Abstract
This study was designed to determine the residual trichothecene mycotoxins in cereal samples. The optimal solvent for extraction was 84% (v/v) aqueous acetonitrile with 1% (v/v) formic acid. The best performing clean-up method was dispersive-solid phase with a mixture octadecyl silica and primary-secondary amine. The recoveries for the studied mycotoxins ranged from 83.3 to 92.8%. The methodology was successfully applied for monitoring 100 cereal samples obtained from a Korean market. The bean sample were found to be co-contamination with deoxynivalenol and HT-2 toxin. Deoxynivalenol possessed the highest detection freauency (4/100) and amount (727.38 µg/kg) among the trichothecene mycotoxins. The hazard index was less than 1.0 for all the observed mycotoxins in all cereal samples except one white rice sample (1.2681). This results indicated that periodic risk assessments of trichothecene mycotoxin through cereal intake are necessary for the health and safety.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mycotoxins are secondary toxic substances produced by mold during the storage, processing, and distribution of food. Mycotoxins affect growth, impair immune function, and cause cytotoxicity, carcinogenicity, and mutations in humans and animals (Agriopoulou et al., 2020; Wen et al., 2016). The diversity of the trichothecene group (types A, B, C, and D) stems from the number and position of hydroxylation at the basic trichothecene nucleus. Notably, epoxidation of C-9 and C-10 of some macrocyclic trichothecenes can increase the toxicity in mammalian systems (Wu et al., 2013). Trichothecene A compounds include neosolaniol (NEO), diacetoxyscirpenol (DAS), HT-2 toxin (HT-2), and T-2 toxin (T-2); type B compounds include nivalenol (NIV), deoxynivalenol (DON), 3-acetyldeoxynivalenol (3-AcDON), 15-acetyldeoxynivalenol (15-AcDON), and fusarenon-X (FUS-X) (Agriopoulou et al., 2020; Ostry et al., 2020). There is also a type D (including verrucarol, VER), but this type is rarely found in food and feed.
Cereals such as wheat, barley, corn, and nuts often contain trichothecene mycotoxins. In recent years, contamination by trichothecene mycotoxins has been reported in various cereals and cereal commodities worldwide (Lanza et al., 2019; Lee et al., 2021; Mishra et al., 2013; Ostry et al., 2020). An investigation of DON, T-2, and HT-2 in cereal-based foods and beer in Czech Republic indicated that the DON levels were below 72.4 ng/g, and the combined levels of T-2 and HT-2 ranged from 0.3 to 15.5 ng/g (Ostry et al., 2020). Another reported that the incidence of DON was 40% (20/50) in wheat, 24% (6/25) in maize, and 16% (4/25) in barley in India (Mishra et al., 2013). Therefore, some places, including the EU and USA, have established MLs for individual trichothecene mycotoxins in food and feed (European Commission, 2005, FAO, 2004).
Examples of methods used to detect mycotoxins to ensure food safety are enzyme-linked immunosorbent assay, HPLC, GC, and LC with MS (Rahmani et al., 2009). Notably, LC-tandem MS (LC-MS/MS) has been increasingly used for the accurate quantitative analysis of mycotoxins in food (Agriopoulou et al., 2020). The accurate detection of target components requires appropriate extraction, clean-up, and analysis technologies for the samples. In the extraction step, high cost, long preparation and analysis times, and matrix effects reduce the efficiency of the analysis. QuEChERS (quick, easy, cheap, effective, rugged, and safe) is an extraction method applied to overcome these issues in analysis using LC-MS/MS (Azaiez et al., 2014). In the clean-up step, IACs and MFCs column have been demonstrated to effectively remove unwanted interfering matrix of the sample extracts for mycotoxin analysis (Iha et al., 2017; Khayoon et al., 2010).
Cereals are a major dietary source worldwide. This means that cereals contaminated with trichothecene mycotoxins pose a widespread risk to food safety. Nevertheless, available data on trichothecene mycotoxins other than DON, HT-2, and T-2 are insufficient. Therefore, the aim of this study is to confirm the contamination levels of trichothecene mycotoxin in cereals from South Korea to protect consumers from the potential health and safety risks associated with cereal consumption.
Materials and methods
Chemicals and materials
Analytical standards of NIV, DON, 3-AcDON, 15-AcDON, FUS-X, NEO, HT-2, VER, and DAS were purchased from Sigma-Aldrich (St. Louis, MO, USA), and T-2 was obtained from Biopure (Buchs, Switzerland). Analytical grade anhydrous magnesium sulfate (MgSO4), sodium chloride, sodium citrate tribasic, sodium hydrogen citrate sesquihydrate, and formic acid (FA; purity > 98%) were purchased from Sigma-Aldrich. The end-capped C18 sorbent and primary-secondary amine (PSA) sorbent were obtained from Agilent Technologies (Santa Clara, CA, USA). Myco 6-in-1 immunoaffinity columns (IACs) were purchased from VICAM (Milford, MA, USA). MultiSep® 226 AflaZon+ multifunctional columns (MFCs) were purchased from Romer Labs GmbH (Gernsheim, Germany). Analytical HPLC grade water, methanol, and acetonitrile (ACN) were purchased from J. T. Baker (Phillipsburg, NJ, USA).
Sample collection
A total of 100 cereal samples were purchased from different local markets in Korea. Representative cereal types were selected, including white rice, black rice, barley, proso millet, sorghum, foxtail millet, beans, oats, adlay millet, and corn. The samples were ground using a laboratory blender (DA700G; Daesung Artlon Co. Ltd., Seoul, Korea) for 3 min and were immediately dispensed into polyethylene bags. Each sample was packaged in aliquots of 500 g and stored at 4 °C until further use.
Extraction procedure
Trichothecene mycotoxin was extracted using a QuEChERS method described by Kim et al. (2017) with modifications. Specifically, ground sample (4 g) was weighed in a 50 mL-conical tube along with MgSO4 (4 g), sodium chloride (1 g), sodium citrate tribasic (1 g), sodium hydrogen citrate sesquihydrate (0.5 g), water (20 mL), and 84% (v/v) aqueous ACN with 0.1% (v/v) FA (20 mL). The mixture was shaken for 15 min. Then, the tubes were centrifuged at 925 × g for 10 min at 4 °C using a CR22N high-speed refrigerated centrifuge (Hitachi Koki Co., Ltd., Tokyo, Japan). The supernatant (ACN phase) was transferred into a dispersive solid-phase extraction (d-SPE) tube.
Clean-up procedure
The extract was purified using three clean-up methods: an IAC column (A), an MFC column (B), and a d-SPE (C). Clean-up method A involved loading 5 mL of the QuEChERS extracts into IACs. The columns were washed with 5 mL of water. After drying the columns with gentle airflow, the QuEChERS extracts were eluted with 5 mL of ACN. The eluates were evaporated to dryness under a stream of N2 at 50 °C, and the residues were re-dissolved in 1 mL of 20% (v/v) aqueous ACN. Clean-up method B involved loading the QuEChERS extract mixture (5 mL extract, 1 mL water, and 60 µL FA) into each of the MFCs and eluting with 4 mL ACN. The eluates were evaporated to dryness under a stream of N2 at 50 °C, and the residues were re-dissolved in 0.5 mL water containing 0.1% (v/v) FA. Clean-up method C involved transferring 4 mL of QuEChERS extract into each of the d-SPE tubes containing 600 mg MgSO4, 200 mg C18, and 400 mg PSA, before immediately vortexing for 1 min. The mixtures were then centrifuged at 925 × g for 10 min at 4 °C. Subsequently, 1 mL of each supernatant was transferred to a 1.5 mL-micro tube. The final extracts were filtered through a 0.22 μm nylon syringe filter (Millipore, Burlington, MA, USA) and analyzed by LC-MS/MS.
LC-MS/MS analytical method
Trichothecene mycotoxins were detected and quantified using an UPLC system (Agilent infinity 1200 LC; Agilent Technologies) with MS (4000 QTRAP; AB SCIEX, Darmstadt, Germany). The trichothecene mycotoxins were ionized in positive and negative electrospray ionization modes and analyzed using multiple reaction monitoring with the following parameters: ion spray voltage: 5,500 V in positive mode and −4,500 V in negative mode; curtain gas pressure: 30 psi (N2); ion source gas-1 pressure: 50 psi; ion source gas-2 pressure: 50 psi; source temperature: 550 °C; the collision activated dissociation gas: medium. Analyst 1.5 software (Applied Bio systems) was used to identify and optimize the precursor ions, product ions, declustering potential, entrance potential, collision energy, and collision cell exit potential for trichothecene mycotoxins (Table 1). A Cadenza CW-C18 column (50 mm × 2 mm, i.d., 3 μm; Imtakt, Kyoto, Japan) was used for chromatographic separation, and the column temperature was maintained at 30 °C. Gradient elution was performed with a mobile phase consisting of 0.1% (v/v) FA in water (solvent A) and 0.1% (v/v) FA in ACN (solvent B). The gradient conditions were 100% A (0 min), 90% A (1 min), 20% A (6 min), 100% A (6.5 min), and 100% A (10 min). The flow rate was 300 μL/min, and the injection volume was 5.0 μL (Fig. 1).
Method validation
The new LC-MS/MS analytical method for the determination of trichothecene mycotoxins in cereal was validated to determine the limits of detection (LOD), limits of quantification (LOQ), linearity, accuracy, and precision according to the ICH/2005/Q2/R1 guidelines (ICH, 2005). To evaluate the linearity of the matrix-matched calibration curves, a mixed standard solution of 10 mycotoxins was diluted to six different concentrations (0.5–150 ng/mL). The LODs and LOQs were calculated as 3.3 and 10 times the standard deviation of the response divided by the gradient of the calibration curve, respectively. The accuracy was estimated by analyzing blank samples (white rice) spiked with standard solutions at three concentrations (low, middle, and high), and then identifying the recovery rate. Precision was measured by analyzing blank samples that were spiked at low, medium, and high concentrations on the inter- and intra-day, and expressed as the relative standard deviation (RSD, %). In addition, the matrix effects were investigated using matrix-matched solvent calibration curves and solvent calibration curves (Eq. 1). Matrix-matched calibration was prepared using blank samples, spiked in the same range of concentration as for the solvent (ACN) calibration.
Estimation of exposure and risk assessment
Estimated exposure was calculated using the contamination levels for individual mycotoxins and cereal intake data from the Korea Health Industry Development Institute (KHIDI, 2021). The average body weight (bw) of Korean men aged 25 to 39 years is 75.7 kg according to the Korean Statistical Informational Service (KOSIS, 2021) (Eq. 2).
The non-carcinogenic risk to health was assessed for each mycotoxin in terms of the hazard quotient (HQ) and hazard index (HI) (Reffstrup et al., 2010). HQ and HI were calculated using the following equations (Eq. 3 and 4):
The HQ for each mycotoxin in the cereal is determined by dividing the estimated (detected) exposure by a tolerable daily intakes (TDI) (Feron et al., 2004). The HQs are then combined to produce a hazard index. The HI was applied for mixtures of compounds without interactions (Reffstrup et al., 2010). The European food safety authority (EFSA) published TDIs for DONs (1.0 µg/kg bw/day), DAS (0.65 µg/kg bw/day), and T-2 (0.1 µg/kg bw/day) (EFSA, 2011, 2017, 2018).
Results and discussion
Optimization of extraction and clean-up procedure
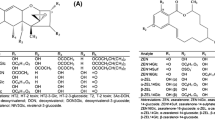
The extraction solvent was optimized in order to minimize the matrix effects of the extracted samples and improve the extraction efficiency of the method. All trichothecene mycotoxins dissolve well in methanol and ACN, but DON dissolves well in water and polar solvents. According to previous study, 84% (v/v) aqueous ACN can be used as an extraction solvent for the simultaneous extraction of DON (Abramović et al., 2005). Thus, the efficiencies of solvent systems containing 0, 1, 5, and 10% (v/v) FA with 100% ACN and 84% (v/v) aqueous ACN, were evaluated by spiking blank rice samples with trichothecene mycotoxins to achieve a concentration of 100 µg/kg.
Figure 2A show that the recoveries of all trichothecene mycotoxins extracted with 1% (v/v) FA in 84% (v/v) aqueous ACN were satisfactory and ranged between 70 and 120%. In addition, the extraction efficiencies of analyses using 84% (v/v) aqueous ACN were superior to those using 100% ACN. The addition of more than 5% (v/v) FA reduced the recovery rate. These results indicate that the addition of water and a small amount of FA are beneficial for mycotoxin extraction. The aqueous ACN (80%, v/v) with 1% (v/v) FA provided better extraction recovery than 80% (v/v) aqueous methanol with 1% (v/v) acetic acid for mycotoxins in food samples (Zhou et al., 2018). Form these results, aqueous ACN acidified with FA is most suitable for the extraction of multiple trichothecene mycotoxins from cereals.
The clean-up methods following extraction with 84% (v/v) aqueous ACN with 1% (v/v) FA are shown in Fig. 2B. The d-SPE clean-up procedure achieved the desired recovery rate (70–100%) and a higher recovery of trichothecene mycotoxins than that of the other clean-up methods tested. In contrast, the recovery rate of the IAC and MFC procedures was over 120% from half of the trichothecene mycotoxins. Thus, an extraction solvent containing 84% (v/v) aqueous ACN with 1% (v/v) FA and a d-SPE purification method were selected for the extraction of trichothecene mycotoxins.
Method validation
Uncontaminated white rice was confirmed to contain no mycotoxins in the preliminary test. Validation tests were evaluated with uncontaminated white rice. The linear range, LOD, LOQ, and matrix effect for each type of trichothecene mycotoxin are shown in Table 2. The linear regression coefficients of all calibration curves exhibited a good correlation (r2 > 0.999). The LODs and LOQs were in the ranges of 0.13–3.56 and 0.40–10.80 µg/kg, respectively. The LODs and LOQs of DON, FUS-X, and HT-2 were lower than those in the reported methods for toxin analysis in cereal syrups (Arroyo-Manzanares et al., 2015). The matrix effects were between −0.7 and 14.6% for all the mycotoxins, and these were compensated by using matrix-matched calibration. According to the acceptance criteria, the slope ratio (matrix/solvent) should be 0% for a method with no matrix effects, while matrix effects of > 0% and < 0% indicate ionization enhancement and ionization suppression, respectively (Zhou et al. 2017).
The accuracy and precision of the analytical method were evaluated by determining the recoveries and RSDs from blank samples (white rice) spiked at three different concentration levels (Table 3). The recovery values of all trichothecene mycotoxins ranged between 83.3% (15-AcDON) and 92.8% (T-2). The intra-day (n = 3) and inter-day precision (n = 9) of the method were in the RSD range of 0.5–12.6% and lower than 15%. Other studies have demonstrated recovery rates of NIV, DON, 3-AcDON, and 15-AcDON between 83.2% and 114.1% when analyzing maize samples using an LC-MS/MS (Ye et al., 2018). These results prove that our analytical method is appropriate for the identification and quantification of trichothecene mycotoxins in cereals.
Occurrence of trichothecene mycotoxins
A total of 100 cereal samples were analyzed using an optimized and validated method. Among them, seven cereal samples were positive for trichothecene mycotoxins (Table 4). DON had the highest detection frequency and ranged from 226.63 to 727.38 µg/kg. Nevertheless, monitoring results not exceed the admissible maximum level of DON (1,000 µg/kg) recommended by the human food.
Mycotoxins 3-AcDON and 15-AcDON are the main acetylated derivatives of DON. In this study, only one proso millet sample was found to contain 15-AcDON (293.69 µg/kg). FUS-X, DAS, and T-2 contaminations were quantified in one out of cereal samples (431.11, 146.11, and 253.18 µg/kg, respectively). NIV, 3-AcDON, NEO, HT-2, and VER were not found in any of the samples. Here, trichothecene mycotoxins exhibit co-contamination that DON and T-2 was observed in imported from China bean sample. Co-contaminations should be noted because DON and T-2 is more hazardous than T-2 alone (Friend et al., 1992).
The detection frequency of trichothecene mycotoxins was low in the samples domestically in Korean cereal samples monitored in this study (7/100). One factor that affects trichothecene mycotoxins in cereals is the climate. Because of the temperate climate in South Korea (latitude: 37° N and longitude: 128° E), the rate of mycotoxin detection in cereals is lower than that in countries with a subtropical climate. (Zinedine et al., 2006). In contrast, Brazil (latitude: 10° S and longitude: 55° W) is one of the subtropical country and had a detection rate of the most prevalent mycotoxins (AcDON, ZEA, HT-2, and beauvericin) of over 70% in 2014 and 2015 (Moreira et al., 2020). When targeting aflatoxin B1, a representative mycotoxin, the +2 °C climate change due to global warming demonstrably increased the risk of aflatoxin in Europe, including central and southern Spain, southern Italy, Greece, northern and southeastern Portugal, Bulgaria, Albania, Cyprus, and European Turkey (Battilani et al., 2016). This indicates that climate change may affect the detection frequency of trichothecene mycotoxins in cereals. Moreover, there are no established regulations for some of the trichothecene mycotoxins detected in this study (15-AcDON, FUS-X, and DAS). Therefore, continuous monitoring of trichothecene mycotoxins in cereals is necessary to mitigate the effects of rapid climate change and imported products, rather than established regulations.
Assessment of health risk
The health risk assessment was performed using TDI, the national consumption data and the concentration levels of detected trichothecene mycotoxins (DON, 15-AcDON, FUS-X, DAS and T2) in cereal samples. The exposure assessment, HQs, and HI associated with each detected mycotoxin in cereals are summarized in Table 4. Adult exposure to the five mycotoxins was calculated to be between 0.0002 and 0.8350 μg/kg bw/day through the consumption of cereals. Exposure levels of all mycotoxin were below the TDI.
The HI was determined by summing the HQs for mycotoxins. The HIs ranged from 0.0026 to 1.2681 for adults. The HI values were less than 1.0 for all the observed mycotoxins in all cereal samples except white rice. The high HQ and HI values of white rice are attributed to its relatively high consumption compared to other cereals. However, DON and DAS toxins in white rice were detected in only one sample each. Therefore, the results in Table 4 do not mean that the risk index (e.g. HI) of all commercially available white rice is high on average. In addition, the samples monitored here were not reflected the years of production and storage conditions. The mycotoxin concentration in rice is affected by storage period and conditions (Tang et al., 2019). Therefore, mycotoxins can be detected in large quantities in some samples.
Table 4 indicated the possibility of unintentional exposure to mycotoxins in cereals, although at a low frequency. However, these results were obtained from raw kernels without considering the usual pretreatment process such as washing and cooking. The process of soaking and washing in water can effectively remove substances including damaged kernels, fine material, and dust with a lot of mycotoxins (Adebo et al., 2021; Karlovsky et al., 2016). Moreover, the process of cooking, heating, or steaming are reduced mycotoxins in cereal by 34–70% (Karlovsky et al., 2016). Nevertheless, the health risks posed by the daily intake of mycotoxins through cereals should not be overlooked, and continuous contamination surveys of mycotoxins are required to protect consumers from the risk caused by exposure to mycotoxins.
This study aimed to confirm the contaminations from 10 trichothecene mycotoxin species in cereals from South Korean markets using the QuEChERS extraction combined with LC-MS/MS. QuEChERS extraction using 84% (v/v) aqueous ACN containing 1% (v/v) FA. The best performing clean-up method was d-SPE with a mixture C18 and PSA. The developed method demonstrated good selectivity, accuracy, and precision for the analysis of trichothecene mycotoxins in cereals. The frequency of detection of trichothecene mycotoxins in cereals was low (7/100), but the admissible HI was exceeded (1.2681) in one white rice sample. This is the value of the raw kernel without taking into account the storage period and storage conditions. Nevertheless, periodic risk assessments of trichothecene mycotoxin through cereal intake are necessary for the health and safety of Korean consumers.
References
Abramović BF, Jajić IM, Jurić VB, Gaál FF. Optimization of the determination of deoxynivalenol in corn samples by liquid chromatography and a comparison of two clean-up principles. Journal of the Serbian Chemical Society. 70: 1005-1013 (2005)
Adebo OA, Molelekoa T, Makhuvele R, Adebiyi JA, Oyedeji AB, Gbashi S, Adefisoye MA, Ogundele OM, Njobeh PB. A review on novel nonthermal food processing techniques for mycotoxin reduction. International Journal of Food Science and Technology. 56: 13-27. (2021)
Agriopoulou S, Stamatelopoulou E, Varzakas T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods. 9: 137 (2020)
Arroyo-Manzanares N, Huertas-Pérez JF, Gámiz-Gracia L, García-Campaña AM. Simple and efficient methodology to determine mycotoxins in cereal syrups. Food Chemistry. 177: 274-279 (2015)
Azaiez I, Giusti F, Sagratini G, Mañes J, Fernández-Franzón M. Multi-mycotoxins analysis in dried fruit by LC/MS/MS and a modified QuEChERS procedure. Food Analytical Methods. 7: 935-945 (2014)
Battilani P, Toscano P, Van der Fels-Klerx H, Moretti A, Leggieri MC, Brera C, Rortais A, Goumperis T, Robinson T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Scientific Reports. 6: 24328 (2016)
EFSA, European Food Safety Authority Panel on Contaminants in the Food Chain (CONTAM) EFSA scientific opinion on the risks for animal and public health related to the presence of T‐2 and HT‐2 toxin in food and feed. EFSA Journal. 9: 2481 (2011)
EFSA, European Food Safety Authority Panel on Contaminants in the Food Chain (CONTAM). EFSA scientific opinion on the risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA Journal. 15: e04718 (2017)
EFSA, European Food Safety Authority Panel on Contaminants in the Food Chain (CONTAM) EFSA scientific opinion on the risks to human and animal health related to the presence of 4, 15‐diacetoxyscirpenol in food and feed. EFSA Journal. 16: e05367 (2018)
European Commission. Commission Regulation (EC) No. 856/2005 of 6 June 2005 amending Regulation (EC) No. 466/2001 as regards Fusarium toxins. Official Journal of the European Union L143: 3-8 (2005)
Food and Agriculture Organization of the United Nations (FAO). Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper. 81:165 FAO, Rome, Italy (2004)
Feron VJ, van Vliet PW, Notten WR. Exposure to combinations of substances: A system for assessing health risks. Environmental Toxicology and Pharmacology. 18: 215-222 (2004)
Friend D, Trenholm H, Hartin K, Panich P, Thompson B, Boermans H. Toxicity of T-2 toxin and its interaction with deoxynivalenol when fed to young pigs. Canadian Journal of Animal Science. 72: 703-711 (1992)
ICH, I. Q2 (R1): Validation of analytical procedures: text and methodology. Paper presented at the International Conference on Harmonization, Geneva (2005)
Iha M, Mini C, Okada I, de Cássia Briganti R, Trucksess M. The use of regenerated immunoaffinity columns for aflatoxins B1, B2, G1 and G2 in peanut confection. Journal of Chromatography A. 1483: 1-7 (2017)
Karlovsky P, Suman M, Berthiller F, De Meester J, Eisenbrand G, Perrin I, Oswald IPm Speijers G, Chiodini A, Recker T, Dussort P. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Research. 32: 179-205 (2016)
Kim S, Lee S, Nam T-G, Seo D, Yoo M. Comparison of a newly developed liquid chromatography with tandem mass spectrometry method and enzyme-linked immunosorbent assay for detection of multiple mycotoxins in Red pepper powder. Journal of Food Protection. 80: 1347-1354 (2017)
Khayoon W, Saad B, Yan C, Hashim N, Ali A, Salleh M, Salleh B. Determination of aflatoxins in animal feeds by HPLC with multifunctional column clean-up. Food Chemistry. 118: 882-886 (2010)
KHIDI—Korea Health Industry Statistics System The national nutrition statistics. Available from:https://www.khidi.or.kr/kps/dhraStat/result1?menuId=MENU01652&year=2018. Accessed Oct. 31, 2021.
KOSIS—Korean Statistical Information Service Estimated population by weight. Statistics Korea Available from: National Health Measurement Statistics by Weight. Statistics Korea. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=113&tblId=DT_ 113_STBL_1020278&vw_cd=MT_ZTITLE&list_id=113_11304_009&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_path=MT_ZTITLE. Accessed Oct. 31, 2021.
Lanza A, da Silva RC, dos Santos ID, Pizzutti IR, Cence K, Cansian RL, Zeni J, Valduga E. Mycotoxins’ evaluation in wheat flours used in Brazilian bakeries. Food Science and Biotechnology. 28: 931-937 (2019)
Lee J-G, Lee Y, Kim CS, Han SB. Codex Alimentarius commission on ensuring food safety and promoting fair trade: harmonization of standards between Korea and codex. Food Science and Biotechnology. 30: 1151-1170 (2021)
Mishra S, Ansari KM, Dwivedi PD, Pandey HP, Das M. Occurrence of deoxynivalenol in cereals and exposure risk assessment in Indian population. Food Control. 30: 549-555 (2013)
Moreira GM, Nicolli CP, Gomes LB, Ogoshi C, Scheuermann KK, Silva-Lobo VL, Schurt DA, Riteni A, Moretti A, Pfenning LH, Del Ponte EM. Nationwide survey reveals high diversity of Fusarium species and related mycotoxins in Brazilian rice: 2014 and 2015 harvests. Food Control. 113: 107171 (2020)
Ostry V, Dofkova M, Blahova J, Malir F, Kavrik R, Rehurkova I, Ruprich J. Dietary exposure assessment of sum deoxynivalenol forms, sum T-2/HT-2 toxins and zearalenone from cereal-based foods and beer. Food and Chemical Toxicology. 139: 111280 (2020)
Rahmani A, Jinap S, Soleimany F. Qualitative and quantitative analysis of mycotoxins. Comprehensive Reviews in Food Science and Food Safety. 8: 202-251 (2009)
Reffstrup TK, Larsen JC, Meyer O. Risk assessment of mixtures of pesticides. Current approaches and future strategies. Regulatory Toxicology and Pharmacology. 56: 174-192 (2010)
Tang EN, Ndindeng SA, Bigoga J, Traore K, Silue D, Futakuchi K. Mycotoxin concentrations in rice from three climatic locations in Africa as affected by grain quality, production site, and storage duration. Food Science and Nutrition. 7: 1274–1287 (2019)
Wen J, Mu P, Deng Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicology Research. 5: 377-387 (2016)
Wu Q, Dohnal V, Kuca K, Yuan Z. Trichothecenes: Structure-toxic activity relationships. Current Drug Metabolism. 14: 641-660 (2013)
Ye J, Wu Y, Guo Q, Lu M, Wang S, Xin Y, Xie G, Zhang Y, Mariappan M, Wang S. Development and interlaboratory study of a liquid chromatography tandem mass spectrometric methodfor the determination of multiple mycotoxins in cereals using stable isotope dilution. Journal of AOAC International. 101: 667-676 (2018)
Zhou W, Yang S, Wang PG. Matrix effects and application of matrix effect factor. Bioanalysis. 9: 1839-1844 (2017)
Zhou J, Xu JJ, Cong JM, Cai ZX, Zhang JS, Wang JL, Ren YP. Optimization for quick, easy, cheap, effective, rugged and safe extraction of mycotoxins and veterinary drugs by response surface methodology for application to egg and milk. Journal of Chromatography A. 1532: 20-29 (2018)
Zinedine A, Brera C, Elakhdari S, Catano C, Debegnach F, Angelini S, De Santis B, Faid M, Benlemlih M, Minardi V, Miraglia M. Natural occurrence of mycotoxins in cereals and spices commercialized in Morocco. Food Control. 17: 868-874 (2006)
Acknowledgements
This study was supported by the Main Research Program (E0156500-03) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT.
Funding
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Export Promotion Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (GA119027-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, DB., Jung, Y.S., Nam, T.G. et al. Simultaneous determination of trichothecene mycotoxins in cereals by LC-MS/MS. Food Sci Biotechnol 31, 165–174 (2022). https://doi.org/10.1007/s10068-021-01024-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-021-01024-5