Abstract
The use of unconventional sources is very relevant in the food area. In the present study the development of active films with the addition of bioextract (BE) or microencapsulated bioextract (MBE) from xoconostle (Opuntia oligacantha) on chayotextle starch was investigated. The film formulations were: 4 g of chayotextle starch, 2 g of glycerol and 180 g of water, three films with BE added (0.4, 0.8 and 1.2 g) and three films with MBE added (0.4, 0.8 and 1.2 g). Total phenols, total flavonoids, antioxidant activity (ABTS and DPPH), Salmonella typhimurium inhibition, color and mechanical properties of the films were analyzed. The film with 1.2 g of MBE showed high concentration of total phenols (54.12 ± 0.77 mg EAG/100 g), total flavonoids (16.65 ± 0.10 mg QE/100 g) and antioxidant activity (29.11 ± 0.48 and 41.42 ± 1.81 mg EAA for ABTS and DPPH respectively). The addition of bioextract from xoconostle is an option for the development of active films with antioxidant properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development and constant improvement of packaging materials are very important to maintain food quality (Souza et al., 2013). Increasingly, great efforts are being made to develop biodegradable and environmentally-friendly materials from low-cost natural resources, such as polysaccharides and proteins. The design to edible films should provide improvements in the properties of the matrix and should consider other synergistic effects between components (Gao et al., 2017). The application of natural antioxidants in starch-based films to maintain food quality through antioxidant action is an innovative concept in the food industry (Reis et al., 2015).
Film based carbohydrates are particularly attractive because they have good film forming ability due to their unique colloidal properties (Ghasemlou et al., 2013). Starch is completely biodegradable, without toxic components, low in cost and widely available and (Luchese et al., 2017). The use of new starch alternatives for the development of new materials is of great interest for the industry, such as chayotextle starch (Palma-Rodríguez et al., 2018). Chayotextle has been isolated in central Mexico. This tuber contains an adequate amount of starch (60% dry basis), but the use of this tuber is limited to local consumers (Aila-Suárez et al., 2013).
The cactus pear “xoconostle” is cultivated in countries such as Mexico and have high commercial value due to are used for the manufacture of food products such juices, alcoholic beverages, jams, and natural liquid sweeteners (Kıvrak et al., 2018). It is a source of functional ingredients including phenolic compounds, betacyanins and vulgaxanthins (Hernández-Fuentes et al., 2015). The consumption of xoconostle, due to its content of polyphenols, can help improve human health; it may contribute to the prevention of chronic diseases and other problems in humans such as diabetes, obesity and respiratory illnesses (Morales et al., 2012).
The objective of this study was to develop edible films from an unconventional source of starch (chayotextle) with the incorporation of bioextract (BE) or microencapsulated bioextract (MBE) from O. oligacantha and characterize the antioxidant, antimicrobial and mechanical properties of the resulting films.
Materials and methods
The xoconostle variety Opuntia oligacantha was used with commercial maturity and was obtained from Tezontepec de Aldama, Hidalgo, Mexico, in March 2016, a batch of 100 fruits morphologically homogeneous were used. The chayotextle (Sechium edule Sw.) was obtained in the municipal market of Tulancingo, Hidalgo, Mexico, in March 2016, and homogeneous tubers were used in weight and shape (300 g approximately each). The reagents used were as follows: methanol (HPLC grade), 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) from Sigma-Aldrich (St. Louis, MO, USA), potassium persulphate (analytical-reagent grade), Folin-Ciocalteu reagent (analytical-reagent grade) (Sigma-Aldrich), sodium carbonate (analytical-reagent grade) from J.T. Baker (USA), gallic acid (analytical-reagent grade) (Sigma-Aldrich), ascorbic acid (analytical-reagent grade) (Sigma-Aldrich), 1,1-diphenyl-2-picrylhydrazyl (DPPH) (Sigma-Aldrich), ethanol (HPLC grade), glycerol (analytical-reagent grade), acetone (gradient grade). Rutin (95%), ferulic acid (98%), vanillic acid (98%), quercetin (95%), hydroxybenzoic acid (99%), apigenin (98%), caffeic acid (98%) and kaempferol (98%) from Sigma-Aldrich (St. Louis, MO, USA). Maltodextrin with 10% dextrose from Grain Processing Corporation (Muscatine, IA, USA) and gum arabic from Frutarom (Winter Haven, FL, USA) were also used.
Microencapsulation
Xoconostle fruits morphologically homogeneous and commercial maturity were manually selected and the whole fruit was mixed with water to a proportion 1:2 and homogenized in an industrial blender HGBSS (Waring Commercial, Torrington, CT, USA) for approximately 3 min. The product was vacuum filtered using Whatman paper number 1 to obtain cactus pear bioextract (BE), with 4% of total solids. After, it was mixed with two polymers used as wall materialss: maltodextrin and gum arabic (biopolymers mixed between in a proportion 50-50) to obtain 30% total solids. The mixture was placed in a container and stirred for 24 h at room temperature to dissolve the gum; the mixture was then homogenized and dried on a Mini Spray Dryer (Büchi B-290, Switzerland) following the method developed by Martinez et al. (2015) with some modifications. The drying conditions were as follows: inlet temperature of 160 °C, 4 bar pressure and 10 mL/min inflow. The microencapsulated bioextract (MBE) of cactus pear was stored in sealed amber bags and refrigerated until use.
Isolation of chayotextle starch
The starch was isolated following the methodology of Aila-Suárez et al. (2013). The tubers were cut into 2 × 2 cm cubes and macerated at a low speed (500 g of tuber with 500 g of water) for 2 min. The homogenate was washed and sieved to #50, #100, #200, #270 and #325 mesh (300, 150, 75, 53 and 45 µm, respectively) until the wastewater was observed to be transparent; then the homogenate was pelleted overnight and decanted. This material was dried in a convection oven at 35 °C overnight. The dried starch was ground in a blender to a powder; then, it was passed through a #100 mesh standard sieve and stored in a sealed container until use.
Starch film
The films were made following the methodology of Aila-Suárez et al. (2013) with some modifications. First, 4 g of starch was mixed with 180 mL of water and 2 g of glycerol; this mixture was stirred at 40 °C for 3 min to achieve homogenization. Then, we proceeded to gelatinize the starch, raising the temperature to 90 °C and stirring continuously for 10 min. The BE or MBE was added when the filmogenic solution reached 50 °C (Table 1). After, the mixture was transferred to a plate coated with Teflon and subsequently dried in an oven for 24 h at 35 °C.
Extraction of phenolic compounds from the films
Extraction was realized according to reported by Pérez-Alonso et al. (2015). First, 2 g of film was placed in a 100 mL beaker, and 20 mL of ethanol solution was added (50/50). Then, the mixture was stirred for 10 min, and the supernatant was centrifuged at 10,000 min−1 for 10 min at 4 °C in a centrifuge Z 36 HK (HERMLE Labortechnik GmbH, Wehingen, Germany). The pellet obtained in the previous step was mixed with 20 mL of acetone (70/30) for 10 min and centrifuged at 10,000 min−1 for 10 min at 4 °C. The ethanol and acetone supernatants were combined, stirred for 5 min and centrifuged under the conditions described above. The samples were refrigerated in the dark until analysis.
Profile of phenolic compounds
The phenolic compounds were analyzed by capillary electrophoresis using a P/ACE™ MDQ instrument (Beckman Coulter, Indianapolis, USA) by the method described by Cao et al. (2004) with some modifications. The extract obtained in the previous section was mixed with 15 mL of HPLC grade methanol for 10 min in an ultrasonic bath. Then, the sample was centrifuged and filtered, and the supernatant was refrigerated. The temperature was kept at 25 °C and the wavelength of UV detector was set at 214 nm. Pressure injection (30 mbar) was for 12 s. A 60.0 cm (distance between injector and detector 50.0 cm) × 75 µm I. D. fused-silica capillary was used. A 5 min wash cycle with 0.1 M NaOH followed by 2 min distilled water, and 5 min running borate buffer pH 8.7 was necessary to condition the capillary. The running time was standardized at 20 min. For the results a curve for rutin, ferulic acid, vanillic acid, quercetin, hydroxybenzoic acid, apigenin, caffeic acid and kaempferol was elaborated with its respective standard, according to reported by Osorio-Esquivel et al. (2011) for phenolic compounds in Opuntia joconostle fruits. Method validation was performed according to guidelines set by International Union of Pure and Applied Chemistry (IUPAC, 2002). The precision was estimated by making five replicate injections of a standard mixture solution. The relative standard derivations (RSDs) peak areas are 4.17%, 4.21%, 4.19%, 4.85%, 4.42%, 4.11% and 4.65% for rutin, ferulic acid, vanillic acid, quercetin, 4-hidroxibenzoic acid, apigenin, caffeic acid and kaempferol, respectively.
Total phenolic content
The phenolic content was assessed using the Folin–Ciocalteu method (Singleton et al., 1999) with some modifications. First, 2 g of film was mixed with 0.5 mL of Foling Ciocalteu (Sigma-Aldrich, USA). and 4.5 mL of water; after 5 min, the mixture was homogenized with 4 mL of 20% sodium carbonate. Then, the resulting mixture was measured at 765 nm after 2 h using Jenway 6715 UV–Vis spectrophotometer (USA). The total phenols are expressed in mg gallic acid equivalents GAE/100 g of film.
Estimation of total flavonoids
Total flavonoid content was determined using the method described by Arvouet-Grand et al. (1994). A solution of aluminum trichloride (AlCl3) in 2% methanol was used. A total of 1 mL of the supernatant obtained in the extraction of the film was added to a 10-mL volumetric flask with methanol and stirred. Then, 2 mL of the sample mixture was added to 2 mL of AlCl3. The samples were read at 415 nm in a spectrophotometer Jenway 6715 UV–Vis (USA). Total flavonoid content was expressed as mg quercetin equivalents QE/100 g of film.
ABTS free radical scavenging activity
The antioxidant activity was determined using the chromogenic compound ABTS according to the protocol of Re et al. (1999) with some minor modifications. This method is based on studying the discoloration of the free radical ABTS by anti-oxidants. The ABTS (Sigma-Aldrich, USA) 7 mM was combined with potassium persulphate to generate the ABTS+ radical (2.45 mM). Then, 980 µL of ABTS was mixed with 20 µL of the supernatant obtained in the extraction of the film. In total, 2 readings per sample were performed; the first measurement was performed at 1 min, and the second measurement was performed at 6 min, both at a wavelength of 740 nm in a spectrophotometer Jenway 6715 UV–Vis (USA). The results were calculated as mg ascorbic acid equivalents of AAE/100 g of film.
DPPH free radical scavenging activity
The antioxidant activity of DPPH was studied according to the protocol previously described by Kuskoski et al. (2005) with some modifications. This method is based on studying the discoloration of DPPH by anti-oxidants. In total, 2.7 mL of DPPH (Sigma-Aldrich, USA) prepared in 80% methanol was added to 0.3 mL of the supernatant obtained in the extraction of the film. Two readings per sample were performed (at 1 min and 1 h) using a spectrophotometer Jenway 6715 UV–Vis (USA) at 515 nm. The results were calculated as mg ascorbic acid equivalents AAE/100 g of film.
Antibacterial activity
Effect against Salmonella typhimurium was determined according to descript to Seydim and Sarikus (2006) with some modifications. The zone of inhibition assay on solid media was used to determine the antimicrobial effects of the films. S. typhimurium (ATCC 43971) was activated using 1 mL of bacteria in 99 mL of nutritious broth until a concentration of approximately 107 CFU/mL (24 h, 37 °C) was obtained. The concentration of the bacteria was determined using the plate count method. Edible film discs were cut to 1 cm diameters using a sterile cutter and then placed in petri dishes containing Salmonella and Shigella agar. After, the petri dishes were inoculated with 100 µL of bacteria and incubated at 37 °C for 24 h. Then, the area of the whole zone was calculated and then subtracted from the film disc area; this difference in area was reported as the zone of inhibition. Inhibition was monitored until the growth of bacteria occurred in the initial halo. The days of protection were determined by observing when the halo of inhibition disappeared.
Film color
The color of the films was determined following the methodology of Saberi et al. (2017), using a Minolta CM-508d colorimeter (Japan). Calibration was performed prior to the sample analysis using the color white. The measurements were taken directly on the glass surface. Three shots were performed for each measurement, and the obtained parameters were L* a* b* and total color change (∆E).
Mechanical properties
The thickness of the films was evaluated using a manual micrometer (Mitutoyo Co., Kawasaki, Kanagawa, JP) at 5 random positions of the film, according to reported by Fakhouri et al. (2015). The average value was used to calculate the area of the cross-sections of the films, where the area is the width per each film thickness. The percentage elongation, tensile strength and Young’s modulus of each film was determined. The mechanical properties were measured according to a standard method, (ASTM, 1995), using CT3 Brookfield texture analyzer with a 50 kg load cell (AMETEC, Harlow, Essex, UK). For the stress tests, the films were cut into rectangles 10 cm long and 1 cm wide. The rectangles were kept in a desiccator for 14 days with saturated sodium bromide (57% RH) solution at 25 °C 5 cm apart to maintain the constant moisture of films until the mechanical tests were performed. The ends of the films were fixed to clamps. The strain rate was 0.13 mm/s.
Statistical analysis
The experiments were arranged in a completely randomized design and were performed in triplicate. The results were processed by analysis of variance. Media comparison was conducted using Tukey’s multiple test (α = 0.05) (Steel and Torrie, 1960).
Results and discussion
Phenolic compounds
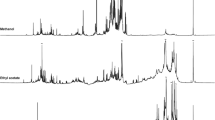
The yield obtained after spray drying was 30%, that is, for every 100 g of sample that was introduced to the dryer (bioextract and gums), 30 g of microencapsulation was obtained, which had a particle size of 14.47 μm. The bioextract (BE) and microencapsulated bioextract (MBE) from Opuntia oligacantha showed rutin, quercetin, kaempferol, apigenin, caffeic acid and ferulic acid (Fig. 1), according to electropherograms, the compound that is in the highest concentration is rutin, while ferulic acid and kaempferol are well preserved once encapsulated. 4-hydroxybenzoic acid was not identified in BME (Fig. 1B), neither showed presence starch films. The films with MBE had the highest concentrations of phenolic compounds, this result suggest that the extract when encapsulated receives protection and is in an adequate form to act as soon as it is released. Rutin had the highest concentration (0.97 ± 0.12 mg/g) in the film with 1.2 g of MBE (Table 2). Our results are similar to those of previous reports regarding 4-hydroxybenzoic acid, caffeic acid, rutin and quercetin (Cortez-García et al., 2015) in samples of O. joconostle, all of which are indicators of possible antioxidant or antimicrobial activity. Phenolic compounds identified in BE and MBE have been associated with some biological activity, antioxidant activity or antimicrobial activity among others, resulting successful this identification method. The results obtained in the present study reaffirm that xoconostle is an important source of phenolic compounds.
(A) Electropherogram of phenolic compounds of bioextract (BE) from Opuntia oligacantha at 214 nm. Identified peaks: (1) rutin, (2) ferulic acid, (3) quercetin, (4) 4-hydroxybenzoic acid, (5) apigenin, (6) caffeic acid, (7) kaempferol. (B) Electropherogram of phenolic compounds of microencapsulated bioextract (MBE) from Opuntia oligacantha at 214 nm. Identified peaks: (1) rutin, (2) ferulic acid, (3) quercetin (4) apigenin, (5) caffeic acid, (6) kaempferol
The total phenolic content showed significant differences between the films (p < 0.05) (Table 3). The film with the addition of 1.2 g of BE had 28.06 ± 0.71 mg GAE/100 g, while the film with the addition of 1.2 g of MBE had 54.12 ± 0.77 mg GAE/100 g, a more than 1.5-fold increase. Cortez-García et al. (2015) found that the total phenols decreased in O. joconostle when subjected to boiling. However, the microencapsulation preserved the phenolic compounds for application in food products, because the biopolymers of the wall of the microcapsules have a thermoprotector effect (Pérez-Alonso et al., 2015). The increase in total phenols in relation to the addition of BE and MBE are consistent with the results reported by Wang et al. (2013) in making chitosan films with different concentrations of polyphenols from tea. Phenolic compounds help protect plants against ultraviolet light, and act as defenses against pathogenic microorganisms (Osorio-Esquivel et al., 2011).
Significant differences between the films were found in terms of total flavonoids (p < 0.05), showing the highest concentration in the film with 1.2 g of MBE (11.65 ± 0.17 mg QE/100 g) (Table 3). According to Reis et al. (2015) the antioxidant activity of flavonoids is completely related to their structure, acting as primary antioxidants when donating a hydrogen atom and they can also act as chelating agents.
Antioxidant activity
It is well known that the anti-oxidant activity of plant extracts is derived primarily from phenolic compounds, which are highly effective antioxidants and free radical scavengers (Wang et al., 2013). Osorio-Esquivel et al. (2011) mention that kaempferol and rutin are powerful antioxidants in the oxidation of LDL. The antioxidant activity measured for ABTS and DPPH were similar. The films showed significant differences (p < 0.05) with the addition of BE or MBE (Table 3) against starch film. High antioxidant activity was observed for films treated with 1.2 g of MBE, 29.11 ± 0.48 mg EAA/100 g for ABTS, and 41.42 ± 1.81 mg EAA/100 g for DPPH. O. oligacantha has been demonstrated to have antioxidant activity due to the phenols and flavonoids it contains (Hernández-Fuentes et al., 2015). Moradi et al. (2012) added the phenolic compounds of grape seeds in a matrix based on chitosan and observed that the antioxidant properties of the matrix increased considerably.
The films with added BE showed no significant differences (p > 0.05) in DPPH (Table 3), perhaps due to the interaction between the phenols and polymers of the carbohydrates of the film. Saura-Calixto (2010) reported interactions between phenolic acids and polysaccharides. These interactions could be affecting the antioxidant activity of the phenolic acid so the increase of BE would not be reflected in increment of antioxidant activity, Corrales et al. (2009) mention that hydroxyl groups of phenolic compounds, mainly responsible for antioxidant properties, may interact with OH− groups from starch films resulting in the reduction of their properties. The films with 0.4, 0.8 and 1.2 g of MBE showed significant differences (p < 0.05), which may be due to the interactions between amylose and maltodextrin/gum arabic allowing the phenolic compounds are free for provide a greater antioxidant capacity. The results of antioxidant activity in the added films indicate that the incorporation of phenolic compounds from natural sources are an effective alternative for their addition in complex matrices.
Antibacterial activity
Figure 2 shows the zones of inhibition resulting in the films by the addition of bioextract, in the starch film no effect is observed, showing growth of the colonies even in the contact zone (a), in section b of the image can be observed that at the maximum concentration of BE the zone of inhibition is well defined, although few colonies managed to develop there, and section c shows a zone of inhibition that is greater and better defined with respect to the others. The films with MBE exhibited protective effects for a longer time than films with added BE. The films with 0.4 g of BE and 0.4 g of MBE showed no had inhibitory against S. typhimurium (Table 3). The maximum protection of a film against S. typhimurium was found for the film containing 1.2 g of MBE, which exhibited a halo of inhibition of 2 mm for 4.66 days, this due to the micro-encapsulates free the anti-bacterial compounds more slowly. Hayek and Ibrahim (2012) reported the anti-microbial effect of O. matudae against Escherichia coli O157: H7, showing halos of inhibition of up to 9.8 ± 1.01 mm. Further, Espinosa-Muñoz et al. (2017) reported that extracts from O. oligacantha contain phenols and flavonoids with inhibitory effects on S. typhimurium and Staphylococcus aureus. The inhibitory effect of BE and MBE is due of the phenolic compounds that contain, which attack cell walls and cell membranes, thereby affecting their permeability and the release of intracellular constituents causing the dead of pathogenic bacteria (Bajpai et al., 2008). According to the results it found that the higher the antioxidant activity increased antimicrobial activity was observed, therefore, can be attributed to the antibacterial effect synergy of different phenolic compounds added to the films by BE and MBE, demonstrating that the bioactive compounds present in the xoconostle provide beneficial effects for use in the food industry.
Film color
Table 4 shows the results obtained for the color properties, where L*, a* and b* presented significant differences (p < 0.05) among starch films. Parameter L*, a* and ∆E increased with the addition of BE and MBE. The films with BE and MBE presented negative values for parameter b*. The BE from O. Oligacantha have phenols and flavonoids that confer color to the extract.
Mechanical properties
Mechanical properties were also altered, depending on the levels of BE and MBE incorporated. Table 4 shows the significant (p < 0.05) differences between the starch film, the film with 0.4 and 0.8 of BE added, the film with 1.2 of BE added and the films with 0.4, 0.8 and 1.2 of MBE added in terms of thickness, which is related to breaking resistance. The films with 0.4 g and 0.8 g of BE were not significantly different (p < 0.05) from the starch film (Table 4) in terms of tensile strength. The films with major phenol and flavonoid contents showed decreased tensile strength. Thus, the molecular properties of phenolic compounds affect the strength of the film matrix considerably, the interaction between the BE and MBE, and starch promotes the formation of discontinuities in the structure of the film. For example, the tensile strength of zein films reduces as the phenolic concentration in the films increases (Arcan and Yemenicioğlu, 2011). Similar results were observed by Moradi et al. (2012) with chitosan films incorporating grape seed extract.
The films with 1.2 g of BE and those with 0.4, 0.8 and 1.2 g of MBE showed significant differences (p < 0.05) from the starch film in terms of the percentage elongation (Table 4). The lowest percentages of elongation may be attributed to the natural free sugars in O. oligacantha, which interact with the film network causing a percentage elongation less, as reported by Reis et al. (2015) in their study packaging films based on cassava starch with added yerba mate and mango pulp.
The films incorporating 1.2 g of BE and those with 0.8 and 1.2 g of MBE showed decreases (p < 0.05) in the Young’s modulus compared to the starch film. Arcan and Yemenicioğlu (2011) reported that the Young’s modulus of zein films reduced as the phenolic concentration in the films increased, and the films in our study with high concentrations of phenols and flavonoids showed the same effect. The plant extracts rich in polyphenols affect the properties of starch in different ways. For example, they either increased or decreased setback and cool paste viscosity. The effect appears to be dependent on the type of starch, chemical composition of the extract or the structure of the specific phenolic compound (Zhu, 2015).
In Conclusion, it is possible to develop starch films added with bioextract of O. oligacantha. The addition of bioextract to films resulted in higher antioxidant activity, being higher which were added with BME. Only the films with 0.8 and 1.2 of BE and MBE had antibacterial properties against S. typhimurium. The films with MBE had higher functional properties than films with added BE. The films with 0.4 and 0.8 of BE retained all the mechanical properties of starch film. Thus, the addition of BE or MBE is an option for the development of active films with functional properties.
References
Aila-Suárez S, Palma-Rodríguez HM, Rodríguez-Hernández AI, Hernández-Uribe JP, Bello-Pérez LA, Vargas-Torres A. Characterization of films made with chayote tuber and potato starches blending with cellulose nanoparticles. Carbohydr. Polym. 98: 102–107 (2013)
Arcan I, Yemenicioğlu A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 44: 550–556 (2011)
Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardisation d’un extrait de propolis et identification des principaux constituants Standardization of a propolis extract and identification of the main constituents. J. Pharm. Belg. 49: 462–468 (1994)
ASTM. Designation D 882-95a. Standard test method for tensile properties of thin plastic sheeting. In: Annual Book of ASTM Standards. American Society for Testing and Materials, Philadelphia, USA (1995)
Bajpai VK, Rahman A, Dung NT, Huh MK, Kang SC. In vitro inhibition of food spoilage and foodborne pathogenic bacteria by essential oil and leaf extracts of Magnolia liliflora Desr. J. Food Sci. 73: M314–M320 (2008)
Cao YH, Wang Y, Yuan Q. Analysis of flavonoids and phenolic acid in propolis by capillary electrophoresis. Chromatographia. 59: 135–140 (2004)
Corrales M, Han JH, Tauscher B. Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. Int. J. Food Sci. Technol. 44: 425–433 (2009)
Cortez-García RM, Ortiz-Moreno A, Zepeda-Vallejo LG, Necoechea-Mondragón H. Effects of cooking methods on phenolic compounds in Xoconostle (Opuntia joconostle). Plant Food Hum. Nutr. 70: 85–90 (2015)
Espinosa-Muñoz V, Roldán-cruz CA, Hernández-Fuentes AD, Quintero-Lira A, Almaraz-Buendía I, Campos-Montiel RG. Ultrasonic-assisted extraction of phenols, flavonoids, and biocompounds with inhibitory effect against Salmonella typhimurium and Staphylococcus aureus from Cactus Pear. J. Food Process. Eng. 40: e12358 (2017)
Fakhouri FM, Martelli SM, Caon T, Velasco JI, Mei LHI. Edible films and coatings based on starch/gelatin: film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 109: 57–64 (2015)
Gao P, Wang F, Gu F, Liang J, Li N, Ludescher RD. Preparation and characterization of zein thermo-modified starch films. Carbohydr. Polym. 157: 1254–1260 (2017)
Ghasemlou M, Aliheidari N, Fahmi R, Shojaee-Aliabadi S, Keshavarz B, Cran MJ, Khaksar R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 98: 1117–1126 (2013)
Hayek SA, Ibrahim SA. Antimicrobial activity of xoconostle pears (Opuntia matudae) against Escherichia coli O157: H7 in laboratory medium. Int. J. Microbiol. 2012: 1–6 (2012)
Hernández-Fuentes AD, Trapala-Islas A, Gallegos-Vásquez C, Campos-Montiel RG, Pinedo-Espinoza JM, Guzmán-Maldonado SH. Physicochemical variability and nutritional and functional characteristics of xoconostles (Opuntia spp.) accessions from Mexico. Fruits 70: 109–116 (2015)
IUPAC. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC technical report). Pure Appl. Chem. 74: 835–855 (2002)
Kıvrak Ş, Kıvrak İ, Karababa E. Analytical evaluation of phenolic compounds and minerals of Opuntia robusta J.C. Wendl. and Opuntia ficus-barbarica A. Berger. Int. J. Food Prop. 21: 229–241 (2018)
Kuskoski EM, Asuero AG, Troncoso AM, Mancini-Filho J, Fett R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 25: 726–732 (2005)
Luchese CL, Spada JC, Tessaro IC. Starch content affects physicochemical properties of corn and cassava starch-based films. Ind. Crops Prod. 109: 619–626 (2017)
Martinez ML, Curti MI, Roccia P, Llabot JM, Penci MC, Bodoira RM, Ribotta PD. Oxidative stability of walnut (Juglans regia L.) and chia (Salvia hispanica L.) oils microencapsulated by spray drying. Powder Technol. 270: 271–277 (2015)
Moradi M, Tajik H, Rohani SMR, Oromiehie AR, Malekinejad H, Aliakbarlu J, Hadian M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT Food Sci. Technol. 46: 477–484 (2012)
Morales P, Ramírez-Moreno E, de Cortes Sanchez-Mata M, Carvalho AM, Ferreira IC. Nutritional and antioxidant properties of pulp and seeds of two xoconostle cultivars (Opuntia joconostle FAC Weber ex Diguet and Opuntia matudae Scheinvar) of high consumption in Mexico. Food Res. Int. 46: 279–285 (2012)
Osorio-Esquivel O, Álvarez VB, Dorantes-Álvarez L, Giusti MM. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 44: 2160–2168 (2011)
Palma-Rodríguez HM, Alvarez-Ramírez J, Vargas-Torres A. Using modified starch/maltodextrin microparticles for enhancing the shelf life of ascorbic acid by the spray-drying method. Starch-Stärke 1700323 (2018)
Pérez-Alonso C, Campos-Montiel RG, Morales-Luna E, Reyes-Munguía A, Aguirre-Álvarez G, Pimentel-González DJ. Stabilization of phenolic compounds from Opuntia oligacantha Först by microencapsulation with agave SAP (aguamiel). Revista Mexicana de Ingeniería Química 14: 579–588 (2015)
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26: 1231–1237 (1999)
Reis LCB, de Souza CO, da Silva JBA, Martins AC, Nunes IL, Druzian JI. Active biocomposites of cassava starch: the effect of yerba mate extract and mango pulp as antioxidant additives on the properties and the stability of a packaged product. Food Bioprod. Process. 94: 382–391 (2015)
Saberi B, Chockchaisawasdee S, Golding JB, Scarlett CJ, Stathopoulos CE. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 104: 345–359 (2017)
Saura-Calixto F. Dietary fiber as a carrier of dietary antioxidants: an essential physiological function. J. Agric. Food Chem. 59: 43–49 (2010)
Seydim AC, Sarikus G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 39: 639–644 (2006)
Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Vol. 299, pp. 152–178. In: Oxidants and Antioxidants Part A. Packer L (ed). Academic press, San Diego, California, USA (1999)
Souza AC, Goto GEO, Mainardi JA, Coelho ACV, Tadini CC. Cassava starch composite films incorporated with cinnamon essential oil: antimicrobial activity, microstructure, mechanical and barrier properties. LWT Food Sci. Technol. 54: 346–352 (2013)
Steel RGD, Torrie JH. Principles and Procedures of Statistics: with Special Reference to the Biological Sciences. 2nd ed. McGraw-Hill Company, New York, USA. pp. 145–216 (1960)
Wang L, Dong Y, Men H, Tong J, Zhou J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 32: 35–41 (2013)
Zhu F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 43: 129–143 (2015)
Acknowledgements
This work was financially supported by CONACYT, Mexico. Basic Science, Code 183807 Project: “Nano-encapsulación de polifenoles de (Opuntia oligacantha) aplicados en biopelículas comestibles a partir de almidón obtenido de chayotextle (Sechium edule Sw.)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cenobio-Galindo, A., Pimentel-González, D.J., Del Razo-Rodríguez, O.E. et al. Antioxidant and antibacterial activities of a starch film with bioextracts microencapsulated from cactus fruits (Opuntia oligacantha). Food Sci Biotechnol 28, 1553–1561 (2019). https://doi.org/10.1007/s10068-019-00586-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-019-00586-9