Abstract
Guhong injection (GHI), composed of aceglutamide and safflower aqueous extract, has been used clinically for the treatment of cerebrovascular diseases such as cerebral embolism, hemorrhage and mental deterioration. In this paper, we reported the results of the first study on the anti-inflammatory effects of GHI in murine focal cerebral ischemia–reperfusion (I/R) injury. Adult male SD rats were randomly divided into six groups: Sham group, I/R group, GHI-L group (2.5 mL/kg), GHI-M group (5 mL/kg), GHI-H group (10 mL/kg) and Nimodipine group. I/R injury was induced by middle cerebral artery occlusion (MCAO) for 1.5 h followed by reperfusion for 24 h. Compared with I/R group, rats treated with GHI showed dose dependent reductions in neurological defect scores and cerebral infarct volume. GHI obviously down-regulated nitric oxide (NO), inducible NO synthase (iNOS), myeloperoxidase (MPO), interleukin-1β (IL-1β), TNF-α (tumor necrosis factor-α) and C reactive protein (CRP) levels in serum. Moreover, histological examination by H&E staining showed that clear cell outline, less vacuolated spaces and largely surviving neurons were observed in GHI-treated rats. The immunohistochemical staining revealed that GHI administration significantly diminished the positive expressions of intercellular cell adhesion molecule-1 (ICAM-1) and nuclear factor-κB p65 (NF-κB p65) in brain tissues. Western blot analysis for ICAM, NF-κB p65 and iNOS further solidified the above findings. All these results demonstrate that GHI exerts a strong and ameliorative effect on cerebral I/R injury in rats possibly through the inhibition of inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke, accounts for 88 % of all strokes (Van der Worp and Van Gijn 2007), is a major cause of death, disability, morbidity and mortality in the world (Elkind 2009; Sun et al. 2010a). At present, recombinant tissue plasminogen activator (rtPA), the only FDA-approved stroke medication (Young et al. 2007), is a early thrombolytic therapy limited to patients within 4 ~ 5 h of stroke onset (Garcia-Bonilla and Iadecola 2012). But later reperfusion after cerebral ischemia causes more deleterious brain damage than cerebral occlusion itself (Yang and Betz 1994). As a major and crucial pathophysiological mechanism following focal cerebral I/R, the excessive activation of inflammation could lead to more serious damage in stroke (Lambertsen et al. 2012; Ouyang 2013). The cerebral inflammatory reaction occurs via an amplification cascade and is characterized by leukocyte infiltration, microglial cells and macrophages activation, and upregulated expression of mediators such as chemokines and cytokines (Morganti-Kossmann et al. 2001). Alleviating inflammatory injury plays a vital role in neuroprotection during the early stages after focal cerebral I/R (Qin et al. 2013). Many studies have demonstrated that inhibiting inflammatory injury during reperfusion could bring better therapeutic results (Barone and Parsons 2000; Gao et al. 2013), therefore anti-inflammatory treatment has good prospects in alleviating cerebral I/R injury.
Recently, study on western medicine has started from a single drug model to the multi-targets (combination drugs) model and drug combination therapy gradually becomes a trend for new drugs development. Polypharmacology has been used to the study of drug discovery (Liu et al. 2013) and pharmacological mechanisms (Zhang et al. 2011) in China. Guhong injection (GHI) is a novel compound preparation consisted of aceglutamide and safflower extract. It combines the features of western medicine and traditional Chinese medicine and possesses efficacies of anticoagulant, antithrombotic, relaxing blood vessels, improving microcirculation and antioxygen free radicals, etc. (Sun and Wei 2012; Zhang and Ning 2015). Aceglutamide (Fig. 1) is a glutamine acetylated derivative. With very similar pharmacological action to glutamine (Zhou and Wu 2005), it can significantly improve the clinical prognosis of patients with stroke (Wasa et al. 2005; Wang 2011). Safflower (Carthamus tinctorius L., dried florets) has been long used in traditional medicine as a remedy for stroke and its main ingredient hydroxysafflor yellow A (HYSA, Fig. 1) has good anti- inflammatory effects on cerebral I/R injury (Ye and Gao 2008; Sun et al. 2010b). On the basis of previous researches, GHI has good clinical manifestations on stroke (Zhao et al. 2006; Wang and Wu 2013) and Jiang et al. proved that GHI could reduce the serum levels of inflammation factors IL-6 and TNF-α in patients with acute cerebral infarction (Jiang et al. 2007). However, how GHI modulates celluar and molecular inflammatory reactions is still unclear even though it has good clinical application. The functional targets and exact treatment mechanism of GHI for inflammatory injury after cerebral I/R need to be elucidated.
In our previous work, we confirmed the protective effect of GHI on focal cerebral I/R injury in SD rats induced by middle cerebral artery occlusion (MCAO) for 1.5 h followed by reperfusion for 24 h (Shu et al. 2014). In this paper, we particularly explored whether GHI could inhibit the secretion of inflammation related factors in the early stage of focal cerebral I/R to alleviate cerebral I/R injury, that might be one of the mechanisms of GHI therapy for focal cerebral I/R injury. Nimodipine, which exhibited anti-inflammatory activity through the control of cytokine levels in cerebral injury (Babu and Ramanathan 2011; Justin et al. 2014; Zhang et al. 2012), was chosen as a positive control drug.
Materials and methods
Preparation of GHI
GHI, manufactured by Tonghua Guhong Pharmaceutical Co., Ltd. with country medicine accurate character H22026582, is a sterile solution of aceglutamide and safflower aqueous extract. Safflower was collected from Jimusaer county (Xinjiang Province, China), and was authenticated by Prof. Luqi Huang, a pharmacognosist worked at Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, where the voucher specimen was deposited. DHI was prepared according to the Chinese national standard [No. WS-10001-(HD-1506) -2004] promulgated by Chinese Pharmacopoeia Commission and the process was described as follows. Safflower (500 g) was subjected to boiling water extraction for three times (60, 50 and 30 min, respectively) and the aquous extraction was vacuum evaporated to the relative density of 1.16 ~ 1.26 (50 ~ 60 °C). Ethanol was added to make the physic liquor containing 70 % ethanol and filtered after 48 h, then the filtrate was concentrated to the relative density of 1.10 ~ 1.14 (50 ~ 60 °C). Ethanol was added again to make its content up to 80 %. After the filtrate was condensed to the relative density of 1.16 ~ 1.20 (50 ~ 60 °C), 10-fold water was added and the mixture was stored at 4 °C for 16 ~ 24 h, and the filtrate was consequently vacuum distilled to the relative density of 1.02 ~ 1.04 (50 ~ 60 °C). The purified safflower aqueous solution (about 150 mL) was blended with aceglutamide (30 g) dissolved in 10 % sodium hydroxide solution. Finally, the mixed liquor was adjusted the pH value 5.0 ~ 7.0, then added water for injection into 1000 mL, sterilized and encapsulated into ampoules.
According to the corresponding quality control standard, the content of aceglutamide (molecular formula: C7H12N2O4) should be in the range of 27–33 mg/mL and the content of HYSA (molecular formula: C27H32O16) should not be lower than 0.15 mg/mL when detected by HPLC.
Establishment of GHI fingerprint
The establishment of GHI fingerprint was carried out using an Agilent 1260 Infinity HPLC system consisted of a G1322A on-line degasser, a G1311C quarternary pump, a G1316A column oven, a G1329B autosampler and a G1314F variable wavelength detector. An Alltima™ C18 column (250 mm × 4.6 mm, 5 µm) was used with mobile phase consisted of 0.1 % formic acid aqueous solution (A) and acetonitrile (B). The GHI samples were eluted with a linear gradient: 0–5 min, 2 %; 5–10 min, 2–6 % B; 10–12 min, 6–7 % B; 12–20 min, 7–10 % B; 20–26 min, 10–11 % B; 26–34 min, 11–12 % B; 34–47 min, 12–20 % B; 47–50 min, 20–22 % B; 50–55 min, 22–25 % B; 55–60 min, 25–30 % B; 60–65 min, 30–36 % B; 65–75 min, 36–42 % B. The flow rate was 0.9 mL/min and the column temperature was kept at 30 °C. The UV detection wavelength was first set at 239 nm from 0 to 7 min, and at 260 nm from 7 to 36 min, then switched to 280 nm after 36 min. HPLC Fingerprint of DHI was shown in Fig. 2, and seven effective components were identified by HPLC and comparison with reference substances. These representative ingredients included aceglutamide, uridine, adenosine, guanosine, syringing, hydroxysafflower yellow A and anhydrosafflor yellow B, and their simultaneous quantification were achieved in our research (He et al. 2015).
Experimental animals
Adult male Sprague–Dawley rats (body weight, 260 ~ 300 g) were obtained from Animal Central of Zhejiang Chinese Medical University, Hangzhou, China (Laboratory animal certificate: scxk 2013–0016). All animals were cared for according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (National Research Council of the National Academies, 2011). The rats were fed standard rodent chow and water ad libitum in a house with a 12 h light/dark cycle (temperature 22 ~ 24 °C and humidity 50 ~ 60 %) for 10 days to adapt to the experimental environment. Formal approval to conduct the experiments was obtained from the Animal Subjects Review Board of Zhejiang Chinese Medical University.
Reagents and chemicals
GHI was provided by Tonghua Guhong Pharmaceutical Co., Ltd (Jilin, China). Nimodipine injection was manufactured by Bayer Schering AG (Leverkusen, Germany). 2,3,5-triphenyltetrazolium chloride (TTC) was produced by Beijing Dingguo Changsheng biotech Co., Ltd (Beijing, China). The kits for biochemical analysis, including NOS, MPO and NO were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Rat IL-1β, TNF-α and CRP ELISA kits were bought from Shanghai Xinfan Biotechnology Co., Ltd (Shanghai, China). NF-κB p65 (Santa Cruz SC-372, USA) and β-actin (1:1000 dilution, Santa Cruz SC-130656, USA) were rabbit polyclonal antibodies. ICAM-1 (Clone:1A29, BD Pharmingen™ NO:554967, USA) and iNOS (Abcam ab49999, USA) were mouse monoclonal antibodies.
Induction of focal cerebral I/R injury
All rats were anesthetized by intraperitoneal (i.p.) injection of chloral hydrate (10 %, 3.5 mL/kg) after weighing. Focal cerebral ischemia was induced according to the Longa’s method (Longa et al. 1989). The skin of neck was cut to expose the right common artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA), respectively. The CCA and ECA were ligated and the nylon wire with a rounded tip (diameter of 0.28 mm, Beijing Sunbio Biotech Co., Ltd. Beijing, China) was carefully inserted into the ICA until the cerebral middle artery (18 ± 2 mm). After 90 min of MCAO, the nylon filament was removed to achieve a reperfusion. The rats of sham group were performed using the same surgical exposure procedures except inserting the nylon suture. A successful model was judged when the rats awoke demonstrating right eye Horner syndrome, left foreleg buckling when lifting the tail, climbing in a circle to the left. Rats that showed excessive bleeding, subarachnoid hemorrhage and early death were excluded. Room temperature was maintained at 37 ± 0.5 °C throughout the whole surgery. Focal cerebral I/R injury in rats were induced by MCAO for 1.5 h followed by 24 h reperfusion.
Experimental design
All rats were randomly divided into six kinds of groups (n = 6 per group): sham group, I/R group, GHI-L group (GHI 2.5 mL/kg), GHI-M group (GHI 5 mL/kg), GHI-H group (GHI 10 mL/kg) and nimodipine group (10 mL/kg). The dosages of GHI for rat were 10, 20, 40 times the dose for human being of 0.25 mL/kg, respectively. GHI and nimodipine groups were i.p. administered at 0, 6, 23 h after reperfusion, respectively. Sham-operated and I/R groups were given physiological saline (10 mL/kg) undergoing the same procedures.
Evaluation of neurological deficits
The neurological deficits were evaluated as Longa’s method (Longa et al. 1989) by an observer blinded to all groups after 24 h of reperfusion on a five-point scale: rats with no neurologic deficit scored 0, left forepaws of rats with flexion, adduction and failure to extend fully scored 1, rats with circling to the left when crawling scored 2, rats with falling to the left when standing scored 3, rats with not walking spontaneously and had a depressed level of consciousness scored 4.
Measurement of cerebral infarct volume
The brain samples were collected at 24 h after reperfusion and frozen at −20 °C for 15 min. Frozen brains were sliced into 2 mm coronal slices starting 1 mm from the frontal pole and immediately stained with 2 % TTC solution at 37 °C for 30 min and then fixed in 4 % paraformaldehyde overnight before analysis. Brain slices were scanned using a flat-bed scanner and infarct volume was quantified by Image J2x. Infarct volume was expressed as a percentage of the total volume of slices.
Biochemical estimation in serum
At 24 h after reperfusion, the rats were deep anesthetized with 10 % chloral hydrate. 4 mL blood were drawn from the abdominal aorta and centrifuged at 4000 rpm for 15 min at 4 °C. Serum samples were carefully collected into two EP tubes to avoid repeated freezing and thawing and then stored at −80 °C until assayed. The levels of NO, iNOS and MPO in serum were assayed by colorimetry through commercially available kits, which the levels of IL-1β, TNF-α and CRP in serum were measured by ELISA according to the manufacturer’s instructions.
Histopathology and immunohistochemistry
After 24 h of reperfusion, deeply anesthetized rats were perfused first with 200 mL physiological saline solution at 4 °C till two forelimbs became white and the right atrial effluent became clear, followed fixed by freshly prepared 200 mL 4 % paraformaldehyde in 0.1 M phosphate buffered saline (pH 7.4). Finally, the brains were removed normatively and soaked in 4 % paraformaldehyde for 24 h at 4 °C. After dehydration in graded ethanol and transparency with xylene, the brain block was embedded in paraffin and cut into 3–4 μm coronal sections on a rotary microtome (Microm HM340E, Germany).
For histological study, the sections were stained with hematoxylin and eosin (H&E) using standard methods, and the pathological change of brain was examined by a light microscope at ×200 magnification. For immunohistochemical analysis, the slices were incubated with relevant primary antibodies at 37 °C for 60 min. After washed in PBS for 5 min × 3, sections were incubated with secondary antibody (EnVision™ Two-Step kit, DAKO 1701777A, Denmark) at 37 °C for 40 min. Subsequently, the sections were incubated with diaminobenzidine (DAB) and hematoxylin staining solution until brown products appeared under the light microscope.
The stained sections were observed under a fluorescence microscope (Olympus BX60, Japan), and then 5 microscopic horizons (×200 magnification) around the infarction was chosen and photographed. The positive expressions of ICAM-1 and NF-κB p65 were measured by the average integrated optical density (IOD) of positive cells using the Image-ProPlus 5.0 software (Media Cybernetics, USA).
Western blot analysis for ICAM, NF-κB p65 and iNOS
24 h after reperfusion, murine brain tissues were collected and homogenized with RIPA lysis buffer. After centrifugated at 15000 rpm for 10 min, the supernatant was taken and the immunoblot assay of ICAM, NF-κB p65 and iNOS was achieved with the instructions of previous reports (Joh and Kim 2011; Wang et al. 2015). The applied primary antibodies of ICAM-1and NF-κB p65 were consistent with those in immunohistochemistry, only had different dilution ratio. The IOD of the objective band was detected using the image analysis system of Pro-Plus 5.0. Taking β-actin expression as an internal reference, the ratio of the IOD values of the objective protein band and β-actin protein band was relative expression of the target protein.
Statistical analysis
All statistical analyses were performed using IBM SPSS statistics 19 and all data were shown as Mean ± SD. One way ANOVA was used to compare the differences among multiple groups. P value <0.05 was considered statistically significant.
Results
Effects of GHI on neurological deficits in rats
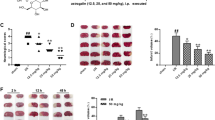
There were significant differences in neurological deficit scores among the sham group, I/R group and treated groups, as illustrated in Fig. 3. After MCAO for 1.5 h followed by 24 h reperfusion, neurological deficit score of the I/R group was significantly higher than that of the sham group (P < 0.01), whereas all the other treatment groups were lower than the I/R group (P < 0.01 or P < 0.05).
Effects of GHI on cerebral infarct volume
As shown in Fig. 4a, viable tissue is stained deep red in color by TTC staining, whereas area of infarction in the right cerebral hemisphere is white. The infarct volume in the I/R group was increased in comparison to the sham group (P < 0.01), and the infarct volume was significantly decreased in a dose dependent manner in the GHI-treated groups and nimodipine group (all P < 0.01) (Fig. 4b).
Effects of GHI on the levels of iNOS, NO and MPO in serum
As shown in Fig. 5, the levels of iNOS, NO and MPO were significantly increased (all P < 0.01) in rat serum after MCAO for 1.5 h followed by 24 h reperfusion. Compared with the I/R group, the GHI-M group, GHI-H group and Nimodipine group had significantly lower the secretion of iNOS and NO (P < 0.01 or P < 0.05), but no significant differences in iNOS activity and NO content were observed between the GHI-L group and the I/R group. Moreover, treated with GHI (2.5, 5 and 10 mL/kg) and nimodipine injection could significantly decrease the expression of MPO (P < 0.01 or P < 0.05) induced by cerebral I/R injury.
Effects of GHI on levels of IL-1β, TNF-α and CRP in serum
The expressions of IL-1β, TNF-α and CRP in serum were measured by ELISA. The levels of IL-1β, TNF-α and CRP in I/R group were significantly higher than the sham group (all P < 0.01). The levels of IL-1β and CRP in the GHI treated groups (2.5, 5 and 10 mL/kg) were significantly lower than that in I/R group (P < 0.01 or P < 0.05). Meanwhile, the level of TNF-α was restrained in the GHI-M group, GHI-H group and Nimodipine group (P < 0.01 or P < 0.05). These results indicated that the treatment of GHI could alleviate inflammatory injury (Table 1).
Effect of GHI on histopathology in brain tissue
H&E staining was used to observe histological changes. Figure 6 showed the pathologic changes in each group. No histopathological abnormalities were observed in the sham group, and microscopic pictures showed a clear cell outline, obvious nucleolus and no intracellular edema. At 24 h after reperfusion, many cells in the ischemic zone appeared shrunken with eosinophilic cytoplasm and triangulated pyknotic nuclei. Meanwhile, edematous cells, a fuzzy cell outline, loose cytoplasm, condensed nuclear, swelling, severe cell deformation and necrosis were also observed. Furthermore, numerous vacuolated spaces were found in infarct core (Fig. 6b). Compared with I/R group, the abnormalities above-mentioned were markedly alleviated in other treated groups (Fig. 6c–f), indicating that GHI could attenuate the cerebral I/R injury.
Effects of GHI on cerebral cortical histopathology in rats after 24 h of reperfusion by H&E staining (×200 magnification). a Sham group; b I/R group; c GHI-L group; d GHI-M group; e GHI-H group; f Nimodipine group. The pyknotic nuclei (arrows) and edematous cells (arrowheads) in MCAO group were marked, respectively
Effects of GHI on expressions of ICAM-1 and NF-κB p65 in IHC
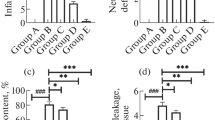
Immunohistochemical staining of ICAM-1 (Fig. 7a–f) and NF-κB p65 (Fig. 8a–f) was performed to determine whether GHI has anti-inflammatory effects against cerebral I/R injury. Positive expression appeared yellow or brown in color after immunostaining. After MCAO for 1.5 h followed by 24 h reperfusion, the positive immunological reaction of ICAM-1 was mainly located in cerebrovascular endothelial cells within infarct related zone. NF-κB p65 positive cells were found in nucleus and cytoplasm obviously. According to the corresponding immunohistochemical staining figures, more brown spots and vacuoles come out in I/R group than in sham group. Administration of GHI (2.5, 5 and 10 mL/kg) reduced the expressions of ICAM-1 and NF-κB p65 gradually. As well, similar effect was found in the nimodipine group.
All data of IHC were summarized in Fig. 9a, b. Mean IOD values analysis of ICAM-1 and NF-κB p65 immunoreactive products showed that IOD values in I/R group were significantly increased compared with the sham group (all P < 0.01). Treatment with GHI (5 and 10 mL/kg) and nimodipine injection effectively lessened the IOD values of ICAM-1 (all P < 0.01) and NF-κB p65 (P < 0.01 or P < 0.05) compared with the I/R group.
Effect of GHI on protein expressions of ICAM, NF-κB p65 and iNOS in western blot analysis
It was clear from the results of western blot analysis that the expression levels of ICAM, NF-κB p65 and iNOS were upregulated in the I/R group when compared with those in the sham group (all P < 0.01). However, compared with model group, these proteins were markedly inhibited in the GHI-M and DHI-H groups (P < 0.01 or P < 0.05). DHI attenuated their I/R–induced increases in expression (Table 2).
Discussion
Cerebral I/R injury is a rapid cascade process, including intracellular calcium overload, excessive release of excitatory amino acids, generation of oxygen free radicals, secretion of inflammatory cytokines and adhesion molecules and activation of apoptosis, etc. (Kato and Kogure 1999; An et al. 2014). These procedures closely linked to each other cause and effect, overlapping each other, form a vicious cycle leading to brain cell apoptosis or necrosis finally. Among these procedures, the inflammatory response is a critical factor and anti-inflammatory activities play an important role in neuroprotective effects (Wang et al. 2014). In this study, we used a rat model of MCAO to investigate the therapeutic effects of GHI on inflammatory injury in the early stage of focal cerebral I/R. For all we know, it was the first time to study the inflammatory reaction in rats of cerebral I/R injury after GHI treatment. We demonstrated that rats treated with GHI had decreased the expressions of inflammation-related factors (NO, iNOS, MPO, IL-1β, TNF-α, CRP, ICAM-1 and NF-κB p65) at 24 h of reperfusion after MCAO for 1.5 h. GHI significantly attenuated cerebral I/R injury, which might be associated with its anti-inflammatory activities.
The neurological score was used to evaluated the neuroprotective effects (Garcia et al. 1995) in MCAO rats at first. The neurological function recovery was improved by treatment with GHI after 24 h of reperfusion. By assessment of cerebral infarct volume, we found that the infarct was significantly reduced in a dose dependent manner in the GHI-treated groups in comparison to the I/R group. The results of HE staining also confirmed that GHI progressively extenuated cerebral pathological damage in the ischemic area of rats after cerebral I/R injury.
NO is a key factor in the pathophysiological response after I/R injury and it is connected with multiple inflammatory reactions in vivo. Excess NO can activate neurotoxic cascade reaction, lead to cell death and damage brain tissue (Chen et al. 2004). iNOS, mainly distributed in macrophages, white blood cells and other inflammatory cells, can induce a large amount of NO after I/R injury and is one of the most important inflammatory mediators in the ischemic diseases (Ha et al. 2008). Pro-inflammatory cytokines of TNF-α and IL-1β play vital roles in glia activation and inflammatory cell infiltration, which further aggravates cerebral I/R injury (Wang et al. 2009). Previous studies have found that injecting the antagonists of TNF-α and IL-1β could alleviate cerebral I/R injury in rats (Zhang et al. 1998; Liang et al. 2005). With the development of I/R injury and inflammatory cells infiltration, iNOS can be induced by the increased secretion of TNF-α and IL-1β and thus mediates neurotoxicity, resulting in further damage in neurons (Anctil et al. 2005). In the present study, our results showed that the levels of NO, iNOS, TNF-α and IL-1β in rats were reduced significantly after GHI treatment (especially in high-dose GHI group), when compared to I/R group. These changes indicated that anti-inflammatory property of GHI might act as a protective mechanism by inhibiting the expressions of NO, iNOS, TNF-α and IL-1β after cerebral I/R.
MPO is the specific enzyme of polymorphonuclear neutrophils (PMN), taking responsible for altering the kinetics of blood–brain barrier (BBB) disruption and its activity can reflect the degree of PMN infiltration (Sadeghi et al. 2011). Once MPO is released into the blood participating the inflammatory reaction, a positive feedback loop circuit will be formed simultaneously, which would further promote the release of MPO, thereby aggravate the inflammatory response continually. CRP as a marker for vascular inflammation is closely related to the size of infarction area (Liu et al. 2009). Studies have revealed that inflammatory response may be an independent risk factor for ischemic (Elkind et al. 2006) and CRP can be used as an independent evaluation indicator (Di Napoli et al. 2000). In clinical trials, GHI was reported to have the effect of decreasing the expression of CRP in patients with coronary heart disease of blood stasis type (Wang and Wu 2013). In our experiment, the contents of MPO and CRP were increased notably after MCAO for 1.5 h followed by 24 h reperfusion. However, treatment of GHI (2.5, 5 and 10 mL/kg) markedly decreased MPO and CRP levels compared with the I/R group and GHI exerted a dose-dependent inhibitory effect. GHI has distinct protective effect on cerebral I/R injury by suppressing the expressions of inflammatory markers MPO and CRP.
Besides, IHC and western blot analysis were utilized to evaluate the positive expressions of ICAM-1 and NF-κB p65 in MCAO rats after the treatment of GHI. The expression of ICAM-1 has been proved to be induced by cytokines, inflammation and ischemia (Wilcox et al. 1990). According to a study of Vemuganti (Vemuganti et al. 2004), inhibiting the expression of ICAM-1 can protect brain tissue and reduce infarct volume. Moreover, in order to regulate the inflammatory injury induced by focal cerebral I/R, it is necessary to forbid the activation of NF-κB signaling pathway (Qin et al. 2013). In our study, the IHC positive expression of NF-κB p65 was predominantly located in nucleus, while ICAM-1 was mainly in cytoplasm after focal cerebral I/R. Compared with the sham group, positive cells of ICAM-1 and NF-κB p65 in the I/R group were significantly increased and arranged loosely and disorderly as reported in previous study (Zhai et al. 2013). On the contrary, the IOD values of ICAM-1 and NF-κB p65 were reduced by GHI administration at moderate or high dose, and at the same time, cells outline became clear, arranged orderly and the numbers of vacuoles were relatively decreased. GHI might contribute to the recovery of cerebral I/R injury due to regulating the expressions of ICAM-1 and NF-κB p65.
In summary, the result of this investigation strongly supports that GHI has beneficial effects on cerebral I/R injury, which is particularly linked to its inhibition of inflammatory response. Consequently, GHI could be considered as an alternative drug for cerebrovascular diseases.
References
An C, Shi Y, Li P, Hu X, Gan Y, Stetler RA, Leak RK, Gao Y, Sun BL, Zheng P, Chen J (2014) Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurobiol 115:6–24
Anctil M, Poulain I, Pelletier C (2005) Nitric oxide modulates peristaltic muscle activity associated with fluid circulation in the sea pansy Renilla koellikeri. J Exp Biol 208:2005–2017
Babu CS, Ramanathan M (2011) Post-ischemic administration of nimodipine following focal cerebral ischemic–reperfusion injury in rats alleviated excitotoxicity, neurobehavioural alterations and partially the bioenergetics. Int J Dev Neurosci 29:93–105
Barone F, Parsons A (2000) Therapeutic potential of anti-inflammatory drugs in focal stroke. Expert Opin Investig Drugs 9:2281–2306
Chen L, Gao S, Hu CM, Peng L, Li R, Li J (2004) Investigation of NO and NOS in cerebral ischemia. Anhui J Med Pharm 8:3–6
Di Napoli M, Di Gianfilippo G, Sollecito A, Bocola V (2000) C-reactive protein and outcome after first-ever ischemic stroke. Stroke 31:238–239
Elkind MS (2009) Outcomes after stroke: risk of recurrent ischemic stroke and other events. Am J Med 122:S7–13
Elkind MS, Coates K, Tai W, Paik MC, Boden-Albala B, Sacco RL (2006) Levels of acute phase proteins remain stable after ischemic stroke. BMC Neurol 6:37
Gao D, Kawai N, Nakamura T, Lu F, Fei Z, Tamiya T (2013) Anti-inflammatory effect of D-allose in cerebral ischemia/reperfusion injury in rats. Neurol Med Chir 53:365–374
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26: 627–634; discussion 635
Garcia-Bonilla L, Iadecola C (2012) Peroxiredoxin sets the brain on fire after stroke. Nat Med 18:858–859
Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park JH, Lee EH, Ryu JH, Lee KR, Kim SY (2008) Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int 52:878–886
He Y, Zhou HF, Huang LN, Zhao T, Fu W, Wan HT (2015) Simultaneous quantification of seven components in Guhong injection by HPLC-DAD. Chin J Pharm Anal 35:954–959
Jiang XL, Zhang X, Lei HX (2007) Effect of Kingtag on the Levels of IL-6 and TNF-α in Serum of Patients with Acute Cerebral Infarction. J Neurol Neurorehabil 4(65–67):87
Joh EH, Kim DH (2011) Kalopanaxsaponin A ameliorates experimental colitis in mice by inhibiting IRAK-1 activation in the NF-kB and MAPK pathways. Brit J Pharmacol 162:1731–1742
Justin A, Sathishkumar M, Sudheer A, Shanthakumari S, Ramanathan M (2014) Non-hypotensive dose model by attenuating brain cytokine levels. Pharmacol Biochem Behav 122:61–73
Kato H, Kogure K (1999) Biochemical and molecular characteristics of the brain with developing cerebral infarction. Cell Mol Neurobiol 19:93–108
Lambertsen KL, Biber K, Finsen B (2012) Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 32:1677–1698
Liang QC, Wu Y, Shi SJ, Lü HY (2005) Effect of tumor necrosis factor alpha treatment on cerebral ischemia-reperfusion injury in rats. Chin J Clin Rehabil 9:168–171
Liu QC, Zhuang ZH, Zeng K, Cheng ZJ, Gao F, Wang ZQ (2009) Prevalence of pancreatic diabetes in patients carrying mutations or polymorphisms of the PRSS1 gene in the Han population. Diabetes Technol Ther 11:799–804
Liu H, Wang J, Zhou W, Wang Y, Yang L (2013) Systems approaches and polypharmacology for drug discovery from herbal medicines: an example using licorice. J Ethnopharmacol 146:773–793
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T (2001) Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock (Augusta, Ga.) 16: 165-177
National Research Council of the National Academies (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington
Ouyang YB (2013) Inflammation and stroke. Neurosci Lett 548:1–3
Qin WY, Luo Y, Chen L, Tao T, Li Y, Cai YL, Li YH (2013) Electroacupuncture could regulate the NF-kappaB signaling pathway to ameliorate the inflammatory injury in focal cerebral ischemia/reperfusion model rats. Evid-Based Compl Alt 2013:924541
Sadeghi H, Hajhashemi V, Minaiyan M, Movahedian A, Talebi A (2011) A study on the mechanisms involving the anti-inflammatory effect of amitriptyline in carrageenan-induced paw edema in rats. Eur J Pharmacol 667:396–401
Shu MC, Wan HT, Zhou HF, Yang JH, Zhao T, Fu W, He Y (2014) Effect and mechanism of Guhong injection against cerebral ischemia reperfusion injury. Chin J Chin Mater Med 39:4829–4833
Sun Y, Wei WL (2012) Protective effect of Guhong Zhusheye on ischemic/reperfusion injury in rats. Dig World Latest Med Inf 12:22–28
Sun K, Hu Q, Zhou CM, Xu XS, Wang F, Hu BH, Zhao XY, Chang X, Chen CH, Huang P, An LH, Liu YY, Fan JY, Wang CS, Yang L, Han JY (2010a) Cerebralcare Granule, a Chinese herb compound preparation, improves cerebral microcirculatory disorder and hippocampal CA1 neuron injury in gerbils after ischemia-reperfusion. J Ethnopharmacol 130:398–406
Sun X, Wei X, Qu S, Zhao Y, Zhang X (2010b) Hydroxysafflor Yellow A suppresses thrombin generation and inflammatory responses following focal cerebral ischemia–reperfusion in rats. Bioorg Med Chem Letts 20:4120–4124
Van der Worp HB, Van Gijn J (2007) Clinical practice. Acute ischemic stroke. New Engl J Med 357:572–579
Vemuganti R, Dempsey RJ, Bowen KK (2004) Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke 35:179–184
Wang Y (2011) The clinical application of aceglutamide injection. Heilongjiang Med J. 24:272–273
Wang J, Wu ZF (2013) Effects of safflower extract and aceglutamide injection on expressions of CD62P and CRP in patients with coronary heart disease of blood stasis type. Shanghai J Trad Chin Med 47:47–48
Wang Q, van Hoecke M, Tang XN, Lee H, Zheng Z, Swanson RA, Yenari MA (2009) Pyruvate protects against experimental stroke via an anti-inflammatory mechanism. Neurobiol Dis 36:223–231
Wang H, Zhang K, Zhao L, Tang J, Gao L, Wei Z (2014) Anti-inflammatory effects of vinpocetine on the functional expression of nuclear factor-kappa B and tumor necrosis factor-alpha in a rat model of cerebral ischemia-reperfusion injury. Neurosci Lett 566:247–251
Wang YS, Li YX, Zhao P, Wang HB, Zhou R, Hao YJ, Wang J, Wang SJ, Du J, Ma L, Sun T, Yu JQ (2015) Anti-inflammation effects of oxysophoridine on cerebral Ischemia–reperfusion injury in mice. Inflammation 38:2259–2268
Wasa M, Soh H, Shimizu Y, Fukuzawa M (2005) Glutamine stimulates amino acid transport during ischemia-reperfusion in human intestinal epithelial cells. J Surg Res 123:75–81
Wilcox CE, Ward AM, Evans A, Baker D, Rothlein R, Turk JL (1990) Endothelial cell expression of the intercellular adhesion molecule-1 (ICAM-1) in the central nervous system of guinea pigs during acute and chronic relapsing experimental allergic encephalomyelitis. J Neuroimmunol 30:43–51
Yang G, Betz A (1994) Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke 25: 1658-1664; discussion 1664-1655
Ye SY, Gao WY (2008) Hydroxysafflor yellow a protects neuron against hypoxia injury and suppresses inflammatory responses following focal ischemia reperfusion in rats. Arch Pharm Res 31:1010–1015
Young A, Ali C, Duretête A, Vivien D (2007) Neuroprotection and stroke: time for a compromise. J Neurochem 103:1302–1309
Zhai KH, Liu BU, Lu H (2013) Effect of NF-κB p65 and ICAM-1 in chronic cerebral ischemia rats by 3-n-butylphthalide. J Med Forum 34:37–38
Zhang B, Ning Y (2015) Clinical efficacy and safety of guhong injection in the treatment of acute cerebral infarction. Pract Pharm Clin Remedies 18:1129–1132
Zhang Z, Chopp M, Goussev A, Powers C (1998) Cerebral vessels express interleukin 1beta after focal cerebral ischemia. Brain Res 784:210–217
Zhang YJ, Huang X, Wang Y, Xie Y, Qiu XJ, Ren P, Gao LC, Zhou HH, Zhang HY, Qiao MQ (2011) Ferulic acid-induced anti-depression and prokinetics similar to Chaihu-Shugan-San via polypharmacology. Brain Res Bull 86:222–228
Zhang XL, Zheng SL, Dong FR, Wang ZM (2012) Nimodipine improves regional cerebral blood flow and suppresses inflammatory factors in the hippocampus of rats with vascular dementia. J Int Med Res 40:1036–1045
Zhao HD, Wang XZ, Huang SL, Zhai HF (2006) Clinical observation of guhong injection in treatment of 60 cases with acute cerebral hemorrhage. Appl J Gene Pract 4:630–631
Zhou CK, Wu XH (2005) The research progress of acetylglutamine. J Strait Pharm 17:15–17
Acknowledgments
This work was supported by the National Science Foundation of China (No. 81373898), Zhejiang Provincial Natural Science Foundation of China (No. LR13H270001), and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ai, J., Wan, H., Shu, M. et al. Guhong injection protects against focal cerebral ischemia–reperfusion injury via anti-inflammatory effects in rats. Arch. Pharm. Res. 40, 610–622 (2017). https://doi.org/10.1007/s12272-016-0835-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0835-4