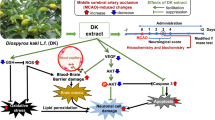
Abstract
To investigate the effects of Gastrodia elata Blume (GEB) and 4-hydroxybenzyl alcohol (HBA) on brain damage, GEB or HBA was administered orally for 14 days before middle cerebral artery occlusion (MCAO). After 24 h reperfusion, the proportion of circling was significantly reduced in the GEB (79%) or HBA (69%) group compared to the MCAO group (100%) in the corner test, and the removal time in the adhesive removal test was significantly decreased in the GEB (117 ± 21.0 s) and HBA (101 ± 20.9 s) groups compared to the MCAO group (161 ± 12.6 s). GEB treatment significantly reduced infarct volume compared to the MCAO group. In the GEB and HBA group, necrosis of nerve cells in hippocampus and cortex, expressions of TNF-α and TUNEL positive cells were significantly reduced compared to the MCAO group. These results suggest that GEB and HBA prevents brain damage by anti-inflammatory and anti-apoptotic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke causes cell death and long-term disability. Ischemic stroke is blockage of blood flow in some areas of the brain due to embolism or thrombosis (De Keyser et al., 1999; Dirnagl et al., 1999). When the blocked blood flow is restored, the oxygen increased to generate free radicals. The formation of free radicals plays an important role in the mechanism of cell damage (Korenkov et al., 2000).
The middle cerebral artery occlusion (MCAO) surgery is used by the method of described previously (Long et al., 1989). This technique does not require a neurosurgical procedure that removes some of the skull that can affect craniectomy, intracranial pressure and temperature. It has been the most frequently used method of imitating the permanent transient focal cerebral ischemia of the rat (Chiang et al., 2011).
Gastrodia elata Blume (GEB) is a traditional herb that has been used as an anticonvulsant, analgesic and sedative to treat paralysis, epilepsy, dizziness and tetanus in the Orient for centuries. Based on previous studies, 4-hydroxybenzyl alcohol (HBA) and vanillin are the major components of GEB (Kim et al., 2007; Liu and Mori, 1993) and GEB is capable of reducing lipid peroxide levels and has free radical scavenging activity (Taguchi et al., 1981). HBA facilitates memory consolidation and retrieval (Hsieh et al., 1997). HBA blocks oxidative stress and excitotoxicity through increased gamma-aminobutyric acid (GABA) transaminase (Kim et al., 2007). As a result, this effect of GEB was mainly due to the action of vanillin and HBA, which are major components.
The aim of this study was to verify whether pretreatment with GEB or HBA, reduces brain infarction related pathological changes in the brain and improves neurological outcome induced by MCAO.
Materials and methods
Preparation of Gastrodia elata Blume extracts
Aqueous extract of GEB was kindly provided by MJ Health Foods Co. (Muju-gun, Jeollabuk-do, Korea). Briefly, roots of GEB were dried by hot air-drying. Dried roots of GEB were cut and extracted with 10 times distilled water at 110 °C for over 20 h (samples:water = 1:10). After removal of the insoluble portion by filtration twice, with 25 µm and 5 µm diameter cartilage respectively, the extra solid component was removed and the filtrate was concentrated under vacuum and lyophilized until it was cooled. Then it was freeze-dried using a freeze dryer to prepare a powder. The yield of GEB was approximately 10.1%. The dried GEB was dissolved in distilled water for dosing and stored at − 20 °C for further use. HBA was purchased from sigma (H20806, St. Louis, MO, USA). The purity of HBA is higher than 99%. HBAs have proven effective in the above studies and are also active markers of GEBs, so in this study HBA have chosen as a positive control of GEB. The HBA content of the GEB extract was 2.4 mg/g, which was the basis for the HBA concentration used in this study.
Animals
Seven-week-old male Sprague–Dawley (SD) rats (Orientbio Co, Seongnam-si, Gyunggi-do, Korea), weighing 300–350 g, were housed in standard conditions (12-h light/day cycle with 22 ± 2 °C temperature and 50 ± 10% humidity) and had free access to commercial food (Purina Inc, Seongnam-si, Gyunggi-do, Korea) and water. Water intake, food intake, and weight were measured twice a week for 14 days. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Eulji University (EUIACUC 14-08).
Experimental design
After a 7-day adaptation period, rats were randomized into 4 groups: sham operating group, MCAO injury group, treatment with GEB (1 g/ml) plus MCAO injury group, and treatment with HBA (2.4 mg/ml) equivalent amount including of GEB plus MCAO injury group. The dose volume was 10 ml/kg body weight and the animals were dosed and examined daily between 9:00 and 10:00. In the present study, the GEB dose level was chosen because it is a maximal dose we can administered. HBA was dissolved and administered by gavage at the same time every day for 14 days (n = 10–12 per each group). After the last administration, brain infarction by MCAO was induced in all the treated rats. The dose level of GEB was used the extract stock solution as the highest concentration. The dose level of HBA was determined by concentration of HBA in GEB extract (Table 1).
Ischemic surgery
The middle part of the neck was cut to expose the common carotid artery. The left common carotid artery (CCA) and external carotid artery (ECA) were exposed. The ECA and CCA were ligated permanently and the ICA was temporarily occluded by suture thread. The 4-0 nylon thread, with a rounded tip and silicon rubber cylinder was used. The thread silk inside the ICA was blocked blood flow. The suture thread on the CCA was removed and the incision closed. Reperfusion was conducted by removing the nylon thread from the ICA after 1 h ischemia. The isoflurane was used for respiratory anesthesia and it took about 20 min to close the MCA. Body temperature was maintained continuously at 37.0 ± 0.5 °C using an electrical blanket and heating lamp throughout the experiment (Kam et al., 2011). The animals undergoing MCAO surgery exhibited eating disorders, walking difficulties, and one side circling. In severe cases, the animal death also occurred. The animals that died after MCAO surgery were not included in the study.
Behavior function assessment
In the corner test, a rat was placed between two 40 cm × 40 cm × 1 cm boards. The board edge is connected to a small hole between the boards at a 30° angle to guide the corner. The rat was placed between the two plates facing the edge and the midpoint of the edge. When the rat reached the corner, both sides of the body were stimulated at the same time. Then the animals were usually lifted to the right or to the left. Each animal was tested in 10 tests and the face selected for rotation was recorded. In the case of ventral turning (i.e., when the animal turned without rearing), the trial was repeated at the end of the session (Ban et al., 2012). The laterality index was calculated according to the formula: laterality index = (number of left turns − number of right turns)/total number of turns (Choi et al., 2010).
In the adhesive removal test (ART), the time taken to remove the sticky tape on the left foot was measured (Schallert et al., 1982; Zhang et al., 2002; Zhang et al., 2010). The square points of the adhesive paper were used as bilateral tactile stimuli occupying the distal-radial region in the wrist of each forelimb. The animals were given three trials with a cut-off time of 180 s (Park et al., 2010). Some animals did not detach the tape completely. We also determined whether they could detach the tape in 90 s.
2,3,5-Triphenyltetrazolium chloride (TTC) staining
The animals in each group were sacrificed 24 h after MCAO and after sacrifice, the brains were removed immediately and placed on a metal brain matrix for tissue slicing. For the detection of the infarction area of the brain, six slices were made at 4, 6, 8, 9 and 11 mm posterior to the olfactory nerve. Each slices were incubated with 2% TTC solution at 37 °C for 30 m and fixed with 10% formalin. The stained slices were photographed with a digital camera (Nikon, Tokyo, Japan), and subsequently, the surface area of the slice and ischemic lesion was measured using Image J software (National Institute of Health, Bethesda, Maryland, USA). The ischemic lesion volumes were calculated as the sum of the areas of the ischemic lesion across the six slices divided by the total cross-sectional area of the six brain slices (Choi et al., 2010).
Histological analysis and immunohistochemistry
After TTC staining, the brains were post-fixed in 10% phosphate-buffered formalin and embedded with paraffin. The brain sections (4 μm) were stained by hematoxylin and eosin. For immunohistochemistry (IHC), sections (4 μm) were mounted on silane-coated slide glasses (Matsunami, Japan). After deparaffinization, the slide were incubated in 1 × PBS containing 0.1% trypsin 20 m. And the slides were incubated in 1% H2O2 in methanol for 30 m at room temperature. The Slides were blocked by normal goat serum (NGS) for 1 h at room temperature. The primary antibody TNF-α (diluted 1:200, Abcam Inc., Cambridge, MA, USA) was incubated overnight at 4 °C for 16 h. After incubation, slides were incubated with biotinylated goat anti-rabbit IgG and avidin–biotin complex (Vector Laboratories, Burlingame, CA, USA) for 30 m at room temperature. The TNF-α was visualized using 3,3-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO, USA).
TUNEL assay
A terminal deoxynucleotidyl transferasemediated d-UTP-biotin nick end (TUNEL) assay was used to assess DNA damage. Apoptosis was detected by the TUNEL assay by means of the in situ cell death detection kit (Roche, Indianapolis, USA).
Statistical analyses
The significance of the differences in mean values among the experimental groups was determined using one-way analysis of variance (ANOVA), followed by Tukey’s multiple-comparison test. The level of statistical significance was set at p < 0.05. SPSS for Windows (version 10.0; SPSS Inc., USA) was used to calculate probability values. All the results in this study were expressed as mean ± standard error (SE).
Results and discussion
General observation
After MCAO surgery, the rats showed abnormality of one eye of the operation side and weak resistance when the forelegs bend and push to the side. Most of rats rotated only in one direction and did not move in severe cases as reported (Hatfield et al., 1991; Richard et al., 1998). The pretreatment of GEB and HBA decreased the number of animals showing those symptoms and dead animals when compared to the MCAO group.
There are many methods making acute ischemia injury on the brain. The MCAO model for inducing ischemia injury was used the present study. The Red ginseng extract protects nerve cells from ischemic brain injury induced by MCAO, which may be due to an increase in endogenous antioxidant defense enzymes as well as inhibition of neurological defects and lipid peroxidation (Ban et al., 2012). This study also showed similar effects. There were many experiments which changed the occlusion time as 30, 60, and 120 m (Popp et al., 2009). In this study, occlusion of the artery was used for 60 m, because occlusion time of 30 m was too short to confirm the ischemic region in TTC stain, while 120 m was considered too long, as many animal models might be die. According to the reports of Yu et al. (2005), GEB extract also has preventive effects which are comparable to the results of this study.
Organ weight and body weight loss changes
The weights of liver, kidney and brain were measured. These were expressed as absolute (g) and relative weight (g/100 g body weight). No alterations were shown in absolute and relative weights of liver, kidney and brain. In the MCAO group, the relative weight of the brain increases compared to the sham group. It seems to be due to cerebral edema after MCAO surgery. In other study, the brain edema increased with reperfusion time (3, 24, 48 h) (Wang et al., 2014). It seems that MCAO causes damage and edema of brain cells, which increases the relative weight of the brain.
There were significant weight changes in the MCAO group, GEB group and HBA group after MCAO surgery compared to the sham group. There were significant weight loss changes in the GEB group, when compared to the MCAO group [Fig. 1]. The Body weight is one of the indicators that can confirm the ideal of the body. Also, the weight loss is one of the symptoms that confirms the operation after MCAO surgery (Wang et al., 2014). There was a significant decrease in the GEB group and a decrease in HBA group in this study. The administration of GEB appears to have protective effects on MCAO, including HBA and other beneficial components such as 4-hydroxybenzyl aldehyde (HBAL) and 4-hydroxy-3-methoxybenzaldehyde (vanillin). Low weight loss means that there was activity. It was related to the damage of the brain neurons, which means that the neurons are not damaged.
Body weight loss after MCAO surgery when compared to the body weight of animals weighed before surgery. The body weight loss of MCAO group was markedly increased when compared to the sham groups. GEB and HBA group treated for 14 days before MCAO reduced body weight loss. Data are expressed as mean ± SE. #Versus sham, *versus MCAO (p < 0.05)
Behavior function assessment
In the corner test, the rats after MCAO showed circling of right direction and the numbers of circling rats were significantly increased in the MCAO group, compared to the sham group. The proportion of circling rats in the sham operation group was 23%, and the MCAO group was 100%. The proportion decreased to 79% and 69% in the GEB and HBA group, respectively [Fig. 2(A)].
Effects of GEB or HBA on behavior test. The pretreatment of GEB of HBA reduced the number of animals circling to the right compared MCAO group to GEB and HBA group on corner test (A). There was a significant reduction of removal time in HBA group compared to MCAO on adhesive removal test (B). The percentage of successful rats increased in the GEB or HBA treated groups compared to the MCAO (C). Data are expressed as mean ± SE. #Versus sham group, *versus MCAO group (p < 0.05)
In this study, behavior changes induced by MCAO surgery were alleviated in the GEB or HBA group. In the corner test, the rat tail was stimulated to move the rat and then cornering was counted. The rat rearing was excluded and was counted to be perfect turning to in any direction. Choi et al. (2010) reported no significant differences among the group at day one, although in this study, there were significant changes at day one. In this study, the occlusion time was 60 m and pretreatment of GEB was administered for 2 weeks before MCAO surgery, while in the study by Choi et al. occlusion time was 120 m and GEB was administered once after MCAO. In Popp et al. (2009) studies where ischemia time and perfusion time are different, the longer ischemia time, the greater damage site of the cell. Because of differences in ischemia time and duration of drug administration, this study showed significant differences after 24 h of surgery.
The ART was employed to measure somatosensory deficits [Fig. 2(B)]. The time of MCAO group (161 s) significantly increased compared to sham group (23 s). Compared to the MCAO group, the removal time in the GEB group (117 s) and HBA group (101 s) was significantly decreased. The percentage of rats completing tape detachment in 90 s was measured. The number of animals detaching the tape in 90 s more decreased in the GEB and HBA group than in the MCAO group [Fig. 2(C)].
Park et al. (2010) reported significant changes in ART on day one. In this study, there were also significant changes and the time of detaching was much longer than their result. The reason for these differences may be related to the drug and MCAO nylon suture. This study used a single substance. Park et al. used 3–0 monofilament nylon suture, while 4–0 monofilament nylon suture used in this study. The other studies have also shown that the hardness of tissue damage varies with the thread used for MCAO (Duan et al., 2015). The thickness of MCAO suture was considered an important factor, and thick nylon used in this study may cause more severe damage to the brain. Behavioral tests are related to motor neurons and sensory nerves in the brain. They exist in the cortex and hippocampus in brain. The damage of these neurons was reduced by the administration of GEB and HBA and the results of the behavioral experiments were observed.
GEB extracts or HBA on brain infarction
After sacrifice, the rat brain promptly removed from the skull. Normal (undamaged) brain was gray and exhibited elasticity. The ischemia-induced brain by MCAO changed from pink to white in color and exhibited a reduction in elasticity [Fig. 3(A)].
TTC-stained coronal sections from ischemic rat brain and calculated infarct volumes. Non-ischemic region is red, and infarct region appears as white color (A). The pretreatment of GEB or HBA significantly reduced infarct volume when compared to MCAO group. The infarct volume was calculated as the infarct area × thickness (2 mm) and expressed as a percentage of the lesion of the brain (B). There is significant decreasing in GEB group compared to MCAO group. Infarct volumes were decreased in the HBA group compare to MCAO group. Data are expressed as mean ± SE. #Versus sham group, *versus MCAO group (p < 0.05). (Color figure online)
After TTC staining, the slides were pictured by digital camera [Fig. 3(A)]. The infarct area was located in the cerebral artery and turned white by TTC staining. The boundary of TTC staining was well defined and surrounded the white infarct area unlike the normal red area. There was a significant difference in infarct size between the MCAO group and pretreated groups [Fig. 3(B)]. The greatest total infarct size was 20.7% in the MCAO group. In the GEB and HBA groups, the area of total brain infarction was decreased (15.4%, 16.9% respectively).
In the TTC test, TTC was used to distinguish between metabolically active and inactive tissues. Due to the activity of the dehydrogenase, it appears red in the biological tissue but remains unreacted at the denatured or denatured necrotic area. In this study, TTC staining revealed that a significant increase in infarct size in the MCAO group. When the brain was sliced, the piece of the third and fourth slices showed the highest injury in the brain. The area sustaining the greatest injury corresponded to the middle cerebral artery. In this study, data were similar to those reported by Zhao et al. (2008). To assess the TTC staining results, the rats sacrificed at 24 h of reperfusion after MCAO as the optimal time for TTC staining is between 24 and 36 h of reperfusion after MCAO. The decrease in the infarcted area means that the cell damage was also reduced, which may be thought to be the role of the neuron. The administration of GEB and HBA reduced the infarct area, which seems to have improved behavioral tests as well as the number of neurons that play a role. There was also a further decrease in GEB administration, which seems to be a combined effect with other components, in addition to the main component, HBA.
Histological finding
The brain of the MCAO group showed neuron and cell necrosis. Particularly in the hippocampus and cerebral cortex [Fig. 4], most of the neuron cells disappeared, and only astrocytes were detected in the MCAO group. There was interstitial edema in the striatum of the brain. There were variable changes in surviving neurons. The most changed cells were pyramid cells in the brain cortex showing cytoplasm shrinkage, decrease in the number of dendrites, shortage of the length and loss of the Nissl body. There was also loss of chromatin in nuclear and variable nuclear size. All these changes decreased in the GEB and HBA groups compared to the MCAO group.
Hematoxylin and eosin stain of the hippocampus and cortex. Pyknotic neurons with enlarged space were observed in the hippocampus and cortex. The pretreatment of GEB or HBA preserve the normal structure of the neuron. In GEB or HBA groups reduced necrotic changes in the neurons significantly decreased, compared to the MCAO group. Sham group (A, E), MCAO group (B, F), GEB group (C, G), HBA group (D, H). HE
The pretreatment of GEB or HBA reduced necrotic changes of neurons significantly, compared to the MCAO cortex. Kim et al. (2007) also reported that HBA, which is an active component of GEB, decreased cell death in the hippocampus. These findings were comparable to those of this study. The necrosis of the cerebral cortex can be improved by the pretreatment of GEB and HBA. After MCAO, cell damage occurred in the striatum and the cortex, but the cell damage significantly reduced in the pretreatment of GEB and HBA (Duan et al., 2015). There are two broad categories in cell death: the necrosis of ischemic core was early phase and delayed phase of cell death in near by neuron, the so-called penumbra (Butler et al., 2002; Zhao et al., 2008). The penumbra exists throughout the cortex of the brain. When the blood is reperfused, the area of greater damage is the penumbra. In this study, the necrosis of penumbra decreased in GEB and HBA groups.
In IHC and TUNEL assay, TNF-α and TUNEL positive cells were expressed in the MCAO group, which were rare in sham animals. TNF-α and TUNEL expression significantly reduced in the GEB or HBA groups compared to MCAO group [Fig. 5].
Immunohistochemistry of TNF-α and TUNEL assay on MCAO brain. TNF-α positive cells (arrow) were increased in the MCAO group, and were rare in sham rats. GEB or HBA rats had a significantly reduced number of TNF-α positive cells compared to the MCAO group. TUNEL positive cells in the MCAO group were more expressed than sham group, and there were significantly decreased in GEB an HBA group. Sham group (A, E), MCAO group (B, F), GEB group (C, G), HBA group (D, H)
IHC and TUNEL assay also demonstrated the effects of GEB extract. TNF-α and TUNEL levels were significantly expressed in the MCAO group. When blood flow is restored, oxygen can generate oxidative stress. Oxidative stress can cause an inflammatory response after an ischemic stroke, which is characterized by increased pro-inflammatory cytokine production. Stimulation by sustained oxidative stress may also induce apoptosis. Of the known cytokines, TNF-α is produced by macrophages, endothelial cells, astrocytes and neurons and plays a crucial role in ischemic brain damage. Reduction of oxidative stress by administration of GEB and HBA may result in the suppressed production of TNF-α as demonstrated by this study (Lipton, 1999) and decreased TUNEL positive cells. The decrease in TNF-α resulted in a decrease in inflammation following GEB and HBA administration. Reduction of inflammation means reduction of cell damage, which can be identified by reduction of necrosis and decreased infarction in TTC stain.
In order to investigate the preventive effect of GEB without any additives, the undiluted solution was used. HBA was used as an analytical marker of GEB and have preventive effects on brain damage in previous study. Also, the concentration of HBA was set to the same amount as the amount of HBA in GEB extraction. GEB contains a number of ingredients. The vanillin, HBA and HBAL are well known components of GEB (Kim et al., 2007). They are also known as phenolic compounds. Vanillin and HBA have been reported to potentially scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, superoxides and hydroxyl radicals (Liu and Mori, 1993). HBAL, an analogue of HBA, exhibited an inhibitory effect on the GABA transaminase and functioned as an anticonvulsant (Liu et al., 2011). Several studies have suggested that HBAs have antioxidant and free radical scavenging activity (Ha et al., 2000; Liu and Mori, 1993). The pretreatment of HBA attenuates drug-induced learning deficits in a passive avoidance task, by suppressing dopaminergic and serotonergic function (Jung et al., 2007). Therefore, HBA was considered as an important ingredient in GEB. In the present study, pretreatment of HBA significantly decreased the score on the behavior test compared to the MCAO group. HBA was a good ingredient preventing infarct volume and cell necrosis. The effects of GEB were consistent with the effects of the major components such as HBA and were considered to the effects of phenolic compounds. The concentration of HBA of the GEB extracts used in this study was 2.4 mg/ml and the dose volume was 10 ml/kg which means 24 mg/kg of HBA when dosing the extract of GEB. According to the reports of Kam et al. (2011) and Yu et al. (2005), the 25 mg/kg of HBA prevented brain ischemic injury and behavioral impairment. The concentration they have used was similar the concentration of HBA used in this study. When GEB was diluted twofold and administered orally for 2 weeks, the weight loss, behavioral tests, brain infarction and histological findings were decreased when compared to MCAO group, but these results did not show significant results (data not shown). Perhaps due to the dilution of GEB extracts, phenolic compound including HBA, which is a major component of GEB, has also been diluted and seems to have less beneficial effects of GEB.
In conclusion, The MCAO model used in this study demonstrated the usefulness of the test system in rats as a screening tool for the detection of ischemic brain damages. The parameters used in this experiment (body weight loss, behavior test, TTC stain, HE stain and IHC) indicated that GEB and HBA had preventive effects on the damaged brain induced by MCAO surgery. Further studies are required to identify the mechanism underlying GEB or HBA treatment. Several study and present study have shown that GEB is effective in preventing stroke. Also, it was confirmed that GEB had a preventive effect in liver and kidney. Because of these effects, GEB can play a role as a health functional food.
References
Ban JY, Kang SW, Lee JS, Chung JH, Ko YG, Choi HS. Korean red ginseng protects against neuronal damage induced by transient focal ischemia in rats. Exp. Ther. Med. 3: 693–698 (2012)
Butler TL, Kassed CA, Sanberg PR, Willing AE, Pennypacker KR. Neurodegeneration in the rat hippocampus and striatum after middle cerebral artery occlusion. Brain Res. 929: 252–260 (2002)
Chiang T, Messing RO, Chou WH. Mouse model of middle cerebral artery occlusion. J. Vis. Exp. (48): 2761 (2011)
Choi HS, Ko YG, Lee js, Kwon OY, Kim SK, Cheong C, Jang KH, Kang SA. Neuroprotective effects of consuming bovine colostrum after focal brain ischemia/reperfusion injury in rat model. Nutr. Res. Pract. 4: 196–202 (2010)
De Keyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing. Trends Neurosci. 22: 535–540 (1999)
Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22: 391–397 (1999)
Duan X, Wang W, Liu X, Yan H, Dai R, Lin Q. Neuroprotective effect of ethyl acetate extract from Gastrodia elata against transient focal cerebral ischemia in rats induced by middle cerebral artery occlusion. J. Tradit. Chin. Med. 35: 671–678 (2015)
Ha JH, Lee JT, Kim JS, Yong CS, Kim JA, Ha JS, Huh K. 4-Hydroxybenzaldehyde from Gastrodia elata Blume is active in the antioxidation and GABAergic neuromodulation of the rat brain. J. Ethnopharmacol. 73: 329–333 (2000)
Hatfield RH, Mendelow AD, Perry RH, Alvarez LM, Modha P. Triphenyltetrazolium chloride (TTC) as a marker for ischemic changes in rat brain following permanent middle cerebral artery occlusion. Neuropathol. Appl. Neurobiol. 17: 61–67 (1991)
Hsieh MT, Wu CR, Chen CF. Gastrodin and p-hydroxybenzyl alcohol facilitate memory consolidation and retrieval, but not acquisition, on the passive avoidance task in rats. J. Ethnopharmacol. 56: 45–54 (1997)
Jung TY, Suh SI, Lee H, Kim IS, Kim HJ, Yoo HS, Lee SR. Protective effects of several components of Gastrodia elata on lipid peroxidation in gerbil brain homogenates. Phytother. Res. 21: 960–964 (2007)
Kam KY, Yu SJ, Jeong NH, Hong JH, Angela M, Lee S, Choi YW, Lee CK, Kang SG. p-Hydroxybenzyl alcohol prevents brain injury and behavioral impairment by activating Nrf2, PDI, and neurotrophic factor genes in a rat model of brain ischemia. Mol. Cells 31: 209–215 (2011)
Kim HJ, Hwang IK, Won MH. Vanillin, 4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent hippocampal CA1 cell death following global ischemia. Brain Res. 1181: 130–141 (2007)
Korenkov AI, Pahnke J, Frei K, Warzok R, Schroeder HW, Frick R, Muljana L, Piek J, Yonekawa Y, Gaab MR. Treatment with nimodipine or mannitol reduces programmed cell death and infarct size following focal cerebral ischemia. Neurosurg. Rev. 23: 145–150 (2000)
Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 79: 1431–1568 (1999)
Liu J, Mori A. Antioxidant and pro-oxidant activities of p-hydroxybenzyl alcohol and vanillin: effects on free radicals, brain peroxidation and degradation of benzoate, deoxyribose, amino acids and DNA. Neuropharmacology 32: 659–669 (1993)
Liu Y, Liu W, Sun X, Li R, Sun Q, Cai J, Kang Z, Lv S, Zhang JH, Zhang W. Hydrogen saline offers neuroprotection by reducing oxidative stress in a focal cerebral ischemia. Med. Gas Res. 1: 15 (2011)
Long EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral cerebral artery occlusion without craniectomy in rat. Stroke 20: 84–91 (1989)
Park SI, Jang DK, Han YM, Sunwoo YY, Park MS, Chung YA, Maeng LS, Im R, Kim MW, Jeun SS, Jang KS. Effect of combination therapy with sodium ozagrel and panax ginseng on transient cerebral ischemia model in rats. J. Biomed. Biotechnol. 2010: 893401 (2010)
Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS ONE 4: e4764 (2009)
Richard CG, Gerz CM, Kasberben BH. Rat middle cerebral artery occlusion by an intraluminal thread compromise collateral blood flow. Brain Res. 791: 223–228 (1998)
Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, Vaughn D, Wilcox RE. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol. Biochem. Behav. 16: 455–462 (1982)
Taguchi H, Yoshioka I, Yamasak K, Kim IH. Studies on the constituents of Gastrodia elata Blume. Chem. Pharm. Bull. 29: 55–62 (1981)
Wang G, Huang H, He Y, Ruan L, Huang J. Bumetanide protects focal cerebral ischemia-reperfusion injury in rat. Int. J. Clin. Exp. Pathol. 7: 1487–1494 (2014)
Yu SJ, Kim JR, Lee CK, Han JE, Lee JH, Kim HS, Hong JH, Kang SG. Gastrodia elata blume and an active component, p-hydroxybenzyl alcohol reduce focal ischemic brain injury through antioxidant related gene expressions. Biol. Pharm. Bull. 28: 1016–1020 (2005)
Zhang C, Chopp M, Cui Y, Wang L, Zhang R, Zhang L, Lu M, Szalad A, Doppler E, Hitzl M, Zhang ZG. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J. Neurosci. Res. 88: 3275–3281 (2010)
Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Zhang L, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke 33: 2675–2680 (2002)
Zhao H, Mayhan WG, Sun H. A modified suture technique produces consistent cerebral infarction in rats. Brain Res. 1246: 158–166 (2008)
Acknowledgements
This study was supported by Brain Korea 21 Plus (No. 31Z20130012916), Jeonju University Industry-Academia Collaboration Foundation (Shin, 2015) and Eulji Univeristy (Shin, 2018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seok, P.R., Oh, S.J., Choi, J.W. et al. The protective effects of Gastrodia elata Blume extracts on middle cerebral artery occlusion in rats. Food Sci Biotechnol 28, 857–864 (2019). https://doi.org/10.1007/s10068-018-0516-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0516-9