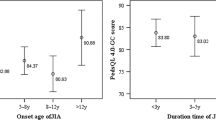
Abstract
Objectives
The objective of this study is to investigate extraarticular manifestations (EAMs) in patients with juvenile idiopathic arthritis (JIA) and assess their impact on health-related quality of life (HRQoL) among these patients.
Methods
This cross-sectional analytic study was carried out on 117 patients with JIA. EAMs were identified clinically by history and examination. Sicca symptoms, peripheral neuropathy, enthesitis, and skin lesions were picked up during clinical examination. Pulmonary involvement was evaluated by high-resolution CT chest. Patients were assessed by abdominal ultrasonography to assess the size of liver and spleen. Atlantoaxial subluxation was evaluated by cervical spine x-rays. Patients were evaluated by Pediatric Quality of Life Inventory-4 (PedsQL-4) and PedsQL-3 arthritis module.
Results
The median age of patients was 14 years with a median disease duration 4 years, 82.9% were females. Of the studied 117 JIA patients, 85 patients (72.6%) had at least one EAM. Persistent fatigue (51.3%) was the most prevalent EAM, followed by recurrent skin rash (16.2%), enthesitis (15.4%), recurrent fever (13.7%), and uveitis (12%). Patients with EAMs scored significantly lower in physical functioning (p = 0.001), emotional functioning (p < 0.001), social functioning (p = 0.005), and school functioning (p = 0.001). Regarding PedsQL arthritis module, patients with EAM had also significantly lower scores than did patients without EAM on the domains of pain and hurt (p < 0.001), daily activities (p = 0.008), and worry (p = 0.001).
Results
EAMs are prevalent among JIA patients and have a negative impact on their HRQoL. So, early identification and treatment are highly recommended.
Key Points • A large percentage of JIA patients experienced at least one extraarticular manifestation (EAM). • Persistent fatigue and recurrent skin rash are the most prevalent EAMs in JIA patients. • JIA patients with EAMs have worse scores in almost all domains of HRQoL. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic arthritis (JIA) is characterized by the presence of arthritis with an unknown cause, which starts before the age of 16 years and lasts for a minimum of 6 weeks [1]. It is characterized by articular inflammation of the synovium, which causes stiffness, pain, and joint swelling [2]. JIA includes several types of chronic arthritis in children, which not only affects the joints but also other structures such as the eyes, skin, and internal organs [1]. JIA impacts around 2 million children globally [3], and if left untreated, it may lead to significant functional limitations and disability with a decline in health-related quality of life (HRQoL) [4].
Many patients with JIA experience extraarticular manifestations (EAMs), which are extra disorders that occur either before or during the clinical progression of JIA [5], with some being temporary and resolving medical conditions while others becoming persistent. It is possible that these EAMs are associated with JIA itself, such as uveitis, or with the therapy of JIA, such as an increase in the number of serious infections [6]. Additionally, patients with different categories of JIA may also exhibit noticeable systemic symptoms, such as fever, rash, and serositis as seen in systemic arthritis [7]. Multiple EAMs may occur by chance or have common risk factors with JIA. These factors can increase the intricacy of the case since the combined effects of different diseases can contribute to the total severity of the illness for the patient, including socio-economic, cultural, environmental, patient behavior, and psychological characteristics [8].
The extent of EAMs in JIA is not well known and there is still a lack of data on the relationship between EAMs and HRQoL in JIA. To gain a more comprehensive insight into the frequency of EAMs in patients with JIA, it is crucial to determine the primary comorbidities and their prevalence within this specific patient cohort. Precise data regarding the prevalence of important coexisting medical conditions in JIA could be valuable for healthcare providers, healthcare authorities, and healthcare insurance companies.
The aim of this study was to evaluate the prevalence of EAMs in JIA patients and assess the relationship between these manifestations and HRQoL in patients with JIA in a well-phenotyped cohort from Egypt.
Patients and methods
Study design and settings
This cross-sectional, single-center study was conducted on 117 JIA patients between 2016 and 2020. The patients were recruited from the Mansoura Rheumatology and Immunology Unit (inpatient and outpatient). The fulfillment of the International League of Associations for Rheumatology (ILAR) classification criteria was used as inclusion criteria [7]. All eligible patients were included in the study. Overlapping connective tissue diseases diagnosed before the onset of symptoms of JIA, e.g., systemic lupus erythematosus or myositis, were excluded from the start. Patients with diabetes mellitus (DM) or pre-existing renal disease were also excluded.
One hundred and thirty consecutive patients fulfilled the inclusion criteria and were initially enrolled in this study. Of them, 13 patients were excluded: 3 patients with overlapping connective tissue diseases, 2 with DM, 3 with incomplete clinical data, and 5 patients refused to participate in the study. Finally, 117 patients were included in the study. A flow chart illustrating participant selection in the study is demonstrated in Fig. 1.
Ethical consideration
This research was conducted in compliance with the principles of the Helsinki Declaration [9], and the study protocol was approved by the Institutional Research Board of the Faculty of Medicine at Mansoura University (approval registration number: R.24.01.2476). A waiver of consent to review medical records was received. All participants and their parents were informed of the objectives and scope of the research and their rights. Informed written consent was obtained from all parents and participants of 12 years and older.
Sociodemographic, clinical, and therapeutic data
The patients’ demographic, clinical, and therapeutic data were extracted from the clinical computerized or written medical records obtained from Mansoura University Hospital. The demographic data included age and gender while clinical data included the subtype of JIA, duration of the disease, and age at the onset of JIA.
Clinical and laboratory evaluation
A general clinical examination, focusing on the number of swollen and tender joints, was done by an experienced rheumatologist. Patient-reported global assessment of overall well-being (PRgloVAS) and patient-reported pain (PRpainVAS) within the last week on a 10-cm visual analog scale (VAS) were collected. On this scale, 0 indicates no activity, no pain, or the best global health, and 10 indicates the maximum activity, worst pain, or poorest global health, respectively. Using the Juvenile Arthritis Disease Activity Score in 27 joints (JADAS-27), disease activity was assessed [10]. Juvenile arthritis damage index (JADI) was used to measure articular (JADI-A) and extra-articular damage (JADI-E) [11]. Also, the status of rheumatoid factor positivity was also registered. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), liver functions tests, and serum creatinine were assessed.
Extraarticular manifestations
Patients and/or their parents were asked about any manifestations suggestive of EAMs, like persistent fatigue, recurrent attacks of fever, recurrent, unexplained skin rash, and significant weight loss in the previous 6 months.
Information on the presence of other EAMs was based on the evaluation of the rheumatologist and on the previous medical records with a standardized method from the medical charts, either written or electronic, with the support of objective examinations and the exclusion of other causes. Also, EAMs were only recorded when a description of uveitis and dry eye was given by an ophthalmologist, chronic heart disease by a cardiologist, peripheral neuropathy by a neurologist, inflammatory bowel disease and celiac disease by a gastroenterologist, and psoriasis and dry mouth by a dermatologist.
Each patient’s clinical evaluation was used to evaluate whether there was any evidence of any other EAMs, with an emphasis on enthesitis and lymphadenopathy, and if so, ultrasound evaluation of the suspected enthesis and lymph nodes was performed.
Radiological assessment
Abdominal ultrasonography (US) was done by an experienced radiologist to evaluate the liver and spleen using a low-frequency 3 to 5 MHz curvilinear transducer. The US evaluation was done on the same day or day after the clinical evaluation. The patient had fasted for more than 4 h prior to the examination and was examined in a supine position. Time-gain compensation was set to give uniform reflectivity [12, 13].
To determine if there was any evidence of atlantoaxial subluxation, an X-ray spine was done. Antero-posterior views with an open mouth and a closed mouth, as well as lateral views in flexion and extension, were done. The atlanto-dens interval, which is the horizontal distance between the anterior arch of the atlas and the dens of the axis, was measured.
Any patients with chronic chest complaints underwent chest CT imaging without a contrast agent on the day of clinical evaluation on a 16-detector CT scanner (Bright Speed; GE Healthcare). All of the patients were examined in a supine position, with images acquired during a single inspiratory breath hold. The scanning range was from the base of the neck to the level of the upper pole of the kidneys. The CT scan parameters were as follows: X-ray tube parameters: 120 KVp, 350 mAs; rotation time, 0.5 s; pitch, 1.0; section thickness, 5 mm; intersection space, 5 mm; and additional reconstruction with a slice thickness of 1.5 mm. Scans were reviewed at a window width and level of 1000 to 2000 HU and 7000 to 50,000 HU, respectively, to assess the lung parenchyma. The images were reconstructed in axial, coronal, and sagittal reformats. Demonstrative cases are illustrated in Fig. 2.
Contrast-enhanced CT axial scans of the chest and abdomen shows A mild pericardial effusion (arrow) in a 14-year-old male JIA patient, B multiple reticulonodular densities at the right upper lobe (arrow) in a 10-year-old female JIA patient, C multiple enlarged para-aortic lymph nodes (arrows) in a 20-year-old male JIA patient, and D splenomegaly in a 17-year-old female JIA patient
The pediatric quality of life inventory (PedsQL-4)
The pediatric quality of life inventory (PedsQL-4) measurement model [14] is a modular strategy to evaluating HRQoL in healthy children and adolescents, as well as those suffering from acute and chronic health conditions. The PedsQL-4 evaluates both global (generic) and disease-specific (disease-specific) quality of life. This generic battery consists of four scales: physical functioning (eight items), emotional functioning (five items), social functioning (five items), and school functioning (five items). These four main scales can be used to calculate three standardized summary scores: a Total Quality of Life Score, a Physical Health Summary Score (based on physical functioning items), and a Psychosocial Health Summary Score (which includes emotional, social, and school items). The respondent (participants or their parents) is asked to rate how difficult each item has been in the previous month on each of the PedsQL-4 scales, with response options of 0, never; 1, almost never; 2, sometimes; 3, often; and 4, very always. To generate scale and summary scores, item scores are first reverse coded, then linearly translated to a scale of 100 points, and then averaged. Greater scale and summary scores imply a higher overall quality of life.
PedsQL3.0 Arthritis module
The 22-item multidimensional PedsQL 3.0 Arthritis module has the following scales: pain and hurt (four items), daily activities (five items), therapy (seven items), worry (three items), and communication (three items). A 5-point Likert scale was used for patient self-reporting and for proxy reporting (0, never a problem; 1, almost never a problem; 2, sometimes a problem; 3, often a problem; and 4, almost always a problem). To increase the ease of young children’s (age, 5–7) self-reporting, the Likert scale was simplified to a 3-point scale (0, not a problem at all; 2, sometimes a problem; and 4, a big problem), with each response anchored to a happy-to-sad face scale [15, 16]. Given the developmental limitations on self-reporting by children younger than 5 years of age, the parent’s proxy report did not include a self-report form and included only 3 subscales: pain and hurt, daily activities, and treatment [17, 18].
Statistical analysis
To analyze the collected data, the Statistical Package for Social Science (SPSS) version 22 program was utilized. We presented quantitative data using mean and standard deviations (SD) for parametric variables and median (min–max) for nonparametric variables. We presented qualitative data using percentages and numbers. The Shapiro–Wilk test was performed to ascertain whether the distribution of the variable was normal. The independent samples t test was used to determine whether there was a statistically significant difference between two groups when the data were normally distributed; however, the Mann–Whitney test was utilized when the variables in question were not parametric. To do comparisons across qualitative variables, we either used the chi-square test or the Fisher exact test, as appropriate. Univariate logistic regression analysis was used to determine factors associated with EAMs. A p value of less than 0.05 was considered statistically significant.
Results
A total of 117 patients with JIA were included in this cross-sectional study. The median age was 14 years. Most of them were below the age of 20 (77.8%), and 97 were female (82.9%). The survey was completed mainly by parents (78.6%). In about 80% of cases, the disease started below the age of 10 years.
According to the presence of associated EAMs, 32 patients were classified in the non-EAM group and 85 in the EAM group. The characteristics of those with and without EAMs are illustrated in Table 1. There were no statistically significant differences between the two groups regarding gender (p = 0.270), age of disease onset (p = 0.056), or the status of rheumatoid factor (p = 0.922).
Those with EAMs had statistically significant higher median age (15 vs. 10, p = 0.011), longer disease duration (p = 0.021), lower prevalence of oligoarticular JIA type (17.6% vs. 43.8%, p = 0.013), higher number of both tender joints (p = 0.013), and worse PRgloVAS (p = 0.041), as illustrated in Table 1.
As shown in Table 2, the most used antirheumatic drugs among JIA patients were non-steroidal anti-inflammatory drugs (85.5%), methotrexate (82.9%), and corticosteroids (65%). By comparing the therapeutic data between those with and without EAMs, we found that those with EAMs were prescribed infliximab (20% vs. 3.1%, p = 0.023) and adalimumab (50.6% vs. 28.1%, p = 0.029) more than those without EAMs. The number of biological drugs received was statistically significantly higher among those with EAMs (p = 0.041).
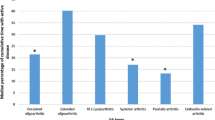
When evaluating the EAMs among our cohort, we found that persistent fatigue (51.3%) was the most prevalent EAM, followed by recurrent skin rash (16.2%), enthesitis (15.4%), recurrent fever (13.7%), and uveitis, as illustrated in Fig. 3.
The univariate logistic regression analysis showed that disease duration (OR 1.141 [95% CI, 1.014–1.285], p = 0.029), tender joint count (OR 1.166; [95% CI, 1.017–1.338], p = 0.028), and JADAS-27 (OR 1.048 [95% CI 1.016–1.080], p = 0.003) were the main predictors of EAMs in JIA patients, as shown in Table 3.
The study employed the score of PedsQL-4.0 generic core scale domains according to the presence or absence of EAMs. Patients with EAMs had statistically significantly lower scores than did patients without in all domains. Additionally, when we employed the PedsQL3.0 arthritis module, we found that those with EAMs had statistically significant lower scores in almost all domains, as shown in Table 4.
Discussion
To the best of our knowledge, this is one of the first studies to assess the added burden of EAMs in Egyptian patients with JIA. The percentage of patients with EAMs was high (72.65%). Persistent fatigue (51.3%) was the most prevalent followed by recurrent skin rash (16.2%), enthesitis (15.4%), recurrent fever (13.7%), and uveitis (12%). Patients with EAMs had statistically significantly lower scores in almost all domains of HRQoL.
The current study revealed that a large percentage of JIA patients, approximately three-quarters, experienced at least one EAM. There is limited data available regarding the JIA patients in this matter. In a study from Tukey that included 459 JIA patients, almost one-third of our patients had at least one comorbidity [19]. In another study conducted on patients with JIA who were undergoing biological therapies, it was shown that 62% of them had at least one comorbidity. The prevailing conditions were uveitis, allergic rhinitis, and migraine [20]. However, individuals undergoing biological treatment may have a higher likelihood of having comorbidities because of their potentially resistant disease progression and more rigorous treatment protocols. In another study including adult patients diagnosed with RA, a high prevalence of comorbidity was observed, with 75% of the participants affected [21].
In this study, we found that more than half of the patients suffer from a feeling of persistent fatigue. When we talk about physical fatigue, we are referring to a feeling of exhaustion, such as feeling weak. The study of fatigue in individuals with rheumatoid arthritis (RA) has received more comprehensive attention compared to patients with JIA [22]. In general, patients diagnosed with JIA frequently experience fatigue, which is linked to the time of day, the disease activity, the level of discomfort, psychosocial variables, and sleep [23]. Approximately 60–76% of JIA patients experience fatigue, with 15–19% reporting moderate to severe fatigue [24, 25]. Fatigue persists not only in young patients but also continues into adulthood, even after the disease has been extinguished [20].
The results of this study revealed that 16% of patients had recurrent skin rash. This was not surprising since dermatological manifestations are frequently present in both systemic and polyarticular JIA, as well as in psoriatic arthritis [26]. Additionally, more than 80% of systemic JIA patients experience a transitory, salmon-colored macular or maculopapular rash alongside their fever [27].
When we evaluated the JIA patients in this study, we found evidence of enthesitis at least at one site in 15.4% of the patients. Enthesitis refers to the inflammation that occurs at the points where tendons, ligaments, joint capsules, or fascia adhere to bone. This inflammation is a defining characteristic of enthesitis-related arthritis (ERA), which is a subtype of JIA [28]. Out of the 1371 patients with JIA who were observed for a median duration of 35.3 months, 214 individuals (16%) experienced enthesitis. Among these, 137 patients (64%) were diagnosed with ERA [29]. Enthesitis has also been documented in other subtypes of JIA, such as psoriatic arthritis and undifferentiated arthritis [30]. Children who have enthesitis, regardless of their category of JIA, experience more negative patient-reported outcomes compared to those who do not have enthesitis. Therefore, it is imperative to evaluate enthesitis in all children diagnosed with JIA [29].
A significant portion of the patients in this study (13.7%) complained of recurrent attacks of fever. An increasing body of evidence demonstrates that fever is a complicated adaptive response of the host to numerous immunological stressors, whether infectious or non-infectious [31]. Fever is almost universal among individuals with systemic JIA during initial presentation [32]. The most captivating and severe complication of systemic JIA is macrophage activation syndrome (MAS), which occurs more frequently in individuals with systemic JIA. A prominent characteristic of MAS is the presence of persistent high fever [33]. On the other hand, fever may also occur with other subtypes of JIA. In a study from China that included 146 consecutive ERA, 52 patients (35.6%) had fever as one of the first symptoms at disease onset [34].
When we talk about uveitis, the most common cause of its occurrence in children is JIA [35]. The development of uveitis is more likely to occur within the first 4 years following the onset of arthritis; nonetheless, cases have been observed even after 10 years have passed [36, 37]. About 10% of children are first diagnosed with uveitis, and JIA develops later [38]. In our cohort, the prevalence of uveitis was found to be 12%. This is in concordance with rates reported in the existing literature [35, 39]. A 2019 meta-analysis found a 13% prevalence of uveitis in JIA, with rates ranging from 19% in Northern European countries to 5% in Southeast Asia [40]. The cause of the geographical variation in uveitis prevalence among JIA patients is unknown and could be attributed to genetic factors, environmental variables, or changes in uveitis screening techniques [35].
Biological agents inhibit immune system activation and control by targeting specific cytokines or cellular interactions. Since the introduction of these medications into clinical practice, the prognosis for children with JIA has significantly improved [1]. In our cohort, we found that those with EAMs were prescribed infliximab and adalimumab more than those without EAMs. This is not strange, as it is expected that some biological medications would be added to manage EAMs. In this regard, Germany reported elevated rates of uveitis in patients undergoing biologic therapy compared to those receiving methotrexate therapy [37]. Also, treatment using TNF inhibitors (TNFi) and other biological diseases modifying antirheumatic drugs has repeatedly shown an elevated risk of severe infections [41]. There have been reports of several additional cases of paradoxical autoimmune renal involvement following biologic therapy. Biologics-induced autoimmune renal disorders are uncommon, with only 0.9 cases per 1000 patient years reported [42].
In general, musculoskeletal and rheumatic diseases are linked to reduced physical function and mental health, as well as poor HRQoL [43]. In the context of HRQoL, comorbidity has been identified as a significant element that differentiates between the different degrees of functioning of individuals [44]. Also, patients with arthritis and other chronic comorbidities may be more likely to experience poor physical and mental health [45]. Our study demonstrated JIA patients with EAMs had worse scores of HRQoL in almost all domains when compared with those without EAMs. Our findings demonstrate a degree of agreement with previous studies that have examined the impact of comorbidity on the HRQoL of individuals with various inflammatory rheumatic disorders. For example, comorbidities in psoriatic arthritis were the subject of a multicentric cross-sectional study by Bavière W. and colleagues. The researchers found that the type of comorbidity had a stronger correlation with the additional impact of comorbidities on patients’ perceived mental health in PsA [46]. Comorbidities are linked to lower physical health-related quality of life (HRQOL) in individuals with RA and osteoarthritis. Additionally, having multiple chronic diseases simultaneously has a detrimental impact on HRQOL scores for patients with arthritis [45].
To the best of our knowledge, few studies have been published on the JIA cohort to assess HRQoL according to the presence of EAMs. This study presents the most current data on the prevalence of EAMs among the Egyptian JIA cohort and their impact on the HRQoL of these patients. An important strength of the study was the relatively large sample size. Also, our results enrich the knowledge of epidemiology, thereby contributing to the comprehension of the disease and its management.
However, a few limitations of this study deserve mention. A general limitation was the cross-sectional study, which does not allow conclusions on causality. It will be important to include EAMs in longitudinal analyses to understand causality. Second, there are potential confounders that were not controlled for, such as polypharmacy, disease knowledge, medication adherence, and social support. Because we did not account for some of these variables, we are unable to substantiate their associations with multimorbidity and their potential impact on these study findings. Third, we did not look at the types of EAMs that grouped together, which might have had a different effect on HRQoL, and physical functioning compared to single diseases or groups of different EAMs that were linked at the same time. Finally, it would be better to conduct an in-depth evaluation of the spinal pathologies. Nevertheless, it will be considered in future studies including these populations.
Conclusion
The evidence of our study is important and adds to the literature demonstrating poorer outcomes in JIA patients with EAMs. In this sample of JIA patients, patients with EAMs had significantly worse outcomes compared to patients with no EAMs. These results point to the need for appropriate clinical guidelines, future research, and improved policies to address HRQoL and outcomes in JIA patients with EAMs.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DM :
-
Diabetes mellitus
- EAMs :
-
Extraarticular manifestations
- ERA :
-
Enthesitis-related arthritis
- HRQoL :
-
Health-related quality of life
- ILAR :
-
International League of Associations for Rheumatology
- JIA :
-
Juvenile idiopathic arthritis
- MAS :
-
Macrophage activation syndrome
- PedsQL-4 :
-
Pediatric quality of life inventory
- PRgloVAS :
-
Patient-reported pain
- PRpainVAS :
-
Patient-reported global assessment of overall well-being
- RA :
-
Rheumatoid arthritis
- TNFi :
-
TNF inhibitors
- US :
-
Ultrasonography
- VAS :
-
Visual analogue scale
References
Cimaz R, Maioli G, Calabrese G (2020) Current and emerging biologics for the treatment of juvenile idiopathic arthritis. Expert Opin Biol Ther 20:725–740. https://doi.org/10.1080/14712598.2020.1733524
Zaripova LN, Midgley A, Christmas SE et al (2021) Juvenile idiopathic arthritis: from aetiopathogenesis to therapeutic approaches. Pediatr Rheumatol Online J 19:135. https://doi.org/10.1186/s12969-021-00629-8
Dave M, Rankin J, Pearce M, Foster HE (2020) Global prevalence estimates of three chronic musculoskeletal conditions: club foot, juvenile idiopathic arthritis and juvenile systemic lupus erythematosus. Pediatr Rheumatol Online J 18:49. https://doi.org/10.1186/s12969-020-00443-8
Martini A, Lovell DJ, Albani S et al (2022) Juvenile idiopathic arthritis. Nat Rev Dis Primer 8:5. https://doi.org/10.1038/s41572-021-00332-8
Ording AG, Sørensen HT (2013) Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol 5:199–203. https://doi.org/10.2147/CLEP.S45305
Swart J, Giancane G, Horneff G et al (2018) Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res Ther 20:285. https://doi.org/10.1186/s13075-018-1780-z
Petty RE, Southwood TR, Manners P et al (2004) International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31:390–392
Valderas JM, Starfield B, Sibbald B et al (2009) Defining comorbidity: implications for understanding health and health services. Ann Fam Med 7:357–363. https://doi.org/10.1370/afm.983
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194. https://doi.org/10.1001/jama.2013.281053
Consolaro A, Ruperto N, Bazso A et al (2009) Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 61:658–666. https://doi.org/10.1002/art.24516
Viola S, Felici E, Magni-Manzoni S et al (2005) Development and validation of a clinical index for assessment of long-term damage in juvenile idiopathic arthritis. Arthritis Rheum 52:2092–2102. https://doi.org/10.1002/art.21119
Watson N, Jones H (2017) Chapman & Nakielny’s Guide to Radiological Procedures E-Book. Elsevier Health Sciences
Riestra-Candelaria BL, Rodriguez-Mojica W, Jorge JC (2018) Anatomical criteria to measure the adult right liver lobe by ultrasound. Sonography 5:181–186. https://doi.org/10.1002/sono.12162
Marsac ML, Alderfer MA (2017) Pediatric Quality of Life Inventory (PedsQL). In: Gellman M, Turner JR (eds) Encyclopedia of Behavioral Medicine. Springer, New York, NY, pp 1–2
Sprangers MA, Cull A, Bjordal K et al (1993) The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 2:287–295. https://doi.org/10.1007/BF00434800
Varni JW, Thompson KL, Hanson V (1987) The Varni/thompson pediatrie pain questionnaire. I. Chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain 28:27–38. https://doi.org/10.1016/0304-3959(87)91056-6
Development of the Waldron/Varni pediatric pain coping inventory - PubMed. https://pubmed.ncbi.nlm.nih.gov/8895242/. Accessed 8 Jan 2024
Thompson KL, Varni JW (1986) A developmental cognitive-biobehavioral approach to pediatric pain assessment. Pain 25:283–296. https://doi.org/10.1016/0304-3959(86)90233-2
Haşlak F, Guliyeva V, Hotaman B et al (2023) Non-rheumatic chronic comorbidities in children with juvenile idiopathic arthritis. Turk Arch Pediatr 58:212–219. https://doi.org/10.5152/TurkArchPediatr.2023.22303
Raab A, Sengler C, Niewerth M et al (2013) Comorbidity profiles among adult patients with juvenile idiopathic arthritis: results of a biologic register. Clin Exp Rheumatol 31:796–802
Parodi M, Bensi L, Maio T et al (2005) Comorbidities in rheumatoid arthritis: analysis of hospital discharge records. Reumatismo 57:154–160. https://doi.org/10.4081/reumatismo.2005.154
Cutolo M, Kitas GD, van Riel PLCM (2014) Burden of disease in treated rheumatoid arthritis patients: going beyond the joint. Semin Arthritis Rheum 43:479–488. https://doi.org/10.1016/j.semarthrit.2013.08.004
Armbrust W, Siers NE, Lelieveld OTHM et al (2016) Fatigue in patients with juvenile idiopathic arthritis: a systematic review of the literature. Semin Arthritis Rheum 45:587–595. https://doi.org/10.1016/j.semarthrit.2015.10.008
Minden K, Niewerth M, Listing J et al (2002) Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum 46:2392–2401. https://doi.org/10.1002/art.10444
Ostlie IL, Aasland A, Johansson I et al (2009) A longitudinal follow-up study of physical and psychosocial health in young adults with chronic childhood arthritis. Clin Exp Rheumatol 27:1039–1046
Chua-Aguilera CJ, Möller B, Yawalkar N (2017) Skin manifestations of rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritides. Clin Rev Allergy Immunol 53:371–393. https://doi.org/10.1007/s12016-017-8632-5
Lee JJY, Schneider R (2018) Systemic juvenile idiopathic arthritis. Pediatr Clin North Am 65:691–709. https://doi.org/10.1016/j.pcl.2018.04.005
Di Gennaro S, Di Matteo G, Stornaiuolo G et al (2023) Advances in the diagnosis and treatment of enthesitis-related arthritis. Child Basel Switz 10:1647. https://doi.org/10.3390/children10101647
Rumsey DG, Guzman J, Rosenberg AM et al (2020) Worse quality of life, function, and pain in children with enthesitis, irrespective of their juvenile arthritis category. Arthritis Care Res 72:441–446. https://doi.org/10.1002/acr.23844
Stoll ML, Zurakowski D, Nigrovic LE et al (2006) Patients with juvenile psoriatic arthritis comprise two distinct populations. Arthritis Rheum 54:3564–3572. https://doi.org/10.1002/art.22173
Ogoina D (2011) Fever, fever patterns and diseases called ’fever’–a review. J Infect Public Health 4:108–124. https://doi.org/10.1016/j.jiph.2011.05.002
Behrens EM, Beukelman T, Gallo L et al (2008) Evaluation of the presentation of systemic onset juvenile rheumatoid arthritis: data from the Pennsylvania Systemic Onset Juvenile Arthritis Registry (PASOJAR). J Rheumatol 35:343–348
Woerner A, von Scheven-Gête A, Cimaz R, Hofer M (2015) Complications of systemic juvenile idiopathic arthritis: risk factors and management recommendations. Expert Rev Clin Immunol 11:575–588. https://doi.org/10.1586/1744666X.2015.1032257
Guo R, Cao L, Kong X et al (2015) Fever as an initial manifestation of enthesitis-related arthritis subtype of juvenile idiopathic arthritis: retrospective study. PLoS ONE 10:e0128979. https://doi.org/10.1371/journal.pone.0128979
Clarke SLN, Sen ES, Ramanan AV (2016) Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol Online J 14:27. https://doi.org/10.1186/s12969-016-0088-2
van Straalen JW, Giancane G, Amazrhar Y et al (2021) A clinical prediction model for estimating the risk of developing uveitis in patients with juvenile idiopathic arthritis. Rheumatol Oxf Engl 60:2896–2905. https://doi.org/10.1093/rheumatology/keaa733
Heiligenhaus A, Niewerth M, Ganser G et al (2007) Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatol Oxf Engl 46:1015–1019. https://doi.org/10.1093/rheumatology/kem053
Sen ES, Dick AD, Ramanan AV (2015) Uveitis associated with juvenile idiopathic arthritis. Nat Rev Rheumatol 11:338–348. https://doi.org/10.1038/nrrheum.2015.20
Kearsley-Fleet L, Klotsche J, van Straalen JW et al (2022) Burden of comorbid conditions in children and young people with juvenile idiopathic arthritis: a collaborative analysis of 3 JIA registries. Rheumatol Oxf Engl 61:2524–2534. https://doi.org/10.1093/rheumatology/keab641
Hayworth JL, Turk MA, Nevskaya T, Pope JE (2019) The frequency of uveitis in patients with juvenile inflammatory rheumatic diseases. Joint Bone Spine 86:685–690. https://doi.org/10.1016/j.jbspin.2019.06.001
Chiu Y-M, Chen D-Y (2020) Infection risk in patients undergoing treatment for inflammatory arthritis: non-biologics versus biologics. Expert Rev Clin Immunol 16:207–228. https://doi.org/10.1080/1744666X.2019.1705785
Piga M, Chessa E, Ibba V et al (2014) Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: systematic literature review and analysis of a monocentric cohort. Autoimmun Rev 13:873–879. https://doi.org/10.1016/j.autrev.2014.05.005
Branco JC, Rodrigues AM, Gouveia N et al (2016) Prevalence of rheumatic and musculoskeletal diseases and their impact on health-related quality of life, physical function and mental health in Portugal: results from EpiReumaPt- a national health survey. RMD Open 2:e000166. https://doi.org/10.1136/rmdopen-2015-000166
Charlson ME, Charlson RE, Peterson JC et al (2008) The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol 61:1234–1240. https://doi.org/10.1016/j.jclinepi.2008.01.006
Geryk LL, Carpenter DM, Blalock SJ et al (2015) The impact of co-morbidity on health-related quality of life in rheumatoid arthritis and osteoarthritis patients. Clin Exp Rheumatol 33:366–374
Bavière W, Deprez X, Houvenagel E et al (2020) Association between comorbidities and quality of life in psoriatic arthritis: results from a multicentric cross-sectional study. J Rheumatol 47:369–376. https://doi.org/10.3899/jrheum.181471
Author information
Authors and Affiliations
Contributions
Conceptualization: ST, Mona KN, and KMS. Investigation, all authors. Data curation and formal analysis: KMS and Mohammed KN. Writing—original draft: ST. Writing—review and editing: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was conducted in compliance with the principles of the Helsinki Declaration [9], and the study protocol was approved by the Institutional Research Board of the Faculty of Medicine at Mansoura University (approval registration number: R.24.01.2476). A waiver of consent to review medical records was received. All participants and their parents were informed of the objectives and scope of the research and their rights. Informed written consent was obtained from all parents and participants of 12 years and older.
Consent for publication
Not applicable.
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tharwat, S., Nassar, M.K., Salem, K.M. et al. Extraarticular manifestations of juvenile idiopathic arthritis and their impact on health-related quality of life. Clin Rheumatol 43, 2295–2305 (2024). https://doi.org/10.1007/s10067-024-07008-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-024-07008-0