Abstract
This study aimed to evaluate the virucidal effect and potential use as a disinfectant of undiluted and diluted slightly acidic electrolyzed water (SAEW) on human noroviruses (HuNoVs) using the in vitro suspension test and in food test. The oxidization reduction potential of SAEW gradually decreased with the increase in distilled water volume. Moreover, as the volume of distilled water and the dilution ratio increased, the available chlorine concentration of the samples significantly decreased from 31.2 ± 0.63 (SAEW) to 1.3 ± 0.21 (1:10 dilution of SAEW solution) (p < 0.05). Undiluted SAEW presented the lowest pH (5.56 ± 0.02) and as SAEW was diluted in distilled water, the pH of the sample increased. Considering the standard reduction values of pathogenic virus (> 4.00 log reduction), the reduction value of HuNoVs in cabbage samples was 4.65 (GI.6) and 4.28 (GII.4) log. These results suggest the potential application of SAEW for inactivating HuNoVs in the food industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human noroviruses (HuNoVs) have been identified as the causative agents of acute gastroenteritis and are considered major pathogenic viruses that pose risks for global public health (Han et al., 2020). HuNoVs are generally transmitted through vomit–oral and/or fecal–oral routes, either by consumption of food or vegetables contaminated with human feces, or via direct person-to-person spread (Han et al., 2020). Recently, HuNoVs have been classified into 10 genogroups: HuNoV genogroup I-X (GI-X), based on the genetic differences in their capsid proteins. Human infections are mainly caused by GI and GII, with HuNoV GI genotype 6 (HuNoV GI.6) and GII genotype 4 (HuNoV GII.4) being the causative agents of most infections worldwide, followed by GII.3 or GII.6, and then other genotypes in varying proportions (Diez-Valcarce et al., 2019; Gentry-Shields and Jaykus, 2015; Griffin et al., 2014). To ensure the microbiological safety of fresh food, chemical disinfectant treatments are the primary approaches for decontamination of viral pathogens during various pre-processing steps. Additionally, suitable disinfectant treatments are deemed an effective strategy for preventing the transmission of secondary infections of HuNoVs.
The hygiene practices in the field of the ready-to-eat (RTE) food industry are of considerable importance, and optimal virucidal pre-processes have been established to ensure the virological safety of fresh vegetables. Common chemical agents, such as ethanol, iodine, hydrogen peroxide, organic compounds, chlorine compounds, peracetic-acid organic compounds, and quaternary ammonium compounds, are used in various food-related industrial processes (Hricova et al., 2008).
Among chemical disinfectants, chlorine reportedly presents the most effective virucidal activity; however, there is a risk of formation of potentially corrosive metallic substances and toxic halogenated disinfection by-products such as trihalomethanes and haloacetic acids (Shen et al., 2016). In several cases, chlorine treatment has been restricted owing to its unpleasant odor and discoloration of food. Thus, plasma-activated water, ozonated water, and electrolyzed oxidizing water have been examined for application as alternative disinfectants to ensure food quality and safety (Schwarz et al., 2019). Recently, slightly acidic electrolyzed water (SAEW) with low available chlorine concentration (ACC; 20 to 80 mg/L), oxidization reduction potential (ORP; approximately 1,000 mV), and near-neutral pH (5.0 to 6.0) has been suggested as an appropriate and acceptable disinfectant for application in processes related to the manufacture of RTE food products such as fresh-cut fruit and vegetables, owing to the presence of its main effective form of chlorine compound, i.e., hypochlorous acid (HOCl) (Issa-Zacharia et al., 2011).
According to Rutala and Weber, most users of commercial disinfectants are categorized under “cost per compliant use” as a key consideration, owing to the economic feasibility of disinfection processes (Rutala and Weber, 2014). However, only a few studies have reported the virucidal effect of diluted crude SAEW, although sufficient reports are available on the virucidal effect of undiluted SAEW on pathogens. The purpose of this study was to determine whether diluted SAEW exhibited virucidal activity. We assessed the virucidal effect of diluted SAEW on HuNoV GI.6 and HuNoV GII.4 using the in vitro suspension test and in food test.
Materials and methods
Viral sample preparation
GI.6 and GII.4 HuNoV were provided by the NoroGene Research Center (Seoul, Korea). The clinical aliquots of HuNoV samples were diluted 1:10 in RNase-free water (Sigma-Aldrich, St. Louis, MO, USA) and vortexed for 1 min. The test sample was stored in 500-µL aliquots at − 80 °C. In this study, each volume of viral suspension for inoculation was approximately between 5.5 and 6.0 log10 genomic copies/µL. For the disinfection test, all HuNoV mixtures were prepared using 20 µL of the HuNoV sample. To prepare the food test mixture, HuNoV (20 µL) was mixed with 180 µL of RNase-free water.
Preparation and characterization of SAEW
For SAEW preparation, distilled water (DW) was mixed inside a electrolysis cell with 5.5% hydrochloric acid (HCl) and experiments were performed at 12.8 V and 5.0 A using a SAEW generator (Purester MP-600 T, Morinaga Engineering Co., Ltd., Tokyo, Japan). For available chlorine concentration (ACC), a colorimetric method using a digital chlorine test kit (RC-3F; Kasahara Chemical Instrument Corp., Saitama, Japan) was used to analyze the prepared solution. A dual-scale pH meter (Accumet model 15; Fisher Scientific Co., Fair Lawn, NJ, USA) equipped with pH and oxidization reduction potential (ORP) electrodes was used to determine the pH and ORP values of the prepared solution. SAEW production rates were achieved at a flow rate of 600 L/h.
Virucidal efficacy of SAEW treatment
Following SAEW preparation, SAEW was diluted from 1:10 to 10:1 with DW to prepare the disinfectant. For example, to prepare a 1:10 dilution of SAEW solution, 1 L of SAEW was mixed with 9 L of DW for obtaining a total of 10 L of the test sample. According to the ratio of SAEW:DW, test samples were denoted using the range between S1/W10 (sample 1) and S9/W10 (sample 9). The virucidal efficacy of SAEW was evaluated using the modified dilution-neutralization method (European CEN EN 1276) based on the principles of quantitative suspension testing (Koivunen and Heinonen-Tanski, 2005). The Korean Food and Drug Administration recommends the consideration of CEN EN 1276 as a quantitative suspension test for the evaluation of microbial inactivation of chemical disinfectants in food (Fig. S1). Kimchi cabbage (Brassica rapa subsp.) was purchased from a local market in Gwangju, Korea. Following removal and discarding of the damaged outer leaves, intact and fresh cabbage leaves were cut using a sterile knife into 10 × 5 cm2 slices (approximately 15 g; 0.4 mm in thickness), and each cabbage sample was washed thoroughly with running tap water. Prior to the addition of HuNoVs, each sample was soaked in 4,000 mg/L sodium hypochlorite (NaClO) for 60 s, which has proven to be a suitable disinfectant to completely control the natural flora present in fresh cabbage (Kang et al., 2018; 2019). Sterilized samples were immersed in neutralization buffer (Sigma-Aldrich) for 60 s and subsequently rinsed three times with phosphate-buffered saline (PBS, Sigma-Aldrich). Thereafter, each sample was inoculated with 200 µL viral suspension of HuNoV and stored at 18 ± 2 °C for 30 min until thoroughly dried, such that viral particles in the viral suspension could completely adhere to the sample. The inoculated Kimchi cabbage was added into sterile stomacher bags with 200 mL of the individually prepared SAEW solution, 4,000 mg/L NaClO (positive control), and PBS (negative control). For disinfection activation, all samples were stored for 1 min at 18 ± 2 °C with constant shaking (60 rpm). Following disinfection, the treated cabbage was transferred to a stomacher bag and mixed with 10 mL of a neutralizing buffer for 1 min to stop the sterilization activity of SAEW. Then, the cabbage sample was transferred into 50 mL of PBS and homogenized using the Stomacher BagMixer R400 (Interscience, Saint Nom, France) for 5 min, followed by the conduction of a tenfold serial dilution via addition of 50 mL of the homogenate to a 50-mL conical tube.
Quantification of HuNoV
Figure S1 depicts a detailed flow diagram of virucidal evaluation and quantification protocol for the suspension test of SAEW disinfectants against GI.6 and GII.4 HuNoV using the optimized quantification. Based on the study reported by Lee et al. (2018), magnetic bead separation (MBS)/quantitative reverse transcriptase PCR (RT-qPCR), which combines the specific MBS technique with RT-qPCR, showed the achievement of maximum recovery rate of viral particles from the test samples (Lee et al., 2018). Furthermore, the MBS/RT-qPCR assay together with a pre-treatment, combining propidium monoazide as an intercalating dye of nucleic acids and sodium lauroyl sarcosinate, for enhancing the penetration of monoazide into slightly-damaged capsids was used (Lee et al., 2018).
Viral RNA extraction and quantitative RT-qPCR
Viral RNA extraction from the disinfected samples was performed using the QIAamp Viral RNA MinElute Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The primers and TaqMan probes (Table 1) were optimized for fast one-step RT-qPCR (Jothikumar et al., 2005). To obtain external standard curves for HuNoV GI.6 and GII.4, tenfold serial dilutions from 1 to 7 log10 genomic copies of in vitro-transcribed viral RNA were prepared using RNase-free water as PCR templates. The standard RNA curves of HuNoV GI.6 and GII.4 presented with slopes of − 3.355 (R2 = 0.989) and − 3.403 (R2 = 0.997), respectively (data not shown). The log reduction value for exposure to each test solution was calculated by subtracting the post-exposure log-transformed HuNoV titer from the log-transformed baseline. The experimental data of RT-qPCR were analyzed using the Applied Biosystems 7500 Fast Real-Time PCR detection system software (ver. 3.0).
Statistical analysis
Duplicate samples were used in individual disinfectant treatments, and the experiment was performed in triplicate. For statistical analysis, one-way analysis of variance and Duncan’s multiple range test were used to compare differences among mean values using SPSS Statistics software (ver.8.2; Inc., Chicago, IL, USA). A p-value of < 0.05 was considered statistically significant. The experimental results have been denoted as log10 genomic copies/200μL, and regression analysis was performed using the SigmaPlot software (ver. 14.0; Systat Software Inc., USA). Correlation coefficients were obtained using Microsoft Excel (CORREL statistical function).
Results and discussion
Changes in the physicochemical properties of SAEW after dilution
Figures 1 and 2 show the changes in pH, ACC, and ORP of undiluted and diluted SAEW with DW. The analysis of the initial physicochemical properties of SAEW showed that ACC, ORP, and pH values were 30.71 ± 0.45 mg/L, 875 ± 4 mV, and 5.56 ± 0.01, respectively Overall, the ORP of SAEW gradually decreased along with the increase in DW volume. Under the dilution conditions, ORP values between Sample 5 (S5/W10) and Sample 9 (S9/W10), and undiluted SAEW remained stable between 858 and 871 mV, whereas ORP values between Sample 1 (S1/W10) and Sample 4 (S4/W10) significantly decreased from 839 to 801 mV (p < 0.05). DW had an ORP value of 566 mV. As the dilution ratio increased, the ACC level of the samples significantly decreased (p < 0.05). The ACC levels of undiluted SAEW and DW were 30.71 ± 0.45 mg/L and 0.21 ± 0.05 mg/L, respectively. The ACC level up to Sample 7 (S7/W10) was 20.07 ± 0.11 mg/L and decreased consistently, whereas the ACC level between samples 1 and 6 was lower than 20.00 mg/L. Undiluted SAEW showed the lowest pH, 5.56 ± 0.02. Furthermore, as SAEW was diluted in DW, the pH of the samples consistently increased. The pH of Sample 6 (S6/W10) was maintained at 6.49 ± 0.01, whereas the pH between S1 and S5 continued to increase, above 6.50.
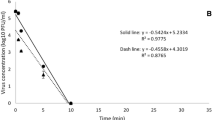
Changes in the physicochemical properties of undiluted and diluted SAEW and HuNoV GI.6 reduction values by disinfectant condition used in the suspension test. A-G indicate a significant (p < 0.05) difference within the log reduction values. SAEW, slightly acidic electrolyzed water; HuNoV, human norovirus
SAEW solutions have been recognized as efficient virucidal agents, along with free chlorine (Issa-Zacharia et al., 2011). Despite the advantages of virucidal efficacy, the use of SAEW has limited potential in applications pertaining to diluting disinfectants owing to the instability of its chemical properties. In general, commercially available disinfectants are provided with information on the appropriate dilution ratio for intended use, whereas there is no scientific evidence available on the use of diluted SAEW for inactivation of foodborne pathogens. Among several previous studies regarding the chemical stability of SAEW, Xuan et al. (2016) demonstrated the stable properties of SAEW under different conditions such as storage time and application period; however, there was no evidence of changes in physicochemical properties regarding the level of dilution of SAEW with DW. Zang et al. (2019) compared the bactericidal effects of different ACCs (i.e., 10, 18, and 26 mg/L) of SAEW diluted in sterile deionized water for the elimination of Escherichia coli and Salmonella Enteritidis on shelled eggs. The authors reported that when SAEW was diluted with sterile deionized water, ACC decreased and the pH increased (Zang et al., 2019), whereas variation in ORP values remained between 647.5 and 675.9 mV and did not show significant differences (p > 0.05).
Virucidal effect of SAEW in the suspension test
The virucidal effects of the prepared SAEW solution against HuNoV GI.6 and GII.4 in the suspension test are shown in Figs. 1 and 2. During this test, the RT-qPCR assay presented a low detection limit, of approximately 20 viral copies per reaction for both HuNoVs. HuNoV GI.6 and HuNoV GII.4 treated with 4,000 mg/L NaClO (positive control) showed reduction values of 6.13 and 5.33 log10 CFU/g, respectively (data not shown). Moreover, the maximum mean reduction values of HuNoV GI.6 and GII.4 titers were 6.13 and 5.33 log10 genomic copies/200μL in undiluted SAEW and Sample 6 (S6/W10) to 9 (S9/W10), respectively; whereas the virucidal efficacy was significantly diminished between Sample 1 (S1/W10) and 5 (S5/W10) (p < 0.05). In addition, DW did not show any virucidal effect against either of the HuNoVs. Thereafter, we investigated the correlation between HuNoV titer reduction values and the changes in ORP, pH, and ACC levels in the suspension test. The three physicochemical properties analyzed were significantly correlated with the reduction in HuNoV titer (p < 0.01). Specifically, the change in pH presented a negative correlation with the reduction in HuNoV titer, whereas ORP and ACC changes showed a positive correlation with that parameter (Fig. 3A-1 and A-2). Furthermore, the ORP value showed the strongest correlation with the reduction in HuNoV GI.6 (R = 0.927) and GII.4 (R = 0.952) titers.
Correlation coefficient matrix of HuNoVs and physicochemical properties. A-1 and A-2 show the suspension test results and B-1 and B-2 the food test results. Positive coefficients represented by blue circles indicate a direct relationship between variables in the matrix; negative coefficients shown by using red circles reflect an inverse relationship. HuNoVs, human noroviruses
Our results showed changes in the physicochemical properties of undiluted SAEW and SAEW diluted with DW, and its virucidal effects. The changes in pH and ACC values were prominent, whereas those of ORP demonstrated a gradual change. These variations in physicochemical properties have a substantial effect on the virucidal activity of SAEW (Miyaoka et al., 2021; Moorman et al., 2017). However, it is not clear whether ACC, ORP, pH, or a combination of two or three of these characteristics affect the efficacy of viral control (Al-Haq et al., 2005). Park et al. (2004) have demonstrated that the low pH of electrolyzed water may inactivate pathogenic microorganisms and disturb the microbial growth rate (Park et al., 2004). The specific pH of SAEW to elicit a virucidal effect is between 5.0 and 6.5, which is assumed to render the viral particles more sensitive to active chlorine by damaging the viral capsid protein to enable HOCl penetration. Therefore, in agreement with our results, it was observed that the reduction values of HuNoVs diminished when the pH of SAEW increased more than 6.5. Additionally, ACC can affect various modes of chlorine disinfection, such as degradation of the capsid protein, reaction with nucleic acids, and decarboxylation of amino acids. HOCl is well known for its major disinfection activity among chlorine compounds (ClO−, Cl2, and HOCl) (Mahmoud, 2007). The loss of chlorine in electrolyzed solutions causes a decrease in its ACC, leading to a reduction in its virucidal effect (Morita et al., 2000). Cui et al. (2009) have reported that ACC reduction due to chlorine loss is dependent on SAEW storage conditions, which naturally reduces the bactericidal activity (Cui et al., 2009). Notably, the relative fraction of chlorine compounds affected the antimicrobial activity of SAEW, especially pH. Eventually, the changes in pH and ACC could be considered as external factors that exert a substantial effect on the virucidal effect of SAEW. In addition, it was possible to confirm the acceptable dilution conditions that ensure a significant virucidal effect, even if SAEW was used diluted. The variation in ORP among SAEW samples remained relatively stable within the range of 743 to 875 mV. The ORP changes as SAEW is diluted because the ORP values of SAEW tend to establish reactions with the oxidized species in electrolyzed water. Cui et al. (2009) suggested that the variation of SAEW could be considerably slow owing to the limited oxidized species in DW (Cui et al., 2009). Therefore, we confirmed that the ORP of SAEW was stable without considerable changes, ranging from Sample 6 (S6/W10) to 9 (S9/W10).
Virucidal effect of SAEW in the food test
The food test showed that HuNoV GI.6 and GII.4 populations in cabbage samples were eliminated, 5.94 and 5.59 log10 genomic copies/200μL, respectively, when subjected to treatment with undiluted SAEW and Sample 5 (S5/W10) to Sample 9 (S9/W10) (Table 2). Moreover, the virucidal effects of Sample 3 (S3/W10) and Sample 4 (S4/W10) presented reduction values of more than 3 log, whereas for Sample 1 (S1/W10) and Sample 2 (S2/W10) s, those values were lower than 2 log for both HuNoVs. In addition, owing to the detection and quantification limit of the procedures developed here to study HuNoVs, the log reduction values displayed by the HuNoVs subjected to treatment with DW were 0.42 log10 genomic copies/200μL (GI.6) and 0.43 log10 genomic copies/200μL (GII.4). ORP and ACC were positively correlated, whereas pH was negatively correlated with HuNoV titer reduction in the food test. ORP values strongly correlated with HuNoV GI.6 (R = 0.939) and HuNoV GII.4 (R = 0.943) titer (Fig. 3B-1 and B-2).
As a criterion for virucidal efficacy, a decrease of at least 4 log could be considered an adequate standard reduction titer (Tarka and Nitsch-Osuch, 2021). Our experimental data regarding the reduction value of HuNoV titer in cabbage samples were 4.65 (GI.6) and 4.28 (GII.4) log for Sample 4 (S4/W10). Our results indicate that, when the mixture ratio of undiluted SAEW solution and DW was 4:6, more than 4 log of norovirus present in cabbage was reduced. These results demonstrate the potential application of SAEW via implementation of the dilution method for inactivating HuNoVs. Furthermore, the scientific evidence based on the stable properties of diluted SAEW can reduce cost per optimum usage conditions. In conclusion, our study demonstrates the potential application of diluted SAEW as a virucidal agent. Thus, it has implications for utilization in the food industry. The scientific evidence of the stable properties of diluted SAEW indicated its potential to save cost per compliant use.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its Additional files].
References
Al-Haq MI, Sugiyama J, Isobe S. Applications of electrolyzed water in agriculture and food industries. industries. Food Science and Technology Research. 11: 135-150 (2005)
Cui X, Shang Y, Shi Z, Xin H, Cao W. Physicochemical properties and bactericidal efficiency of neutral and acidic electrolyzed water under different storage conditions. Journal of Food Engineering. 9: 582-586 (2009)
Diez-Valcarce M, Lopez MR, Lopez B, Morales O, Sagastume M, Cadena L, Kaydos-Daniels S, Jarquin C, McCracken JP, Bryan JP, Vinjé J. Prevalence and genetic diversity of viral gastroenteritis viruses in children younger than 5 years of age in Guatemala, 2014–2015. Journal of Clinical Virology. 114: 6-11 (2019)
European Committee for Standardization (CEN). EN 1276: Chemical disinfectants and antiseptics - Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas - Test method and requirements (phase 2, step 1). Brussels, Belgium (2019)
Griffin SM, Brinkman NE, Hedrick EJ, Rhodes ER, Fout GS. Comparison of nucleic acid extraction and reverse transcription-qPCR approaches for detection of GI and GII noroviruses in drinking water. Journal of Virological Methods. 199: 76-85 (2014)
Han MS, Chung SM, Kim EJ, Lee CJ, Yun KW, Choe PG, Kim NJ, Choi EH. Successful control of norovirus outbreak in a pediatric ward with multi-bed rooms. American Journal of Infection Control. 48: 297-303 (2020)
Hricova D, Stephan R, Zweifel C. Electrolyzed water and its application in the food industry. Journal of Food Protection. 71: 1934-1947 (2008)
Issa-Zacharia A, Kamitani Y, Miwa N, Muhimbula H, Iwasaki K. Application of slightly acidic electrolyzed water as a potential non-thermal food sanitizer for decontamination of fresh ready-to-eat vegetables and sprouts. Food Control. 22: 601-607 (2011)
Gentry-Shields J, Jaykus LA. Comparison of process control viruses for use in extraction and detection of human norovirus from food matrices. Food Research International. 77: 320-325 (2015)
Jothikumar N, Lowther JA, Henshilwood K, Lees DN, Hill VR, Vinjé J. Rapid and sensitive detection of noroviruses by using TaqMan-based one-step reverse transcription-PCR assays and application to naturally contaminated shellfish samples. Applied and Environmental Microbiology. 71: 1870-1875 (2005)
Kang M, Kim SJ, Lee JY, Yoon SR, Kim SH, Ha JH. Inactivation of Pectobacterium carotovorum subsp. carotovorum on Chinese cabbage (Brassica rapa L. subsp. pekinensis) by wash treatments with phenolic compounds. LWT - Food Science and Technology. 93: 229-236 (2018)
Kang M, Kim SJ, Yoon SR, Lee HW, Lee JY, Ha JH. Determination of Transfer Patterns of Pectobacterium carotovorum subsp. carotovorum planktonic cells and biofilms during mechanical cutting of kimchi cabbage. Journal of Food Science. 84: 2603-2609 (2019)
Koivunen J, Heinonen-Tanski H. Inactivation of enteric microorganisms with chemical disinfectants, UV irradiation and combined chemical/UV treatments. Water Research. 39: 1519-1526 (2005)
Lee HW, Lee HM, Yoon SR, Kim SH, Ha JH. Pretreatment with propidium monoazide/sodium lauroyl sarcosinate improves discrimination of infectious waterborne virus by RT-qPCR combined with magnetic separation. Environmental Pollution. 233: 306-314 (2018)
Mahmoud BS. Electrolyzed water: A new technology for food decontamination—A review. Deutsche Lebensmittel-Rundschau. 103: 212-221 (2007)
Miyaoka Y, Kabir MH, Hasan MA, Yamaguchi M, Shoham D, Murakami H, Takehara K. Virucidal activity of slightly acidic hypochlorous acid water toward influenza virus and coronavirus with tests simulating practical usage. Virus Research. 297: 198383 (2021)
Moorman E, Montazeri N, Jaykus LA. Efficacy of neutral electrolyzed water for inactivation of human norovirus. Applied Environmental Microbiology. 83: e00653-e00617 (2017)
Morita C, Sano K, Morimatsu S, Kiura H, Goto T, Kohno T, Hong WU, Miyoshi H, Iwasawa A, Nakamura Y, Tagawa M, Yokosuka O, Saisho H, Maeda T, Katsuoka Y. Disinfection potential of electrolyzed solutions containing sodium chloride at low concentrations. Journal of Virological Methods. 85: 163-174 (2000)
Park H, Hung YC, Chung D. Effects of chlorine and pH on efficacy of electrolyzed water for inactivating Escherichia coli O157: H7 and Listeria monocytogenes. International Journal of Food Microbiology. 91: 13-18 (2004)
Rutala WA, Weber DJ. Selection of the ideal disinfectant. Infect Control and Hospital Epidemiology. 35: 855-865 (2014)
Schwarz KR, Sidhu JPS, Toze S, Li Y, Lee E, Gruchlik Y, Pritchard DL. Decay rates of Escherichia coli, Enterococcus spp., F-specific bacteriophage MS2, somatic coliphage and human adenovirus in facultative pond sludge. Water Research. 154: 62-71 (2019)
Shen C, Norris P, Williams O, Hagan S, Li K. Generation of chlorine by-products in simulated wash water. Food Chemistry. 190: 97-102 (2016)
Tarka P, Nitsch-Osuch A. Evaluating the virucidal activity of disinfectants according to European Union standards. Viruses. 13: 534 (2021)
Xuan XT, Wang MM, Ahn J, Ma YN, Chen S, Ye XQ, Liu DH, Ding T. Storage stability of slightly acidic electrolyzed water and circulating electrolyzed water and their property changes after application. Journal of Food Science. 81: 13230 (2016)
Zang Y, Bing S, Li Y, Shu D, Huang A, Wu H, Lan LT, Wu HD. Efficacy of slightly acidic electrolyzed water on the microbial safety and shelf life of shelled eggs. Poultry Science. 98: 5932-5939 (2019)
Acknowledgements
This work was supported by the World Institute of Kimchi (Grant number KE2102-2), which is funded by the Ministry of Science and ICT, Republic of Korea.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, software, writing of the original draft preparation: H.W.L. and B.P.; software data curation: S.R.Y. and J.S.Y.; conceptualization, reviewing and editing: J.H.H. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, HW., Park, B., Yoon, SR. et al. Physicochemical stability and virucidal effect of diluted, slightly acidic electrolyzed water against human norovirus. Food Sci Biotechnol 31, 131–138 (2022). https://doi.org/10.1007/s10068-021-01011-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-021-01011-w