indentified to species level: Ochrobactrum soli (10%), Erwinia aphidicola (15%), Enterobacter bugandensis (10%), Staphylococcus epidermidis (10%), Curobacterium flaccumfaciens (40%), Pantoea agglomerans (5%), and Pseudomonas graminis (10%) (Fig. 2). Curobacterium flaccumfaciens was the most dominant bacteria amongst this group. Curtobacterium flaccumfaciens is normally dispersed via agricultural practices such as, planting saved seed and through farm equipment, and it is known to survive only a couple of weeks as free bacteria in the soil (Nascimento et al., 2020). Previous studies by Wassermann et al. (2019b) found the overall bacterial community on apple samples assessed by 16S rRNA gene amplicon sequencing, consisted of Rhizobiales (12%), Pseudomonadales (11%), and Enterobacteriales (7%); Pseudomonas (11%), Pantoea (5%), and Hymenobacter (5%) as abundant genera.
(A) PCR products of 16S and 16D primer of band size 100-1500 bp, electrophoresis on 1.5% agarose at 90 V/cm2 for 1:30 h with UV visualization. Lane M: DNA ladder (100 bp), lanes (1–16) PCR products, and (B) PCR products of ITS1 and ITS4 primer of band size 100-1500 bp, electrophoresis on 1.5% agarose at 90 V/cm2 for 1:30 h with UV visualization. Lane M: DNA ladder (100 bp), lanes (1–8) PCR products
Culturable bacteria associated with treated and untreated apple fruit surface during storage at 5 °C for 21 days. *Description of abbreviations for pre-treatments used in the study is provided in Table 1
On day 7, the dominate microbe (C. flaccumfaciens) before treatment and storage notably declined across all treatments and control, with EW4 treatment (200 mg/L) having the most effect in reducing this bacterial community. Futhermore, on day 7, the result showed that total number of identified bacteria increased to eight with the addition of S. capitis. Bacteria, S. capitis has been shown to be commonly isolate from/on human skin (Cui et al., 2013). Therefore, suggests possible human cross-contamination during the furit post-harvest handling processes. This observation emphasises the need for good agricultural practices along the value chains. Furthermore, S. capitis was found only on the surface of apples under control, C1, C2 and EW1 (300 mg/L, 10 min dipping time) treatments. Thus, EW2 to EW4 treatments (200 mg/L) was effective in preventing S. capitis (Fig. 2).
In addition, P. graminis which was found on samples before storage became more prominent from day 7. The observation in this study is consistent with literature. Studies have shown that P. graminis is a natural microbe on the surface apple fruit. Some strains of P. graminis such as P. graminis CPA-7, was identified as an effective biocontrol agent against Listeria monocytogenes, Salmonella enterica, and Escherichia coli O157:H7 on fresh-cut fruit (Alegre et al., 2013a; 2013b). Therefore, the presence of P. graminis on the apple surface in this study could have an inhibitory effect on the growth of other bacteria such as S. capitis and S. epidermidis. Percentage contribution of S. capitis and S. epidermidis declined with the increase in the presence of P. graminis under C1 and C2 treated apples. In addition, results obtained also showed that EW treatment had most impact on S. capitis and S. epidermidis compared to chlorinated water. Both were present in low percentage on EW1 treated samples on day 7 and declined until the end of the storage, while EW2–EW4 effectively removed them. In contrast, C1 and C2 treatment did not change the abundance of both Staphylococci species (Fig. 2). Staphylococci species identified in this study are commonly found on human skin and mucous membranes, however, they are able to contaminate raw and/or processed food products under improper handling (Cui et al., 2013).
At the end of storage day 21, P. graminis and Pantoea agglomerans became the most abundant groups across all the treatments (Fig. 2). Both P. graminis and P. agglomerans strains have been reported as effective biocontrol agent for postharvest management of fruit disease (Abadias et al., 2014; Soto-Muñoz et al., 2014; Mikiciński et al., 2016). Thus, this change in microbial profile is significant and it could have contributed to the maintenance keeping quality of the apples during storage and reducing the prevalence spoilage microorgansims. Overall, the study established that the culturable natural microbes on apple fruit changes in composition along the value chain depending on the postharvest handling practices and storage conditions.
Fungal characterization
RAPD-PCR
Using RAPD-PCR typing with the primer RAPD1283, out of the approximately 30 fungal isolates only a total of 10 distinct band patterns were identified, across all treated and untreated ‘Granny Smith’ apple fruit surfaces during the storage period (S-Fig. 3). Bands obtained ranged from 100 to 3000 bp on each gel. The RAPD analysis compares the whole chromosomal DNA displaying bands of genotypically specific values, and is fast and trust-worthy (Molnár et al., 1993). It uses short (5–10 m) oligonucleotide primers with arbitrary sequences at low annealing temperatures that hybridize at loci distributed randomly throughout the genome, allowing the amplification of polymorphic DNA fragments (Lathar et al., 2010). These patterns, based on the RAPD-PCR typing analyses, indicate that the various fungi (yeast and mold) isolated from of electrolyzed water treated ‘Granny Smith’ apples, are different from each other based on the number of polymorphic bands produced.
Pianzzola et al. (2004) recovered fourteen isolates of Penicillium expansum from rotten apple with blue mold symptoms. RAPD primer gave reproducible RAPD patterns, with 13% being the highest difference in band presence between repetitions for the same isolate. Two different and very homogeneous patterns were revealed for natural isolates, which corresponded to those of P. expansum and P. solitum type strains. RAPD band patterns corresponding to P. expansum and P. solitum isolates showed up to 78% similarity, whereas those corresponding to the closest related species (P. expansum and P. viridicatum) showed similarity levels of about 68%. RAPD analysis proved to be an objective, rapid, and reliable tool to identify Penicillium spp. involved in blue mould of apple. Wei et al. (2017) identified and characterized fungal species on apples for their potential cider-making performance using RAPD-PCR typing. More than 71 different fungal species belonging to 24 different genera were observed following RAPD-PCR sequencing. Younus (2018) isolated Saccharomyces cerevisiae present on different fruits (apple, plum, dates, and peach) and performing RAPD analyses. Based on RAPD assay the data developed from the PCR analysis demonstrated that some primers generate several bands, while other generates only a few bands.
ITS-PCR analysis
The results of ITS1 and ITS4 showed that most of the isolates give single bundle of DNA on agarose gel [Fig. 1(B)]. Out of the 10 isolates characterized via RAPD1283, only four isolates could be successfully amplified using the ITS1 and ITS4 regions. This includes Debaryomyces hansenii, Rhodotorula nothofagi, Phialemoniosis curvata, and Aspergillus inuii (Table 2). The unsuccessful amplification of the other four isolates could be attributed to the conserved nature of ITS1 and − 4 region of the fungal isolates (Manter and Vivanco, 2007), as well as the DNA template quantity and quality or the presence of a DNA inhibitor (Taylor and McCormick, 2008). Grantina-Ievina (2015) obtained baseline information about apple rot-causing fungi, their incidence during fruit storage and evaluated the fungicide sensitivity of most of isolated fungal species. The authors found that in part of the storehouses, apple rot caused by Cadophora luteo-olivacea was observed and its identity was successfully proved by PCR with ITS1 and ITS 4 primers specific to this species. Alternaria spp. and Cladosporium spp. were detected on few apples as secondary infection agents.
Shen et al. (2018a) characterized the superficial fungi of 18 apple samples before and after cold storage by sequencing ITS1 region. A total of 1,319,679 high-quality ITS reads were obtained from eighteen samples. Recently, Madbouly et al. (2020) isolated five endophytic yeasts from ‘Golden Delicious’ apples and identified them according to their microscopic characteristics as Schwanniomyces vanrijiae, Galactomyces geotrichum, Pichia kudriavzevii, Debaryomyces hansenii, and Rhodotorula glutinis. The first three isolates showed appreciable inhibitory potential against by in vitro test. Moreover, these fungal species caused a significant inhibition of germination of pathogen conidia by 67.6–89.2%. Identification of these three potent fungal species in addition to M. fructigena isolate was confirmed by PCR analysis through amplification of ITS region and PCR products of approximately 650 bp amplified with the ITS1/ITS4 primers and corresponding to the ITS1, 5.8S and ITS2 regions of rDNA were obtained from all isolates.
Fungal community composition in this study was affected by pre-treatment and cold storage (Fig. 3). For instance, before storage four equally prevalent fungal species were observed, however, at the end of day 7 storage the dominate microbiota notably shifted occurred with Debaryomyces hansenii > Phialemoniosis curvata ≥ Rhodotorula nothofagi > Aspergillus inuii in untreated control (Fig. 3). Rhodotorula nothofagi became most prominent in untreated control samples on day 14. The phyllo plane yeast Rhodotorula spp., which was found before storage as well as after postharvest treatments (control, C1, C2) during cold storage has been reported to control B. cinerea on geranium seedlings in combination with fungicides (Buck, 2004). In contrast, on day 21 Debaryomyces hansenii and Phialemoniosis curvata were most dominant for EW treated samples. Debaryomyces hansenii has also been recently employed as a biological control against a variety of fruit rot causing diseases, including apples (Madbouly et al., 2020). Notably, chlorinated water was not effective in eliminating A. inuii compared to the EW treatment in this study. The biggest shift in the fungal community composition during storage occurred between day 14 and 21. The effect of treatment on the fungal community was similarly most evident between EW 1, EW2, EW3 and Ew4 treated fruit, with the EW4 exhibiting the highest degree of inactivation (Fig. 3).
Culturable fungi associated with treated and untreated apple fruit surface during storage at 5 °C for 21 days. *Description of abbreviations for pre-treatments used in the study is provided in Table 1
Overall, for practical application, this study demonstrated that the slightly electrolyzed water could be an effective alternative to chlorinated water treatment used in apple packhouses. Additionally, by implication, this study showed that natural microbes on the surface of fresh fruit plays crucial role for safety and preservation during the postharvest period. Several of these microbes serves as competitive inhibitors and the presences of others as safety indicators based on literature. Furthermore, EW treatments were found to be effective in eliminating Staphylococci species compared to the use of chlorinated water on the surface of the ‘Granny Smith’ apples. Microbes such as Pantoea agglomerans, Pseudomonas graminis and Debaryomyces hansenii became predominant, while others declined demonstrate the dynamics on the fruit surface. However, further study is required to investigate the effects of other types of electrolyzed water (slightly acidic, acidic, and/or alkaline) alone or comparatively, and in combination with other hurdle techniques on the changes in microbial profile on ‘Granny Smith’ apples. In addition, a full-scale metagenomic sequencing approach with 16S rRNA gene and 18 S fungal ITS amplicons must be considered to get a complete picture of cultivable and non-cultivable microbial profile on the apple.
References
Abdelfattah A, Wisniewski M, Droby S, Schena L. Spatial and compositional variation in the fungal communities of organic and conventionally grown apple fruit at the consumer point-of-purchase. Horticulture Research. 3: 16047 (2016)
Abdelfattah A, Freilich S, Bartuv R, Zhimo VY, Kumar A, Biasi A, Salim S, Feygenberg O, Burchard E, Dardick C, Liu J, Khan A, Ellouze W, Ali S, Spadaro D, Torres R, Teixido N, Ozkaya O, Buehlmann A, Vero S, Mondino P, Berg G, Wisniewski M, Droby S. Global analysis of the apple fruit microbiome: are all apples the same? Environmental Microbiology. 23: 6038-6055 (2021)
Abadias M, Altisent R, Usall J, Torres R, Oliveira M, Vinas I. Biopreservation of fresh-cut melon using the strain Pseudomonas graminis CPA-7. Postharvest Biology and Technology. 96: 69-77 (2014)
Alegre I, Viñas I, Usall J, Anguera M, Altisent R, Abadias M. Antagonistic effect of Pseudomonas graminis CPA-7 against foodborne pathogens in fresh-cut apples under simulated commercial conditions. Food Microbiology. 33: 139-148 (2013a)
Alegre I, Viñas I, Usall J, Teixidó N, Figge MJ, Abadias M. Control of foodborne pathogens on fresh-cut fruit by a novel strain of Pseudomonas graminis. Food Microbiology. 34: 390-399 (2013b)
Aljindan R, Alsamman K, Elhadi N. ERIC-PCR genotyping of Acinetobacter baumannii isolated from different clinical specimenseric. Saudi Journal of Medicine and Medical Sciences. 6: 13-17 (2018)
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ Basic local alignment search tool. Journal of Molecular Biology. 215: 403-410 (1990)
Argemi X, Nanoukon C, Affolabi D, Keller D, Hansmann Y, Riegel P, Baba-Moussa L, Prévost G. Comparative genomics and identification of an enterotoxin-bearing pathogenicity island, SEPI-1/SECI-1, in Staphylococcus epidermidis pathogenic strains. Toxins. 10: 93 (2018)
Bonachela JA, Nadell CD, Xavier JB, Levin SA. Universality in bacterial colonies. Journal of Statistical Physics. 144: 303-315 (2011)
Buck JW. Combinations of fungicides with phylloplane yeasts for improved control of Botrytis cinerea on geranium seedlings. Phytopathology. 94: 196-202 (2004)
Cellier G, Redondo C, Cubero J, Roselló M, Andrade E, Cruz L, Ince E, Yildiz HN, Güler PG, D’Onghia AM, Yaseen T, Djelouah K, Metz-Verschure E, Gaffuri F, Gottsberger RA, Giovani B. Comparison of the performance of the main real-time and conventional PCR detection tests for ‘Candidatus Liberibacter’ spp., plant pathogenic bacteria causing the Huanglongbing disease in Citrus spp. European Journal of Plant Pathology. 157: 919-941 (2020)
Choi GM, Kim KM, Yun CS, Lee SY, Kim SY, Wee JH, Im WT. Ochrobactrum soli sp. nov., isolated from a Korean Cattle Farm. Current Microbiology. 77: 1104-1110 (2020)
Christopher K, Bruno E. Identification of bacterial species. Vol. 24, pp 103-130. In: Tested studies for laboratory teaching. O’Donnell MA (ed). Proceedings of the 24th Workshop/Conference of the Association for Biology Laboratory Education (ABLE) (2003)
Clayton M, Nevin DA, Nigel HB, Morton RH. Estimation of apple fruit surface area. New Zealand Journal of Crop and Horticultural Science. 23: 345-349 (1995)
Cui B, Smooker PM, Rouch DA, Daley AJ, Deighton MA. Differences between two clinical Staphylococcus capitis subspecies as revealed by biofilm, antibiotic resistance, and pulsed-field gel electrophoresis profiling. Journal of Clinical Microbiology. 51: 9-14 (2013)
Das D, Huntemann M, Han J, Chen A, Kyrpides N, Mavromatis K, Markowitz V, Palaniappan K, Ivanova N, Schaumberg A, Pati A, Liolios K, Nordberg HP, Cantor MN, Hua SX, Woyke, T. Enterobacter bugandensis strain IIT-BT 08 genomic scaffold EntIITBT8DRAFT_scaffold1.1, whole genome shotgun sequence. NCIB. (2013). Available from: https: //www.ncbi.nlm.nih.gov/nuccore/KI911561.1. Accessed Apr. 22, 2021
Farzi N, Behzad C, Hasani Z, Alebouyeh M, Zojaji H, Zali MR. Characterization of Clarithromycin heteroresistance among Helicobacter pylori strains isolated from the antrum and corpus of the stomach. Folia Microbiologica. 64: 143-151 (2019)
Forghani F, Eskandari, M, Oh D-H. Application of slightly acidic electrolyzed water and ultrasound for microbial decontamination of Kashk. Food Science Biotechnology. 24: 1011-101016 (2015)
Flemming HC, Neu TR, Wozniak DJ. The EPSmatrix: the “house of biofilmcells”. Journal of Bacteriology. 189: 7945-7947 (2007)
Fries BC, Goldman DL, Casadevall A. Phenotypic switching in Cryptococcus neoformans. Microbes and Infection. 4: 1345-1352 (2002)
Grantina-Ievina L. Fungi causing storage rot of apple fruit in integrated pest management system and their sensitivity to fungicides. Rural Sustainability Research. 34: 2-11 (2015)
Gu Y, Wang Y, Wang P, Wang C, Ma J, Yang X, Ma D, Li, M. Study on the diversity of fungal and bacterial communities in continuous cropping fields of chinese chives (Allium tuberosum). BioMed Research International. 2020: 1-14 (2020)
Han Q, Song X, Zhang Z, Fu J, Wang X, Malakar PK, Liu H, Pan Y, Zhao, Y. Removal of foodborne pathogen biofilms by acidic electrolyzed water. Frontiers in Microbiology. 8: 988 (2018)
Huang Y, Hsieh H, Lin S, Lin S, Hung, Y, Hwang, D. Application of electrolyzed oxidizing water on the reduction of bacterial contamination for seafood. Food Control. 17: 987-993 (2006)
Huang Y, Hung Y, Hsu S, Huang Y, Hwang D. Application of electrolyzed water in the food industry. Food Control. 19: 329--345 (2008)
Iram A, Wang X, Demirci A. Electrolyzed oxidizing water and Its applications as sanitation and cleaning agent. Food Engineering Reviews. 13: 411-427 (2021)
Issa-Zacharia A, Kamitani Y, Miwa N, Muhimbula H, Iwasaki K. Application of slightly acidic electrolyzed water as a potential non-thermal food sanitizer for decontamination of fresh ready-to-eat vegetables and sprouts. Food Control. 22: 601-607 (2011)
Janssen J, Weyens N, Croes S, Beckers B, Meiresonne L, Peteghem P, Carleer R, Vangronsveld J. Phytoremediation of metal contaminated soil using willow: Exploiting plant-associated bacteria to improve biomass production and metal uptake. International Journal of Phytoremediation. 17: 1123-1136 (2015)
Konecka E, Olszanowski Z. A new Cardinium group of bacteria found in Achipteria coleoptrata (Acari: Oribatida). Molecular Phylogenetics and Evolution. 131: 64-71 (2019)
Kosecka-Strojek M, Sabat AJ, Akkerboom V, Becker K, Zanten E van, Wisselink G, Miedzobrodzki J, Kooistra-Smid AMD, Friedrich AW. Development and validation of a reference data set for assigning Staphylococcus species based on next-generation sequencing of the 16S-23S rRNA Region. Frontiers in Cellular and Infection Microbiology. 9: 278 (2019)
Lathar KP, Sharma A, Thakur I. Isolation and random amplified polymorphic DNA (RAPD) analysis of wild yeast species from 17 different fruits. Journal of Yeast and Fungal Research. 1: 146-151 (2010)
Lee H-W, Park B, Yoon S-R, Yang J-S, Ha J-H. Physicochemical stability and virucida effect of diluted, slightly acidic electrolyzed water against human norovirus. Food Science and Biotechnology. 31: 131--138 (2022)
Lopez-Fernandez M, Vilchez-Vargas R, Jroundi F, Boon N, Pieper D, Merroun ML. Microbial community changes induced by uranyl nitrate in bentonite clay microcosms. Applied Clay Science. 160: 206-216 (2018)
Madbouly AK, Abo E, Ismail IM. Biocontrol of Monilinia fructigena, causal agent of brown rot of apple fruit, by using endophytic yeasts. Biological Control. 144: 104239 (2020)
Maffei DF, Batalha EY, Landgraf M, Schaffner DW, Franco BDGM. Microbiology of organic and conventionally grown fresh produce. Brazilian Journal of Microbiology. 47: 99-105 (2016)
Manter DK, Vivanco JM. Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. Journal of Microbiological Methods. 71: 7-14 (2007)
Mikiciński A, Sobiczewski P, Puławska J, Maciorowski R. Control of fire blight (Erwinia amylovora) by a novel strain 49 M of Pseudomonas graminis from the phyllosphere of apple (Malus spp.). European Journal of Plant Pathology. 145: 265-276 (2016)
Molnár E, Baude A, Richmond SA, Patel PB, Somogyi P, McIlhinney RAJ. Biochemical and immunocytochemical characterization of antipeptide antibodies to a cloned GluR1 glutamate receptor subunit: Cellular and subcellular distribution in the rat forebrain. Neuroscience. 53: 307-326 (1993)
Musa Z. Curtobacterium flaccumfaciens strain LMG 3645(T) 16S ribosomal RNA gene, partial sequence (2019). Available from: https://www.ncbi.nlm.nih.gov/nuccore/MN687837.1. Accessed Apr. 22, 2021
Nan S, Huang X, Wang S, Ye Z, Zhu, S. Investigation on microbicidal potential and action mechanism for Botrytis cinerea of slightly acidic electrolyzed water. Nongye Jixie Xuebao/Transactions of the Chinese Society for Agricultural Machinery. 8: 236 (2019)
Nascimento DM, Oliveira LR, Melo LL, Silva JC, Soman JM, Girotto KT, Eburneo RP, Ribeiro-Junior MR, Sartori MMP, Silva Júnior TAF, Maringoni, AC. Survival of Curtobacterium flaccumfaciens pv. flaccumfaciens in weeds. Plant Pathology. 69: 1357-1367 (2020)
Nyamende NE, Belay ZA, Keyser Z, Oyenihi A, Caleb, OJ. Impacts of alkaline electrolyzed water treatment on physicochemical, phytochemical, antioxidant properties and natural microbial load on ‘Granny Smith’ apples during storage. International Journal of Food Science and Technology. 57: 447-456 (2022)
Pianzzola MJ, Moscatelli M, Vero S. Characterization of Penicillium isolates associated with blue mold on apple in Uruguay. Plant Disease. 88: 23-28 (2004)
Pulpipat T, Viriyarumpa S, Chumsing S, Boonyawiwat V, Wajjwalku W. Lithium acetate (LiOAc)-SDS lysis DNA extraction method of Gram-positive bacteria for PCR templates. Khon Kaen University Veterinary Journal. 23: 24-31 (2013)
Sadeghian M, Shahidi GH, Sharifi GR. Post harvest biological control of apple bitter rot by soil-borne Actinomycetes and molecular identification of the active antagonist. Postharvest Biology and Technology. 112: 46-54 (2016)
Sallman RS, Hussein SS, Ali MR. ERIC-PCR typing, RAPD-PCR fingerprinting and quorum sensing gene analysis of Pseudomonas aeruginosa isolated from different clinical sources. Al-Mustansiriyah Journal of Science. 29: 50 (2018)
Salo EN, Novero A. Identification and characterisation of endophytic bacteria from coconut (Cocos nucifera) tissue culture. Tropical Life Sciences Research. 31: 57-68 (2020)
Shen Y, Nie J, Dong Y, Kuang L, Li Y, Zhang J. Compositional shifts in the surface fungal communities of apple fruits during cold storage. Postharvest Biology and Technology. 144: 55-62 (2018a)
Shen Y, Nie J, Li Z, Li H, Wu Y, Dong Y, Zhang J. Differentiated surface fungal communities at point of harvest on apple fruits from rural and peri-urban orchards. Scientific Reports. 8: 1-12 (2018b)
Siavoshi F, Sahraee M, Ebrahimi H, Sarrafnejad A, Saniee, P. Natural fruits, flowers, honey, and honeybees harbor Helicobacter pylori-positive yeasts. Helicobacter 23: e12471 (2018)
Singh J, Silva KJP, Fuchs M, Khan A. Potential role of weather, soil and plant microbial communities in rapid decline of apple trees. PLoS ONE. 14: e0213293 (2019)
Soto-Muñoz L, Teixidó N, Usall J, Viñas I, Torres R. Detection and quantification by PCR assay of the biocontrol agent Pantoea agglomerans CPA-2 on apples. International Journal of Food Microbiology. 75: 45-52 (2014)
Sousa AM, Machado I, Nicolau A, Pereira MO. Improvements on colony morphology indentification towards bacterial profiling. Journal of Microbioligcal Methods. 95: 327-335 (2013)
Sproer C, Gronow S, Severitt S, Schroder I, Tallon L, Sadzewicz L, Zhao X, Boylan J, Ott S, Bowen H, Vavikolanu K, Mehta A, Aluvathingal J, Nadendla S, Lowell S, Myers T, Yan Y. FDA dAtabase for Regulatory Grade microbial sequences (FDA-ARGOS): Supporting development and validation of Infectious Disease tests. Available from: https://www.ncbi.nlm.nih.gov/nuccore/CP077366.1. Accessed May. 13, 2021.
Taylor DL, McCormick MK. Internal transcribed spacer primers and sequences for improved characterization of basidiomycetous orchid mycorrhizas. New Phytologist. 177: 1020-1033 (2008)
Vu D, Groenewald M, Szöke S, Cardinali G, Eberhardt U, Stielow B, Vries M, Verkleij GJM, Crous PW, Boekhout T, Robert V. DNA barcoding analysis of more than 9000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Studies in Mycology. 85: 91-105 (2016).
Vu D, Groenewald M, Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology. 92: 135-154 (2019)
Wang X, Li C, Liang D, Zou Y, Li P, Ma, F. Phenolic compounds and antioxidant activity in red-fleshed apples. Journal of Functional Foods. 18: 1086-1094 (2015)
Wassermann B, Kusstatscher P, Berg G. Microbiome response to hot water treatment and potential synergy with biological control on stored apples. Frontiers in Microbiology. 10: 1-12 (2019a)
Wassermann B, Müller H, Berg, G. An Apple a Day: which bacteria do we eat with organic and conventional apples? Frontiers in Microbiology. 10: 1-13 (2019b)
Wei J, Niu C, Liu B, Yuan Y, Yue T. Identification and characterization of Epiphytic yeasts on apples in China. Royal Society of Chemistry Advances. 7: 44766-44772 (2017).
Yaguchi T, Sano A, Yarita K, Suh MK, Nishimura K, Udagawa SI. A new species of Cephalotheca isolated from a Korean patient. Mycotaxon. 10: 93 (2006)
Yang J, Song L, Pan C, Han Y, Kang L. Removal of ten pesticide residues on/in kumquat by washing with alkaline electrolysed water. International Journal of Environmental Analytical Chemistry. https://doi.org/10.1080/03067319.2020.1772775 (2020)
Yao C, Conway WS, Sams CE. Purification and characterization of a polygalacturonase produced by Penicillium expansum in apple fruit. Phytopathology. 86: 1160-1166 (1996)
Younus S. RAPD and ISSR analyses of Saccharomyces cerevisiae isolates from different sources. Journal of Biotechnology Research Center. 12: 40-50 (2018).
Youssef K, Hussien A. Electrolysed water and salt solutions can reduce green and blue molds while maintain the quality properties of ‘Valencia’ late oranges. Postharvest Biology and Technology. 159: 111025 (2020)
Acknowledgements
The Innovation Fellowship awarded by the Cape Peninsula University of Technology, South Africa is gratefully acknowledged.
Funding
This work is based upon research supported wholly by the National Research Foundation (NRF) of South Africa (Grant Nos. 137990 and 146360). The opinions, findings, and conclusions or recommendations expressed are those of the author(s) alone and the NRF accepts no liability whatsoever in this regard.
Author information
Authors and Affiliations
Contributions
NEN, JWH, VB, ZAB, ABO, OJC: conceptualized the study. NEN, JWH, VB: collected the data. NEN, ZAB: investigation, writing—first draft of the manuscript. NEN, ZAB, JWH, ABO: methodology, formal analysis. ZAB, ABO, OJC: validation, visualization. VB, JWH resources, software. ZAB, OJC: supervision. JWH, VB, ZAB, ABO, OJC: writing - review and editing of the manuscript. OJC: funding acquisition and project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Not applicable.
Research involving human participants and/or animals
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nyamende, N.E., Hoff, J.W., van Brede, V. et al. Isolation and characterization culturable microbes on the surface of ‘Granny Smith’ apples treated with electrolyzed water during cold storage. Food Sci Biotechnol 31, 1603–1614 (2022). https://doi.org/10.1007/s10068-022-01148-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-022-01148-2