Abstract
In this study, a selected γ-aminobutyric acid (GABA)-rich Malaysian strain Aspergillus oryzae NSK was collected from soy sauce koji. The strain was used to explore the effect of using renewable native sugar syrup, sugarcane, nipa, and molasses as fermentable substrates for developing a novel functional GABA soy sauce. We evaluated the strain using the chosen native sugars for 7 days using shake flask fermentation at 30 °C. The results showed optimum GABA concentration was achieved using cane molasses as the fermentable substrate (354.08 mg/L), followed by sugarcane syrup (320.7 mg/L) and nipa syrup (232.07 mg/L). Cane molasses was subsequently utilized as a substrate to determine the most suitable concentration for A. oryzae NSK to enhance GABA production and was determined as 50% g/L of glucose standard cane molasses. Our findings indicate that cane molasses can be used as a GABA-rich ingredient to develop a new starter culture for A. oryzae NSK soy sauce production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
γ-aminobutyric acid (GABA), a non-protein amino acid, is widely distributed in nature and present in high concentrations in mammalian brains which demonstrated to possess many physiological functions such as the reduction of hypertension and blood pressure [1], lowering blood cholesterol [2, 3], tranquilizing effects [4], improved immune system [5], and enriched muscle growth hormone [6]. Due to its numerous physiological functions, the development of pharmaceutical and functional foods enriched with GABA has been actively pursued, primarily using fermentation techniques [7]. GABA is biosynthesized by glucose-based fermentation from glutamic acid catalyzed by glutamic acid decarboxylase (GAD) [8]. Fermented food products play an important role in human diet because fermentation can enhance nutritional content, mineral bioavailability, and digestibility of proteins and carbohydrates, and improve the organoleptic qualities of a given product.
Various fermented foods such as kimchi [9], yogurt [10], and red mold rice [11] are known to contain microorganisms that can produce a considerable amount of GABA. These strains have been isolated and enhanced for incorporation into novel food systems enriched with GABA such as cheese [12], black raspberry juice[13], soaked-rice germ [14], sourdough bread [15], and tempeh-like fermented soybean [16]. However, the concentration of GABA generated from these fermented foods is typically low, either because of the low biosynthesizing capability of the microbes used, limited availability of carbon sources, or unsuitability of the fermentation system.
Among many carbon sources, native sucrose-based substrates such as sugarcane and nipa are among the most abundant plant products available and renewable in nature. Sugarcane is one of the most important global agricultural crops and has been cultivated in more than 110 countries. The consumption of sugarcane continues to increase, driven by rising household and industrial demand [17]. In addition, the by-product of the cane sugar manufacturing process, sugar cane molasses, has been used as a carbon source in various fermentation processes and has traditionally being used as a raw material for baker’s yeast (Saccharomyces cerevisiae) production [18] as it contains phenolic compounds, minerals, and organic acids [19]. Furthermore, sugarcane juice and sugarcane molasses are the established substrates for the production of D-lactic acid [20]. Nipa juice is a transparent fresh sap with pH 7.0–7.4 [21]. It is widely distributed in the mangrove forests of Southeast Asia and is consumed as fresh juice, syrup, and sugar or fermented to alcoholic drinks. It has also been used as a biofuel [22]. Substrates such as nipa juice may increase the efficiency of GABA-producing soy sauce koji strains.
The purpose of this study was to evaluate the ability of the selected A. oryzae NSK koji strain to ferment sugarcane syrup, nipa syrup, or cane molasses as a substrate for GABA production. This strain was isolated from koji samples and was shown to be capable of producing high GABA concentrations in shoyu (soy sauce) [7]. To the best of our knowledge, this research is the first report of the conversion of native sugars to GABA by A. oryzae NSK with glutamic acid supplementation to GABA in a shake flask fermentation system.
Materials and methods
Koji sample and GABA content
A koji sample, coded koji NSK, was obtained from an established Malaysian soy sauce manufacturer in Selangor as reported previously [7]. The master strain of the green-colored koji sample, designated as A. oryzae NSK, was subcultured and maintained in Nalgene® cryogenic vials (Sigma-Aldrich, Dorset, UK) and stored at − 80 °C when not in use. To maintain a working culture, the strain was subcultured and maintained on slants of potato dextrose agar (PDA) (Merck, USA) at − 20 °C. Spores were harvested using a sterile solution (Tween 80) and the fungus was allowed to grow on PDA slants for 7 days at 30 °C. To avoid contamination, the A. oryzae NSK strain was inspected every 3 weeks.
The GABA content of the chosen A. oryzae NSK koji strain was determined according to a previously modified method [7]. Initially, 30 g of koji from soybean samples was suspended in 90 mL of 50 mM phosphate buffer (pH 7.0) and blended with a Stomacher (Stomacher® 400C Lab Blender) for 10 min. Next, the sample was maintained at 40 °C for 60 min under moderate shaking (around 150 rpm) and the suspension was centrifuged for 30 min at 4 °C (3000 rpm). Whatman No. 2 filter paper was used to filter the supernatant, which was then centrifuged at 10,000 rpm for 10 min. Finally, a 0.22-µm pore-size nylon filter (Fisher Scientific Bishop, Meadow, UK) was used to filter the supernatant prior to high-performance liquid chromatography (HPLC) analysis.
Isolation of A. oryzae NSK koji strain
Spores of Aspergillus sp. were harvested from the surface of a Malaysian soy sauce koji sample [7]. The successfully isolated spores were incubated at 30 °C for 5 days in a sterile microbiological incubator (Sartorius Stedim, Germany). Pure colonies of A. oryzae NSK strain were transferred to Czapek Yeast Agar (CYA) media slants and maintained at − 20 °C until use. For microscopic evaluation, methylene blue staining was used to ensure the sterility and viability of the culture.
Native sugar sources
Sugarcane and nipa syrup were obtained from a small commercial factory in Perlis, Malaysia, while cane molasses was supplied by the Central Sugar Refinery Sdn. Bhd. (CSR; Selangor, Shah Alam, Malaysia).
Culture conditions
A loopful of spore suspension was streaked onto sterile PDA (Merck, USA) slants and cultured for 5 days at 30 °C to develop the spores. The spores were harvested by adding 0.001% (v/v) of sterilized solution (Tween 80) into the culture slant and the resulting sample solution was shaken for 1 min. Spores were counted using a hemocytometer (Copen’s Scientific, Assistent, Germany). For inocula preparation, standardized spore suspensions (approximately 1 × 104 spores/mL medium) were used for all.
The A. oryzae NSK strain was cultured in an Erlenmeyer flask (250 mL) containing a basal medium of native sugar (calculated glucose as 50%), glutamic acid (0.4 g), MgSO4·7H2O (1 g), KH2PO4 (1.5 g), yeast extract (6 g), and CaCl2·2H2O (2 g) in 1000 mL of distilled water at pH 5.5 and autoclaved at 121 °C for 15 min. Batch fermentation was conducted in triplicate in a rotary shaker (Sastec ST-500R) at a constant agitation speed of 200 rpm at 30 °C for 7 days. During fermentation in the shake flask, 10 mL samples were withdrawn at 12 h intervals for analysis. The supernatant was measured for GABA, glutamic acid, and total sugar content, while the residues (biomass) were dried to obtain the dry mycelia weight measurement. Throughout the fermentation process, the supernatant pH of the culture was monitored using a pH meter (Sartorius PB 10).
Analytical methods
Quantitative analysis of total sugar
Total reducing sugar and sucrose concentration were estimated using a dinitrosalicyclic acid (DNS) method as described previously [23]. Next, total sugar concentrations were measured quantitatively by HPLC (Waters 600). Samples of 1 g were weighed and diluted 10× with distilled water. HPLC separations were performed using a Waters 600 apparatus with a refractive index model Waters RID-410 detector. An NH2 column (150 × 2.1 mm I.D., particle size 5/µm; Merck, Malaysia) was used. The stationary and mobile phase of 80% acetonitrile in deionized water as the gradient elution was pumped at 1.0 mL/min and maintained at ambient temperature.
Determination of total nitrogen
The total nitrogen concentration in native sugars was determined using the micro-Kjeldahl method as described previously [24], with slight modifications. Sugarcane syrup, nipa syrup, and molasses (2 g each) were added to 0.8 g of mixed catalyst and 2.5 mL concentrated sulphuric acid, and the digestion process was carried out at 230 °C. Next, the sample was distilled in a distillation flask and condensed in a highly alkaline solution of 10 mL 45% NaOH to separate the two layers of solution. Released ammonium was collected in 10 mL of 2% boric acid. The titer of the ammonium borate formed was measured by the addition of 0.05 N sulfuric acid, using a methyl red and bromocresol green indicator. The titrated volume was recorded and used in the following equation:
Quantitative analysis of GABA and glutamic acid
GABA content in the culture filtrate was determined as described previously [7]. The fermentation broth was filtered using Whatman No. 2 filter paper, followed by centrifugation at 10,000 rpm for 10 min. The resulting supernatant was filtered through a nylon 0.22-μm pore size filter (Fisher Scientific, Bishop Meadow, UK). A 100-μL aliquot of supernatant or standard solution of GABA was dried under vacuum. The resulting residue was dissolved in 20 µL of ethanol:water:triethylamine (2:2:1 v/v) and evaporated to dryness under vacuum. An ethanol–water–triethylamine–phenylisothiocyanate (PITC) solution (7:1:1:1 v/v/v; 30 µL) was added to the residue and the reaction was allowed to proceed for 20 min at room temperature to produce PITC-GABA. Next, excess reagent was removed under vacuum and the resulting dry PTC-GABA residue was dissolved in 200 µL of the mobile phase, containing a combination of 60% solution A (0.7 mL acetic acid in 1000 mL, aqueous solution of 8.205 g sodium acetate and 0.5 mL triethylamine and) adjusted to pH 5.8, 28% solution B (deionized water), and 12% solution C (acetonitrile).
A Shimadzu LC 20AT apparatus: pump system, CT0-10ASVP model oven with 20-µL injection loop injector (Shimadzu Scientific Instruments, Riverwood, MD, USA), and a Model SPD-M20A PDA detector, in conjunction with a DELL OptiPlex integrator (Dell Technologies, Round Rock, Texas, USA), was used for gradient HPLC separations. This process was supported by a Hypersil Gold C-18 column (250 × 4.6 mm I.D., particle size 5/µm; Thermo-Scientific, Loughborough, UK). For detection at 254 nm, the mobile phase was pumped at 0.6 mL/min (room temperature).
Determination of water content
The water content was measured by the method of Dunko and Dovletoglou [25], with slight modifications. Five grams of sample was measured three times using an FD620 infrared moisture determination balance (Mettler Toledo, Columbus, Ohio, USA).
Growth determination
The dry cell weight of the mycelium or the culture growth was determined as described previously [7, 26]. A known volume of sample (150 mL) from the A. oryzae NSK fermentation process was withdrawn and filtered through a pre-weighed GF/C filter Whatman No. 4 (GE Healthcare Life Sciences, Buckingham, UK) using a Buchner funnel filter set attached to a water pump. The mycelium was washed three times with distilled water before drying overnight at 60 °C in an oven. After drying, the filter paper and mycelial cake were re-weighed and the cell dry weight was calculated. All values obtained were based on the average of at least three independent experiments.
Growth kinetics
To describe the growth kinetics, stoichiometric parameters related to batch fermentation were used to evaluate the growth of A. oryzae NSK strains in different native sugars. By plotting ln (Xt − X0) against time, the maximum specific growth rate (Umax) as described in Eq. 1, was obtained. The resulting slope is Umax and Xt denotes the cell concentration at the time while Xi is the initial cell concentration [7].
Equation 2 was used to measure the growth yield coefficient:
where Si is the initial substrate concentration (g/L) and S o is the residual substrate concentration at the fermentation end. Furthermore, the yield of Yp/s and Yp/x was determined by quantifying the maximum GABA and cell concentration and the total amount of substrate consumed, as described previously [7].
and
In Eq. 4, the maximum product concentration is designated as Pmax while the initial product concentration is Pi. For the overall productivity, P (mg/L/h), the total amount of biomass formed over a period of time was measured as:
Statistical analysis
The data were analyzed in triplicate and the resulting mean ± SD was calculated using Statistical Analysis Software (SAS, University Edition, 2012) and shown as error bars. Where error bars do not appear, it is assumed that they are smaller than the size of the symbol. A t test was used to plot fermentation graphs, and one-way ANOVA and Duncan’s new multiple range test (MRT) were used for the kinetic parameters comparison.
Results and discussion
Composition of the selected native sugars
As shown in Table 1, the native sugars were mainly composed of sucrose, glucose, and fructose, with small quantities of total nitrogen, and glutamic acid ranging from 0.02 to 0.29%. The total sugar amount ranged from 9.2 to 12.8% and varied significantly (p < 0.05) between the native sugars. The amounts of glucose, fructose, and sucrose in nipa syrup and molasses were found to be comparable with those of sugarcane syrup. These results differed slightly from those previously reported [27], where the average values were 17.4% total sugar in nipa sap and 17.0% in sugarcane sap. However, the percentage of molasses in this study was different from that reported previously by Oblrich [19] and Nakata [28], where the average values were 62 and 50% for total sugar, respectively. These differences might be due to the different sugar purification processes [22]. Regardless, the sugar content of these native sugars was found to be comparable with that of other commercial sugars in terms of sugar content for fermentation.
Appreciable levels of glutamic acid were found in nipa syrup, followed by sugarcane syrup and molasses (Table 1). Iskandar [29] and Nakata [28] have previously reported a similar range of glutamic acid levels in sugarcane stalk and cane molasses. The amount of GABA in sugarcane (72.78 mg/100 g) was significantly higher than in nipa (30.80 mg/100 g) or molasses (5.30 mg/100 g) and was in agreement with previous studies which reported similar ranges of GABA content for sugarcane [30] and molasses [28]. Moreover, the concentration of GABA in different native sugars varies because the quality of sugarcane and nipa crops is influenced by climate and time of harvesting [17], while molasses is influenced by the processing steps undertaken during sugar purification.
A. oryzae NSK growth in native sugars during fermentation
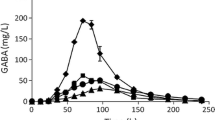
Sugarcane syrup contains a higher initial GABA concentration than nipa syrup and molasses, yet its efficiency during fermentation is crucial. Therefore, the capability of A. oryzae NSK to utilize three different native sugars to enhance GABA production was evaluated through the behavior of A. oryzae NSK in the media in terms of substrate consumption (glutamic acid and glucose), GABA formation, cell formation and pH changes. Typical time courses of strain fermentation in sugarcane syrup, nipa syrup, or molasses were constructed and are shown in Fig. 1[(A), (B) and (C)] and summarized in Table 2.
(A) GABA-producing ability of A. oryzae NSK koji strain using sugarcane. The symbols showed (filled square dashed-line) GABA production, (filled square dotted-line) cell formation, (filled diamond) glutamic acid and (filled square) glucose consumption, and (filled triangle) pH during shake flask fermentation carried out with initial 50 g/L glucose. (B) GABA-producing ability of A. oryzae NSK koji strain using nipa. The symbols showed (filled square dashed-line) GABA production, (filled square dotted-line) cell formation, (filled diamond) glutamic acid and (filled square) glucose consumption, and (filled triangle) pH during shake flask fermentation carried out with initial 50 g/L glucose. (C) GABA-producing ability of A. oryzae NSK koji strain using molasses. The symbols showed (filled square dashed-line) GABA production, (filled square dotted-line) cell formation, (filled diamond) glutamic acid and (filled square) glucose consumption, and (filled triangle) pH during shake flask fermentation carried out with initial 50 g/L glucose
The A. oryzae NSK strain showed a similar growth pattern in all native sugars (Fig. 1). For sugarcane [Fig. 1(A)] and nipa [Fig. 1(B)] syrup substrate, the stationary growth phase commenced after 24 and 48 h, respectively, while in molasses [Fig. 1(C)], the stationary phase was attained after 72 h. However, A. oryzae NSK showed a more diverse substrate consumption profiles. As shown in Table 2, A. oryzae NSK strain in sugarcane syrup resulted in the highest maximum cell concentration, Xmax (9.62 g/L), followed by nipa syrup (7.24 g/L) and molasses (6.25 g/L). However, the calculated cell yield (Yx/s) values showed that fermentation in nipa syrup consumed the largest amount of sugar and glutamic acid for each gram of cells (0.57 g/g), compared with molasses (0.40 g/g) and sugarcane syrup (0.28 g/g).
Since native sugar contains complex sugars, the A. oryzae NSK strain was further evaluated to determine its ability to hydrolyze native sugars into glucose and fructose. This characteristic might be advantageous in extending fungal growth by maintaining the carbon source and thus producing high levels of GABA. The concentrations of sucrose, fructose, and glucose throughout a 7-day course of batch cultivation were analyzed using HPLC (data not shown). It was found that the initial sucrose concentration (as shown in Table 1) accelerated the growth of A. oryzae NSK for up to 24 h and that growth was subsequently supported by fructose and glucose. This was observed during the fermentation of A. oryzae NSK in all native sugars, whereby glucose concentration rose rapidly and the highest concentration was obtained after 24 h in sugarcane (Fig. 1(A): 19.31 g/L), nipa (Fig. 1(B): 13.72 g/L), and molasses (Fig. 1(C): 1.12 g/L). This increase is caused by the ability of A. oryzae to hydrolyze complex sugars to glucose by secreting enzymes with high amylolytic activities, such as α-amylase and glucoamylase, into the fermentation medium [31]. Since the substrates (glucose and glutamic acid) act as the major energy source for the strain for conversion into GABA, glucose was consumed rapidly between 24 and 60 h of fermentation. Inadequate nutrient supply between 48 and 160 h of fermentation in native sugars forced the strain to utilize the available glutamic acid [32] to sustain its metabolite activities. We also found that, during the production of GABA, the pH became more alkaline, likely due to ammonia released by the deamination of amino acids derived from medium composition [32].
GABA changes in native sugars during fermentation
Generally, high levels of GABA were produced during the exponential and stationary phase in all samples. Maximum GABA production all substrates was between 24 and 72 h of fermentation time, indicating that GABA production was associated with secondary metabolites in a similar pattern as described by Su et al. [33] and Ab Kadir et al. [7]. As shown in Fig. 1(A), GABA production started at 72 h for sugarcane syrup and production decreased rapidly afterwards. This observation differed from that of Hirose [34], who reported that GABA production was in line with sugarcane juice content in sugarcane-fermented lactic acid beverages. The decrease in GABA concentration in this study was most likely due to the re-consumption of GABA by A. oryzae NSK [35]. Previous work by Ab Kadir et al. [7] reported a similar finding, whereby A. oryzae NSK showed rapid GABA production after 72 h of fermentation in glucose-containing media. In contrast, the onset of GABA production was faster in nipa syrup (24 h) and molasses (48 h). This may be due to the composition of complex sugars in both substrates, which may have caused stress for the fungal strain to break down the chemical composition, thus triggering the rapid adaptation of the strain to the media. This process is supported by the Krebs cycle metabolic pathway, where the GABA transaminase enzymatic reaction is inhibited by the accumulation of succinic semialdehyde after exposure to complex sugars and eventually leads to the accumulation of GABA [36]. GABA yield then gradually decreases as glucose is depleted, indicating that the depletion of substrate may have affected GABA formation.
In food processing and fermentation processes, fungi that consume the smallest amount of substrate while producing the largest amount of desirable end products are preferable [7]. This study shows that molasses is a suitable fermentable substrate for A. oryzae NSK to enhance GABA production. As summarized in Table 2, the A. oryzae NSK strain grown in molasses consumed less substrate (0.40 g/g) but simultaneously synthesized the highest amount of GABA (354.08 mg/L) as compared with the other native sugars. Although the strain consumed the lowest amount of substrate when grown in sugarcane syrup (0.28 g/g), the highest GABA formation was at 72 h while nipa syrup as substrate gave the highest Yx/s (0.57 g/g) while producing the lowest GABA yield (236.74 mg/L). Overall, A. oryzae NSK in molasses produced 0.20 mg GABA per gram of substrate (Yp/s) used, and each gram of NSK strain cell yielded 0.07 mg of GABA (Yp/x). Furthermore, molasses gave the highest GABA concentrations, with an overall productivity of 7.38 g/L/h (Table 2). Thus, molasses as a substrate was found to be the optimal native sugar for GABA production by A. oryzae NSK.
Effect of molasses concentration as a substrate on A. oryzae NSK performances
Since the most efficient fermentation substrate for A. oryzae NSK was molasses, GABA production and fungal growth were next evaluated based on glucose concentration in the range 10, 50, 100, 150, and 200 g/L (Table 3). The highest specific growth rate (µ) was observed in 10 g/L of glucose (0.356), followed by 50 g/L (0.289), 100 g/L (0.241), 150 g/L (0.241), and 200 g/L (0.229). There was no significant difference observed in µ between 100 g/L and values above this concentration. At a glucose concentration of 10 g/L, a maximum of 65.81 mg/L of GABA was obtained during 24 h of fermentation. However, A. oryzae NSK consumed the highest amount of substrate (sugar and glutamic acid) for each gram of cell (0.85 g/g) in 10 g/L of glucose. The strain then entered the death phase after 72 h because the remaining sugar level was at zero. Thus, the 10 g/L concentration was deemed not suitable for optimum GABA production.
As the glucose concentration increased from 50 to 200 g/L, the fermentation time for maximum GABA production increased from 48 to 72 h while sugar conversion decreased from 84.9% to 72.0%. Moreover, at a concentration of 100 g/L glucose and above, no significant differences were observed in terms of maximum GABA production and final biomass concentration. Increased molasses concentration ultimately suppressed growth, as increased initial concentrations in the solution increased also resulted in increased total initial sugar concentrations. If the fermentation is prolonged, high sugar concentrations may lead to high viscosity and osmotic pressure in the media [37], interrupting the aeration and agitation of the medium and subsequently starving the strain of required oxygen.
Table 3 shows that the specific growth rate (µ) and cell yield (Yx/s) is inversely proportional with molasses concentrations. However, the average GABA yield on substrate (Yp/s) remained relatively constant. This explains why, when molasses concentration increased, the growth of the strain was affected more than the level of GABA production. Therefore, molasses containing a glucose concentration of 50 g/L was deemed appropriate or growth supplementation and maximum GABA production.
Comparison of the current work with literature
The utilization of different carbon sources for GABA production of various fungi species was compared in Table 4. Sun et al. [38] reported that the best carbon sources for GABA production by M. pilosus was ethanol (385 mg/kg) followed by lactose (321 mg/kg), maltose (299 mg/kg), sucrose (284 mg/kg), glucose (233 mg/kg) and starch (203 mg/kg) at 0.5% (w/w) concentration. The closest comparison for this native sugar substrate was the use of different sugars (sucrose, glucose, fructose, and galactose) at 50 g/L on GABA production by A. oryzae NSK [7] and sucrose appears to be the preeminent carbon source, which gave a yield of 327 mg/L as compared to glucose (230 mg/L). In general, although a wide range of carbon source can be used for GABA production, better yield was obtained from glucose and sucrose medium [33, 39]. In this study, molasses, which contains a significant amount of total sugar, was shown to increase GABA yield up to 0.08% when compared to commercial sugar. Besides, molasses and sugarcane syrup are well-known native sugars to be used as a substrate for producing ethanol and citric acid by fungi species whilst no study reported on native sugars as a substrate for GABA production. Therefore, the current work holds the promise as a GABA-rich ingredient in soy sauce production.
To conclude, A. oryzae NSK koji strain demonstrates a high-GABA producing capability by utilizing various native sugars as a fermentable substrate for GABA enhancement in Malaysian soy sauce production. This specific strain produced a higher GABA concentration (354.08 mg/L) using molasses as a substrate than with sugarcane syrup (320.99 mg/L) or nipa syrup (236.74 mg/L) in a controlled fermentation system. Additional investigation revealed that an initial glucose concentration in molasses of 50 g/L was optimal for the growth of A. oryzae NSK and subsequent GABA enhancement. Further scale-up is essential, however, to elucidate the GABA production in a bioreactor fermentation strategy.
Abbreviations
- P :
-
GABA concentration (mg/L)
- S :
-
Substrate concentration (g/L)
- S 0 :
-
Initial substrate concentration (g/L)
- t :
-
Fermentation time (h−1)
- X :
-
Cell concentration (g/L)
- X m :
-
Maximum cell concentration (g/L)
- X 0 :
-
Initial cell concentration (g/L)
- Y p/s :
-
Yield factor for product on cell (mg/g)
- Y x/s :
-
Yield factor for cell on substrate (g/g)
- K s :
-
Monod growth saturation coefficient (g/L)
- μ m :
-
Maximum specific growth rate (h)
References
Zonghua XQTWA. Analysis of the source of antioxidant compounds in Zhenjiang vinegar. Food Ferment Indust 3: 11 (2005)
Roohinejad S, Omidizadeh A, Mirhosseini H, Rasti B, Saari N, Mustafa S, Yusof RM, Hussin ASM, Hamid A, Manap MYA. Effect of hypocholesterolemic properties of brown rice varieties containing different gamma aminobutyric acid (GABA) levels on Sprague-Dawley male rats. J Food Agric Environ 7: 197–203 (2009)
Roohinejad S, Omidizadeh A, Mirhosseini H, Saari N, Mustafa S, Yusof RM, Hussin AS, Hamid A, Abd Manap MY. Effect of pre-germination time of brown rice on serum cholesterol levels of hypercholesterolaemic rats. J Sci Food Agric 90: 245–51 (2010)
Okada T, Sugishita T, Murakami T, Murai H, Saikusa T, Horino T, Onoda A, Kajimoto O, Takahashi R, Takahashi T. Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. J-Jap Soc Food Sci Technol 47: 596–603 (2000)
Han D, Kim HY, Lee HJ, Shim I, Hahm DH. Wound healing activity of gamma-aminobutyric acid (GABA) in rats. J Microbiol Biotechnol 17: 1661–9 (2007)
Choi W-c, Reid SNS, Ryu J-k, Kim Y, Jo Y-H, Jeon BH. Effects of γ-aminobutyric acid-enriched fermented sea tangle (Laminaria japonica) on brain derived neurotrophic factor-related muscle growth and lipolysis in middle aged women. Algae 31: 175–187 (2016)
Ab Kadir S, Wan-Mohtar WA, Mohammad R, Abdul Halim Lim S, Sabo Mohammed A, Saari N. Evaluation of commercial soy sauce koji strains of Aspergillus oryzae for gamma-aminobutyric acid (GABA) production. J Ind Microbiol Biotechnol 43: 1387–95 (2016)
Jorge JM, Nguyen AQ, Perez-Garcia F, Kind S, Wendisch VF. Improved fermentative production of gamma-aminobutyric acid via the putrescine route: Systems metabolic engineering for production from glucose, amino sugars, and xylose. Biotechnol Bioeng 114: 862–873 (2016)
Cho YR, Chang JY, Chang HC. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol 17: 104–9 (2007)
Park K-B, Oh S-H. Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Biores Technol 98: 1675–1679 (2007)
Chiu CH, Ni KH, Guu YK, Pan TM. Production of red mold rice using a modified Nagata type koji maker. Appl Microbiol Biot 73: 297–304 (2006)
Nomura M, Kimoto H, Someya Y, Furukawa S, Suzuki I. Production of gamma-aminobutyric acid by cheese starters during cheese ripening. J Dairy Sci 81: 1486–91 (1998)
Kim JY, Lee MY, Ji GE, Lee YS, Hwang KT. Production of gamma-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int J Food Microbiol 130: 12–6 (2009)
Saikusa T, Horino T, Mori Y. Accumulation of γ-aminobutyric acid (GABA) in the rice germ during water soaking. Biosci Biotechnol Biochem 58: 2291–2292 (1994)
Coda R, Rizzello CG, Gobbetti M. Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of gamma-aminobutyric acid (GABA). Int J Food Microbiol 137: 236–45 (2010)
Aoki H, Furuya Y, Endo Y, Fujimoto K. Effect of gamma-aminobutyric acid-enriched tempeh-like fermented soybean (GABA-Tempeh) on the blood pressure of spontaneously hypertensive rats. Biosci Biotechnol Biochem 67: 1806–8 (2003)
Wisuthiphaet N, Napathorn SC. Optimisation of the use of products from the cane sugar industry for poly(3-hydroxybutyrate) production by Azohydromonas lata DSM 1123 in fed-batch cultivation. Process Biochem 51: 352–361 (2016)
Skountzou P, Soupioni M, Bekatorou A, Kanellaki M, Koutinas A, Marchant R, Banat I. Lead (II) uptake during baker’s yeast production by aerobic fermentation of molasses. Proc Biochem 38: 1479–1482 (2003)
Olbrich H. The molasses. Principles Sugar Technol 3: 511–697 (1963)
Calabia BP, Tokiwa Y. Production of D-lactic acid from sugarcane molasses, sugarcane juice and sugar beet juice by Lactobacillus delbrueckii. Biotechnol Lett 29: 1329–32 (2007)
Law SV, Abu Bakar F, Mat Hashim D, Abdul Hamid A. Popular fermented foods and beverages in Southeast Asia. Intern Food Res J 18: 475–484 (2011)
Sharma M, Patel SN, Lata K, Singh U, Krishania M, Sangwan RS, Singh SP. A novel approach of integrated bioprocessing of cane molasses for production of prebiotic and functional bioproducts. Bioresour Technol 219: 311–318 (2016)
Miloski K, Wallace K, Fenger A, Schneider E, Bendinskas K. Comparison of biochemical and chemical digestion and detection methods for carbohydrates. American J Undergraduate Res 7: 48–52 (2008)
Rossi A, Villarreal M, Juárez M, Sammán N, “Anales de la Asociación Química Argentina,” ed. SciELO Argentina, pp. 99–108 (2004).
Dunko A, Dovletoglou A. Moisture assay of an antifungal by near-infrared diffuse reflectance spectroscopy. J Pharm Biomed Anal 28: 145–54 (2002)
Wan-Mohtar WA, Ab Kadir S, Saari N. The morphology of Ganoderma lucidum mycelium in a repeated-batch fermentation for exopolysaccharide production. Biotechnol Rep (Amst) 11: 2–11 (2016)
Tamunaidu P, Matsui N, Okimori Y, Saka S. Nipa (Nypa fruticans) sap as a potential feedstock for ethanol production. Biomass Bioen 52: 96–102 (2013)
Nakata H, Tamura M, Shintani T, Gomi K. Evaluation of baker’s yeast strains exhibiting significant growth on Japanese beet molasses and compound analysis of the molasses types. J Biosci Bioeng 117: 715–9 (2014)
Iskandar HM, Casu RE, Fletcher AT, Schmidt S, Xu J, Maclean DJ, Manners JM, Bonnett GD. Identification of drought-response genes and a study of their expression during sucrose accumulation and water deficit in sugarcane culms. BMC Plant Biol 11: 12 (2011)
Naik G. Biochemical and molecular analysis of sugarcane glutamate decarboxylase. Intern J Bioas 2: 1011–1016 (2013)
Shibuya I, Tsuchiya K, Tamura G, Ishikawa T, Hara S. Overproduction of an α-amylase/glucoamylase fusion protein in Aspergillus oryzae using a high expression vector. Biosci Biotechnol Biochem 56: 1674–1675 (1992)
Kumar S, Punekar NS, SatyaNarayan V, Venkatesh K. Metabolic fate of glutamate and evaluation of flux through the 4‐aminobutyrate (GABA) shunt in Aspergillus niger. Biotechnol Bioeng 67: 575–584 (2000)
Su YC, Wang JJ, Lin TT, Pan TM. Production of the secondary metabolites gamma-aminobutyric acid and monacolin K by Monascus. J Ind Microbiol Biotechnol 30: 41–6 (2003)
Hirose N, Ujihara K, Teruya R, Maeda G, (2008).
Deacon JW. Fungal biology. John Wiley & Sons (2013)
Shelp BJ, Bown AW, McLean MD. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4: 446–452 (1999)
Park SC, Baratti J. Batch fermentation kinetics of sugar beet molasses by Zymomonas mobilis. Biotechnol Bioeng 38: 304–13 (1991)
Sun B, Zhou L, Jia X, Sung C. Response surface modeling for y-aminobutyric acid production by Monascus pilosus GM100 under solid-state fermentation. African J Biotechnol 7 (2008)
Ratanaburee A, Kantachote D, Charernjiratrakul W, Penjamras P, Chaiyasut C. Enhancement of γ-aminobutyric acid in a fermented red seaweed beverage by starter culture Lactobacillus plantarum DW12. Elect J Biotechnol 14: 1–1 (2011)
Wang J-J, Lee C-L, Pan T-M. Improvement of monacolin K, γ-aminobutyric acid and citrinin production ratio as a function of environmental conditions of Monascus purpureus NTU 601. J Ind Microbiol Biotechnol 30: 669–676 (2003)
Acknowledgements
We would like to extend our gratitude to the Ministry of Higher Education (MOHE) Malaysia for the financial support awarded to Prof Dr Nazamid Saari under the FRGS (Vot No: 5523380).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajar-Azhari, S., Wan-Mohtar, W.A.A.Q.I., Ab Kadir, S. et al. Evaluation of a Malaysian soy sauce koji strain Aspergillus oryzae NSK for γ-aminobutyric acid (GABA) production using different native sugars. Food Sci Biotechnol 27, 479–488 (2018). https://doi.org/10.1007/s10068-017-0289-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0289-6