Abstract
Objectives
The purpose of this study was to investigate the expression of T-cell immunoglobulin and ITIM domain (TIGIT) in peripheral circulation of primary Sjögren’s syndrome (pSS) and its role in the development of pSS.
Methods
The expression of TIGIT on T cells, B cells, natural killer (NK) cells, and CD14 + monocytes was detected by flow cytometry in pSS and healthy control (HC). The correlations between expression of TIGIT and clinical features and laboratory parameters of pSS were analyzed. Meanwhile, we analyzed the change in expression of TIGIT before and after treatment, and its role in the prognosis of pSS treatment was evaluated.
Results
The expression of TIGIT on CD3 + , CD4 + , and CD8 + T cells increased and decreased on CD14 + monocytes in pSS compared to HC; however, there was no significance of B lymphocytes and NK cells. The correlation analysis between the expression of TIGIT on T lymphocytes and CD14 + monocytes and clinical features of pSS showed that the decrease in TIGIT expression on CD14 + monocytes was more closely related to pSS. The expression of TIGIT + CD14 + monocytes negatively correlated with the disease activity of pSS. The expression of TIGIT + CD14 + monocytes of pSS with arthralgia, fatigue, decayed tooth, xerostomia, interstitial lung disease, anti-Ro52 positive, and high IgG decreased compared to that in negative patients. Furthermore, it was significantly lower in active patients than in nonactive patients. After treatment, the expression of TIGIT + CD14 + monocytes tended to increase.
Conclusion
Our study suggested that decreased TIGIT expression on CD14 + monocytes was associated with the clinical manifestations, disease activity, and prognosis of pSS patients. TIGIT + CD14 + monocytes may present as a potential target and a biomarker of the prognosis for immunomodulatory therapy in pSS.
Key Points • The expression of TIGIT+CD14+ monocytes significantly decreased in pSS patients compared to HC. • There was a negative correlation between TIGIT+CD14+ monocytes and the disease activity of pSS. • TIGIT+CD14+ monocyte expression was associated with the clinical manifestations, autoantibodies, IgG, and prognosis of pSS patients. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary Sjögren’s syndrome (pSS) is a chronic and idiopathic inflammatory autoimmune disease characterized by hyperactivation of lymphocytes and progressive damage to exocrine glands [1]. The histological characteristic of pSS is lymphocytic infiltration of the salivary and lacrimal glands. Moreover, activated T cells participate in the pathogenesis of pSS by producing proinflammatory cytokines and inducing the activation of B cells [2]. pSS is also a systemic disease. In addition to the impaired functions of exocrine glands, other organs may also be involved, with clinical manifestations such as purpura, arthritis, ILD, autoimmune hepatitis, renal tubular acidosis, and nervous system and blood system impairment. Some patients have the abovementioned systemic injuries, while others have systemic symptoms such as fatigue, fever, and weight loss.
To date, the pathogenesis of pSS is unclear. However, studies have shown that T-cell hyperactivation may play a key role in the pathogenesis of pSS [3]. The balance between stimulation and inhibition is indispensable for T-cell activation, and costimulation and coinhibition signals are the key regulators of this balance [4]. T-cell immunoglobulin and ITIM domain (TIGIT) plays an important role in immune regulation and is a newly discovered coinhibitory receptor and a transmembrane glycoprotein. This transmembrane glycoprotein consists of three domains, which are an extracellular Ig variable domain, a type I transmembrane domain, and a cytoplasmic tail that includes one immune-receptor tyrosine-based inhibitory motif (ITIM) and one immunoglobulin tyrosine tail (ITT)-like phosphorylation motif. The cytoplasmic tail of TIGIT initiates an inhibitory signal cascade reaction; thus, the expression and signal conduction of TCR are decreased [5]. The ligand of TIGIT is CD155, which was previously identified as a poliovirus receptor (PVR) and later as an immunoglobulin molecule and participates in cell adhesion, proliferation, invasion, and migration [6].
TIGIT is an immune checkpoint coinhibitor that is mainly expressed on T cells and NK cells. It can promote the secretion of IL-10, mediate the inhibitory signal on dendritic cells (DC) and macrophages through CD155, and make them immune-tolerant. As a negative costimulatory molecule, TIGIT exerts its immunosuppressive function by competitively binding its ligands with CD226 [7]. Recently, research data have indicated that TIGIT is involved in the pathogenesis of multiple autoimmune diseases [8,9,10,11,12,13].
However, the relationship between abnormal expression of TIGIT in peripheral circulation and clinical features of pSS is not completely clear. In this study, we focused on TIGIT expression in the peripheral blood of pSS patients and analyzed its correlation with clinical manifestations and laboratory parameters of pSS. It was found that the decrease in TIGIT expression on monocytes was closely related to the clinical features of pSS. Therefore, we investigated the relationship between TIGIT expression on monocytes and the clinical manifestations, autoantibodies, disease activities, and prognosis of pSS and established a foundation for exploring the potential role of TIGIT expression on monocytes in the pathogenesis of pSS in the future.
Materials and methods
Patients and healthy controls
From April 2022 to October 2022, 45 pSS patients (these patients were diagnosed as pSS for the first time and without therapy) were recruited from the Department of Rheumatology and Immunology, The First Affiliated Hospital of Soochow University, and all patients met the classification criteria of primary Sjögren’s syndrome of ACR/European League Against Rheumatism (EULAR) in 2016 [1]. We excluded any patients with a history of head and neck radiotherapy, chronic hepatitis C (HCV), human immunodeficiency virus infection (HIV), sarcoidosis, amyloidosis, graft-versus-host disease (GVHD), IgG4-related diseases, and other rheumatic diseases. In addition, 25 individuals (age- and sex-matched) without chronic and inflammatory disease were all recruited from the physical examination center as healthy controls (HCs). The selection criteria of HCs were as follows: no previous autoimmune disease history, no tumor history and related family history, no symptoms of dry mouth and eyes, and no chronic liver virus infection. We collected and recorded the clinical data of all pSS patients, including clinical manifestations and laboratory parameters, and evaluated disease activity scores, such as ESSDAI and ESSPRI [14, 15]. All participants provided informed consent, and this study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University in 2020 (Ethical No. 2020105).
Flow cytometric analysis
To characterize lymphocytes and monocytes with different TIGIT phenotypes, 50 µl of whole blood samples from the heparinized fresh venous blood of pSS patients and HCs was incubated with anti-CD3-FITC (Biolegend, USA), anti-CD14-FITC (Biolegend, USA), anti-CD56-FITC (Biolegend, USA), anti-CD4 PE/Cy5 (Biolegend, USA), anti-CD8 PE/Cy5 (Biolegend, USA), anti-CD19-PE/Cy5 (Biolegend, USA), and anti-TIGIT-PE/Cyanine7 (Biolegend, USA) at 4 ℃ in the dark for 30 min according to the manufacturer’s instructions. Then, 200 µl/test of erythrolysin (1 ×) was added to the samples with anti-human monoclonal antibodies, and all samples were shaken with an oscillator and placed in a thermostat incubator at 37 ℃ to fully lyse erythrocytes. Then, 2 ml/test sheath fluid was added to all samples for washing and centrifugation, and the waste liquid was carefully poured off. Next, 500 µl/test of sheath fluid was added to resuspend leukocytes to obtain leukocyte suspension for flow cytometry. All samples were processed by flow cytometry (FC500, Beckman Coulter, USA), and the results were analyzed by FlowJo 7.6 software.
The isotype antibodies that we used in our study were as follows: Mouse IgG2a FITC (Biolegend, USA), Mouse IgG1κ (Biolegend, USA), Mouse IgG1 FITC (Biolegend, USA), Mouse IgG1 PE/Cy5 (Biolegend, USA), Mouse IgG2a PE/Cy5 (Biolegend, USA), and Mouse IgG2b PE/Cy5 (Biolegend, USA).
Statistical analysis
FlowJo 7.6 software was used to process and analyze the data of flow cytometry, and GraphPad Prism (version 8.0.2) software was used for statistical analysis and graphic presentations. The Shapiro–Wilk test was used to test the normality of the data (n < 50). Normal distribution data are represented by mean ± standard deviation, and skewed distribution data are represented by median (minimum number, maximum number). T-test was used for the data with normal distribution. The Mann–Whitney U-test was used for the data without normal distribution. The Pearson correlation coefficient analysis was used for the data with normal distribution, and the Spearman correlation coefficient analysis was used for the data without normal distribution. The statistical significance was determined as P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
Clinical characteristics and laboratory parameters of included participants
A total of 45 pSS patients and 25 HC were recruited. Meanwhile, no significant differences were found in age or sex between pSS patients and HC (P > 0.05). The clinical and laboratory characteristics of the enrolled participants are presented in Table 1.
The expression of TIGIT increased on T cells but decreased on CD14 + monocytes in pSS patients
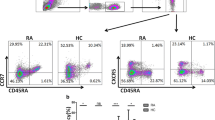
To investigate the expression profile of TIGIT in pSS patients and HC, we used flow cytometry to detect TIGIT expression on CD3 + T lymphocytes, CD4 + T lymphocytes, CD8 + T lymphocytes, CD19 + B lymphocytes, NK cells, and monocytes. Representative FACS plots are shown in Fig. 1A. The results showed that a significantly increased expression of CD3 + TIGIT + T cells was observed in pSS patients compared to HC (27.40% (5.26%, 74.20%) vs. 20.33% (3.98%, 79.80%), P = 0.025) (Fig. 1B). A markedly increased expression of CD4 + TIGIT + T cells was found in pSS patients compared to HC (32.75% (11.63%, 84.10%) vs. 23.55% (7.88%, 81.10%), P = 0.026) (Fig. 1C). We also found that the expression of CD8 + TIGIT + T cells was higher in pSS patients than in HC (30.45% (9.86%, 83.80%) vs. 23.46% (2.10%, 78.60%), P = 0.02) (Fig. 1D). Interestingly, the TIGIT expression on CD14 + monocytes decreased notably in pSS patients compared to HC (56.90% (14.05%, 88.60%) vs. 70.30% (51.99%, 79.00%), P = 0.0015) (Fig. 1G). However, there was no significant difference in TIGIT expression on CD19 + B lymphocytes and NK cells between pSS patients and HC (3.31% (0.64%, 83.30%) vs. 3.00% (1.20%, 72.60%), P = 0.9927 and 19.32% (6.05%, 51.16%) vs. 21.75% (4.21%, 60.90%), P = 0.4072) (Fig. 1E, F). The gating strategies for Fig. 1 are shown in supplementary Fig. 1.
The expression of TIGIT on T lymphocytes, B lymphocytes, NK cells, and monocytes in the peripheral blood of pSS patients and healthy control. A Representative FACS plots. B–D TIGIT expression on CD3 + (B), CD4 + (C), and CD8 + (D) T lymphocytes in pSS patients and HC. E TIGIT expression on B lymphocytes in pSS patients and HC. F TIGIT expression on NK cells in pSS patients and HC. G TIGIT expression on CD14 + monocytes in pSS patients and HC (all of the above used Mann–Whitney U-test statistics. *P < 0.05, **P < 0.01)
Correlation between the TIGIT expression on T cells and CD14 + monocytes and disease activity in pSS patients
According to the above results, we found that the expression of TIGIT on T lymphocytes and CD14 + monocytes was statistically significant between pSS patients and HC. Thus, we analyzed the correlations of TIGIT expression on T lymphocytes and CD14 + monocytes with disease activity in pSS. In this study, the clinical indicators related to the disease activity of pSS mainly included ESSPRI, serum globulin, ESR, IgG, and C4. The specific correlation coefficient and statistical significance are shown in Table 2. The expression of TIGIT on CD14 + monocytes in pSS patients was negatively correlated with ESSPRI (r = − 0.5013, P = 0.0008), serum globulin (r = − 0.5213, P = 0.0006), ESR (r = − 0.5272, P = 0.0011), and IgG (r = − 0.4867, P = 0.0023). However, it was positively correlated with C4 (r = 0.518, P = 0.0014). The above results indicated that TIGIT expression on CD14 + monocytes was negatively correlated with the disease activity and severity of pSS.
Significance of TIGIT expression on CD14 + monocytes in the clinical features of pSS patients
According to the above results, we found that the expression of TIGIT + CD14 + monocytes was closely related to the disease activity of pSS. And then, we analyzed the difference in TIGIT expression on CD14 + monocytes between different clinical features in pSS patients. We compared the difference in TIGIT expression on CD14 + monocytes between pSS patients with and without arthralgia, fatigue, decayed tooth, xerostomia, xerophthalmia, and ILD. Moreover, it was found that the expression of TIGIT on CD14 + monocytes with arthralgia and fatigue decreased significantly in pSS patients (45.36% ± 6.37% vs. 59.93% ± 3.43%, P = 0.0330 and 48.79% ± 3.52% vs. 68.86% ± 5.55%, P = 0.0043; Table 3). Similarly, the expression of TIGIT on CD14 + monocytes with decayed tooth, xerostomia, and ILD was obviously lower in pSS patients than that without decayed tooth, xerostomia, and ILD in pSS patients (43.93% ± 6.10% vs. 62.90% ± 3.24%, P = 0.0054; 48.91% ± 3.32% vs. 70.40% ± 10.75%, P = 0.0318, and 43.60% ± 5.98% vs. 61.94% ± 3.40%, P = 0.0078; Table 3). However, compared with pSS without xerophthalmia, the expression of TIGIT on CD14 + monocytes with xerophthalmia only slightly decreased in pSS patients, and there was no statistical significance (52.81% ± 4.24% vs. 56.39% ± 5.27%, P = 0.6078; Table 3). Our findings demonstrated that the decrease in TIGIT expression on CD14 + monocytes was closely related to the clinical features of pSS.
The expression of TIGIT on CD14 + monocytes decreased in positive anti-SSA/Ro52 and high IgG in pSS patients
Next, we analyzed the difference in the expression of TIGIT + CD14 + monocytes in pSS patients with autoantibodies positive and high IgG and patients with autoantibodies negative and normal IgG. The expression of TIGIT + CD14 + monocytes was significantly lower in anti-SSA/Ro52 positive pSS patients than in anti-SSA/Ro52 negative patients (50.11% ± 3.57% vs. 71.60% ± 6.35%, P = 0.0099; Table 3). Similarly, it also remarkably decreased in pSS patients with high IgG compared with patients with normal IgG (43.25% ± 4.44% vs. 62.30% ± 3.83%, P = 0.003; Table 3). In contrast, the expression of TIGIT + CD14 + monocytes only slightly decreased in anti-SSA/Ro60 positive or anti-La/SSB positive patients compared with anti-SSA/Ro60-negative (53.20% ± 3.79% vs. 57.17% ± 7.62%, P = 0.5636; Table 3) or anti-La/SSB-negative patients (51.54% ± 4.61% vs. 57.27% ± 4.92%, P = 0.4046; Table 3), and there was no statistical significance.
The expression of TIGIT + CD14 + monocytes was negatively correlated with disease activity in pSS patients
Furthermore, we divided pSS patients into an active group (ESSDAI ≥ 5) and nonactive group (ESSDAI < 5) according to the ESSDAI score [15]. We found that the expression of TIGIT + CD14 + monocytes was negatively correlated with disease activity in pSS patients (r = − 0.4122, P = 0.0082; Fig. 2A). In addition, compared with the nonactive pSS patients, we further observed that the expression of TIGIT on CD14 + monocytes of active pSS patients decreased significantly, and the difference was statistically significant (47.81% ± 20.06% vs. 64.47% ± 16.84%, P = 0.0089; Fig. 2B). These results further demonstrated that the TIGIT expression on CD14 + monocytes was negatively correlated with the disease activity and disease severity of pSS.
Correlation of TIGIT expression on CD14 + monocytes with disease activity in pSS patients. A The expression of TIGIT on CD14 + monocytes in pSS patients was negatively correlated with ESSDAI (Spearman correlation coefficient test). B The expression of TIGIT on CD14 + monocytes was significantly lower in active pSS than non-active patients (P = 0.0089) (t-test, *P < 0.05.). C After treatment, TIGIT expression on CD14 + monocytes increased in 6 pSS patients and decreased in 1 patient, and the difference was not statistically significant (P = 0.25) (t-test)
Afterwards, 7 pSS patients who received treatment were followed up for at least 2 months. The therapies in 7 pSS patients were as follows: hydroxychloroquine (HCQ) 0.2 g/d and prednisone 5 mg/d were used in three patients, HCQ 0.2 g/d in two patients, TGP (Total Glucosides of Paeonia) 1.2 g/d in one patient, and methylprednisolone 12 mg/d and hydroxychloroquine 0.2 g/d in another patient. Subsequently, we evaluated the difference in TIGIT expression on CD14 + monocytes in pSS patients before and after treatment. TIGIT expression on CD14 + monocytes increased in 6 pSS patients but decreased in 1 pSS patient; however, there was no statistical significance (42.43% ± 6.02% vs. 52.04% ± 5.17%, P = 0.25; Fig. 2C).
The ROC curve of TIGIT + CD14 + monocytes to predict the occurrence of pSS
Based on the above results, we speculated that the expression of TIGIT + CD14 + monocytes was closely related to the occurrence and development of pSS disease and negatively related to disease activity. Therefore, we draw the ROC curve of TIGIT + CD14 + monocytes to predict the occurrence of pSS, and the results showed that AUC (Area Under the Curve) = 0.735 and P = 0.0018 (Fig. 3), which further indicated that TIGIT + CD14 + monocytes had a certain diagnostic value for pSS.
Discussion
pSS is a systemic chronic autoimmune disease that damages exocrine glands, especially the salivary glands and lacrimal glands. Its main histological feature is lymphocyte infiltration in glandular tissue, which leads to its dysfunction and destruction. In addition to xerostomia and xerophthalmia, the disease usually shows symptoms such as fatigue and pain, systemic injuries such as arthralgia, ILD, nervous system involvement, renal tubular acidosis, and even an increased risk of lymphoma [16]. The potential pathophysiological mechanism of pSS is still ambiguous, which makes its treatment difficult. Compared with other autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), which have made remarkable progress in treatment in recent years, mainly including biotherapy and the introduction of new treatment strategies, pSS lacks an appropriate treatment scheme. Therefore, it is urgent to find new therapeutic targets for pSS.
Increasing evidence shows that TIGIT plays an important role in the pathogenesis of autoimmune diseases [17,18,19,20,21]. Nevertheless, the expression and mechanism of TIGIT in pSS patients are still unknown, and little research has been done on it. To date, only one study has described TIGIT on T lymphocytes in pSS patients [3]. This study showed that the expression of TIGIT + T cells in pSS patients was significantly higher than that in HC, which was consistent with our results, although our research found that TIGIT + CD14 + monocytes were more closely related to the clinical features of pSS. Similar studies have been reported in SLE and RA. Luo et al. found that TIGIT expression on T lymphocytes increased significantly in the peripheral circulation of SLE and RA patients and was correlated with disease activities [22, 23]. In addition, this team also found that the expression of TIGIT on NK cells in SLE patients was significantly lower than that in HC and was negatively correlated with the disease activity and severity of pSS; therefore, TIGIT was a potential negative regulator of NK cells in SLE patients [13]. Another study showed that the expression of TIGIT was negatively correlated with the IFN-γ secretion ability of NK cells in patients with autoimmune diseases and cancer, which indicated that the low expression of TIGIT in vivo contributed to the increased secretion of inflammatory factors [24].
TIGIT exhibits immunosuppressive functions on immune cells via multiple mechanisms to regulate immunity and maintain immune tolerance [25]. Another study showed that TIGIT knockdown in mice enhanced IFN-γ levels and suppressed IL-10 production [26]. Further elucidation of the expression of TIGIT in the peripheral circulation in pSS patients and its correlation with clinical features will contribute to finding new therapeutic targets for the disease. In our study, we showed that TIGIT expression on CD14 + monocytes decreased significantly in pSS patients compared with HC. In addition, our research revealed that the expression of TIGIT on CD14 + monocytes was negatively correlated with disease activity and the severity of pSS. Similar research has also been reported in RA. The expression of TIGIT + CD4 + T cells in synovial fluid of active RA patients was lower than that of inactive RA, and TIGIT + CD4 + T cells were negatively correlated with RA disease activity. In the RA mouse model study, it was found that TIGIT overexpression significantly reduced the severity of the disease, and early regulation of TIGIT could effectively alleviate the severity of the disease, which suggested the potential therapeutic role of TIGIT in RA patients [27]. Likewise, our research indicated that the TIGIT + CD14 + monocytes may be used as a new potential therapeutic target for the treatment of pSS, and regulating the expression of TIGIT + CD14 + monocytes may contribute to the treatment of pSS.
Based on the above data, we focused on analyzing the differences between TIGIT expression on monocytes and the clinical manifestations in pSS patients. As is known to all, the common clinical manifestations of pSS mainly include xerostomia, xerophthalmia, and decayed tooth caused by lesions of the salivary gland and lacrimal gland, as well as arthralgia, fatigue, and ILD, which involve the whole body [28]. Therefore, we compared the difference in TIGIT expression on CD14 + monocytes in pSS patients with or without the above clinical symptoms. The expression of TIGIT on CD14 + monocytes with these symptoms decreased significantly in pSS patients (statistically significant in patients with arthralgia, fatigue, decayed tooth, xerostomia, and ILD but not statistically significant in pSS patients with xerophthalmia), which indicated that the lower expression of TIGIT on CD14 + monocytes was, the more serious the clinical manifestations of pSS patients were. This phenomenon in which the expression of TIGIT is associated with the severity of autoimmune diseases was also described in SLE. It has been reported that increasing TIGIT expression can suppress the function and activation of CD4 + T cells, which is conducive to alleviating the severity of SLE [29]. This result indirectly suggested that we could ameliorate the clinical symptoms of pSS by regulating the expression of TIGIT + CD14 + monocytes.
The other characteristic of Sjögren’s syndrome is the production of autoantibodies such as anti-SSA/Ro52, anti-SSA/Ro60, anti-SSB/La, and IgG in serum [30]. B lymphocytes are considered major players in SS pathogenesis and its main complication (such as lymphoma). Therefore, we investigated whether the production of autoantibodies and IgG was related to the expression of TIGIT + CD14 + monocytes. It was found that the expression of TIGIT + monocytes in pSS patients with anti-SSA/Ro52-positive and high IgG was significantly lower than that in pSS patients with anti-SSA/Ro52-negative and normal IgG (P < 0.01) (Fig. 4A, B). TIGIT is a coinhibitor molecule that can transmit negative signals to immune cells and inhibit the activation of immune cells and the secretion of cytokines. The decrease in TIGIT expression led to the weakening of its immunosuppressive function [31]. Therefore, the decrease of TIGIT + CD14 + monocytes weakened the inhibition of the biological function of monocytes and enhanced the activation of monocytes. We speculated that the decrease in TIGIT expression enhanced the antigen-presenting function of monocytes and indirectly induced the activation of B cells to secrete proinflammatory factors to promote humoral hyperimmunity and the production of autoantibodies. Yoshimoto et al. had the same viewpoint on the production of autoantibodies and IgG in the study of the correlation between BR3 expression on monocytes and clinical features of pSS [32]. However, this study is a single-center, small-sample observational study; in our study, we only observed a certain correlation between TIGIT + monocytes and pSS, and there are still some problems to be solved, such as how the decrease of TIGIT on monocytes can affect the activation of lymphocytes to produce autoantibodies, how it can influence the disease of pSS, and what is the specific pathogenic mechanism of it in pSS? Therefore, our next study will be carried out in-depth from these aspects.
It was reported that TIGIT combined with CD155 on dendritic cells (DCs) could induce immunosuppressive DCs and indirectly inhibit the function of T cells by abating the phosphorylation level of the signal molecule ERK and then triggering the increased production of IL-10 while reducing the secretion of IL-12 and the expression of CD86 [33]. Hence, we presumed that the biological function of monocytes was enhanced via the decrease in TIGIT + monocytes. This was consistent with some previous studies, that is, blocking TIGIT could enhance the proinflammatory T-cell response and lead to the progression of autoimmune diseases [21, 34]. In addition, we followed up with 7 pSS patients who had been treated for 2 months. We analyzed the changes of the expression of TIGIT + CD14 + monocytes, ESSDAI, and ESSPRI between before and after treatment. After treatment, ESSDAI and ESSPRI decreased (P = 0.042, Supplementary Fig. 2), and by contraries, the expression of TIGIT + CD14 + monocytes increased overall, although there was no significant difference. Therefore, the decrease of TIGIT + CD14 + monocytes reflected the prognosis of pSS to a certain extent. This may be due to the small number of pSS patients and the short duration of follow-up in this study, so it is necessary to expand the sample and long follow-up for further study.
However, there are still some limitations in the present research that are worth mentioning. First, the number of patients enrolled in this study was small. Second, our research is a cross-sectional and observational study, and we only observed that the decrease of expression of TIGIT on CD14 + monocytes was correlated with the clinical features and disease activities of pSS, but further longitudinal studies and in-depth elucidation of the regulatory mechanism of TIGIT expression on monocytes will be needed. Third, we only analyzed the expression of TIGIT on CD14 + monocytes in our study, which lacked of TIGIT expression in different subpopulation of monocytes. In our further study, we will label different subsets of monocytes with anti-CD14 and anti-CD16 antibodies to evaluate TIGIT expression in each subtype of monocytes. Finally, this study was lack of histological research on the salivary glands and lacrimal glands. Next, we reserved the lower lip gland tissue of pSS patients for a histopathological study to further investigate the role of TIGIT in the pathogenesis of pSS.
Conclusion
In summary, our study found that the expression of TIGIT + CD14 + monocytes in pSS patients decreased compared to HC. Moreover, the expression of TIGIT + CD14 + monocytes was related to disease activity, severity, and production of autoantibodies and IgG, indicating that TIGIT + CD14 + monocytes may be a biomarker of pSS disease activity. Thus, this study also provided a new idea for us to find new potential therapeutic targets and biomarkers of pSS.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Negrini S, Emmi G, Greco M (2022) Sjögren’s syndrome: a systemic autoimmune disease. Clin Exp Med 22:9–25
Verstappen GM, Kroese F, Bootsma H (2019) T cells in primary Sjogren’s syndrome: targets for early intervention. Rheumatology (Oxford). https://doi.org/10.1093/rheumatology/kez004
Deng C, Chen Y, Li W et al (2020) Alteration of CD226/TIGIT immune checkpoint on T cells in the pathogenesis of primary Sjogren’s syndrome. J Autoimmun 113:102485. https://doi.org/10.1016/j.jaut.2020.102485
Yasutomi M, Christiaansen AF, Imai N et al (2022) CD226 and TIGIT cooperate in the differentiation and maturation of human Tfh cells. Front Immunol 13:840457. https://doi.org/10.3389/fimmu.2022.840457
Yue C, Gao S, Li S et al (2022) TIGIT as a promising therapeutic target in autoimmune diseases. Front Immunol 13:911919. https://doi.org/10.3389/fimmu.2022.911919
Nandi SS, Gohil T, Sawant SA et al (2022) CD155: a key receptor playing diversified roles. Curr Mol Med 22:594–607. https://doi.org/10.2174/1566524021666210910112906
Preillon J, Cuende J, Rabolli V et al (2021) Restoration of T-cell effector function, depletion of Tregs, and direct killing of tumor cells: the multiple mechanisms of action of a-TIGIT antagonist antibodies. Mol Cancer Ther 20:121–131. https://doi.org/10.1158/1535-7163.MCT-20-0464
Yu Y, Chen Z, Wang Y et al (2021) Infliximab modifies regulatory T cells and co-inhibitory receptor expression on circulating T cells in psoriasis. Int Immunopharmacol 96:107722. https://doi.org/10.1016/j.intimp.2021.107722
Battella S, Oliva S, Franchitti L (2019) Finetuning of the DNAM-1TIGIT ligand axis in mucosal t cells and its dysregulation in pediatric inflflammatory bowel diseases (IBD). Mucosal Immunol 2019(12):1358–69
Yang M, Liu Y, Mo B et al (2019) Helios but not CD226, TIGIT and Foxp3 is a potential marker for CD4+Treg cells in patients with rheumatoid arthritis. Cell Physiol Biochem 52(5):1178–9252. https://doi.org/10.33594/000000080
Lucca LE, Axisa PP, Singer ER et al (2019) TIGIT signaling restores suppressor function of Th1 Tregs. JCI Insight 4(3). https://doi.org/10.1172/jci.insight.124427
Wang FF, Wang Y, Wang L et al (2018) TIGIT expression levels on CD4+ T cells are correlated with disease severity in patients with psoriasis. Clin Exp Dermatol 43:675–682. https://doi.org/10.1111/ced.13414
Luo Q, Li X, Fu B et al (2018) Decreased expression of TIGIT in NK cells correlates negatively with disease activity in systemic lupus erythematosus. Int J Clin Exp Pathol 11(5):2408–2418
Seror R, Ravaud P, Bowman SJ et al (2010) EULAR Sjögren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren’s syndrome. Ann Rheum Dis 69:1103–1109. https://doi.org/10.1136/ard.2009.110619
Seror R, Theander E, Brun JG et al (2015) Validation of EULAR primary Sjogren’s syndrome disease activity (ESSDAI) and patient indexes (ESSPRI). Ann Rheum Dis 74:859–866. https://doi.org/10.1136/annrheumdis-2013-204615
Sarrand J, Baglione L, Parisis D et al (2022) The involvement of alarmins in the pathogenesis of Sjögren’s syndrome. Int J Mol Sci 23:5671. https://doi.org/10.3390/ijms23105671
Zhou H, Li B, Li J et al (2019) Dysregulated T cell activation and aberrant cytokine expression profile in systemic lupus erythematosus. Mediators Inflamm 2019:8450911–8450947. https://doi.org/10.1155/2019/8450947
Dean JW, Peters LD, Fuhrman CA et al (2020) Innate inflammation drives NK cell activation to impair Treg activity. J Autoimmun 108:102417. https://doi.org/10.1016/j.jaut.2020.102417
Felix KM, Teng F, Bates NA et al (2019) P2RX7 deletion in T cells promotes autoimmune arthritis by unleashing the Tfh cell response. Front Immunol 10:411. https://doi.org/10.3389/fimmu.2019.00411
Muhammad F, Wang D, McDonald T et al (2020) TIGIT(+) A2Ar-Dependent anti-uveitic Treg cells are a novel subset of Tregs associated with resolution of autoimmune uveitis. J Autoimmun 111:102441. https://doi.org/10.1016/j.jaut.2020.102441
Liu S, Sun L, Wang C et al (2019) Treatment of murine lupus with TIGIT-Ig. Clin Immunol 203:72–80. https://doi.org/10.1016/j.clim.2019.04.007
Luo Q, Deng Z, Xu C et al (2017) Elevated expression of immunoreceptor tyrosine-based inhibitory motif (TIGIT) on T lymphocytes is correlated with disease activity in rheumatoid arthritis. Med Sci Monit 23:1232–41. https://doi.org/10.12659/MSM.902454
Luo Q, Ye J, Zeng L et al (2017) Elevated expression of TIGIT on CD3 + CD4 + T cells correlates with disease activity in systemic lupus erythematosus. Allergy Asthma Clin Immunol 13:15. https://doi.org/10.1186/s13223-017-0188-7
Wang F, Hou H, Wu S et al (2015) TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol 45:2886–2897. https://doi.org/10.1002/eji.201545480
Zhao J, Li L, Yin H et al (2023) TIGIT: an emerging immune checkpoint target for immunotherapy in autoimmune disease and cancer. Int Immunopharmacol 120:110358. https://doi.org/10.1016/j.intimp.2023.110358
Lozano E, Dominguez-Villar M, Kuchroo V et al (2012) The TIGIT/CD226 axis regulates human T cell function. J Immunol 188:3869–3875. https://doi.org/10.4049/jimmunol.1103627
Zhao W, Dong Y, Wu C et al (2016) TIGIT overexpression diminishes the function of CD4 T cells and ameliorates the severity of rheumatoid arthritis in mouse models. Exp Cell Res 340:132–138. https://doi.org/10.1016/j.yexcr.2015.12.002
Negrini S, Emmi G, Greco M et al (2022) Sjögren’s syndrome: a systemic autoimmune disease. Clin Exp Med 22:9–25. https://doi.org/10.1007/s10238-021-00728-6
Mao L, Hou H, Wu S et al (2017) TIGIT signalling pathway negatively regulates CD4(+) T-cell responses in systemic lupus erythematosus. Immunology 151:280–290. https://doi.org/10.1111/imm.12715
Stefanski AL, Tomiak C, Pleyer U et al (2017) The Diagnosis and Treatment of Sjögren ’ s Syndrome. Dtsch Arztebl Int 114:354–361
Jantz-Naeem N, Böttcher-Loschinski R, Borucki K, et al. (2023) TIGIT signaling and its influence on T cell metabolism and immune cell function in the tumor microenvironment. Front Oncol 13:1060112. https://doi.org/10.3389/fonc.2023.1060112
Yoshimoto K, Suzuki K, Takei E et al (2020) Elevated expression of BAFF receptor, BR3, on monocytes correlates with B cell activation and clinical features of patients with primary Sjogren’s syndrome. Arthritis Res Ther 22:157. https://doi.org/10.1186/s13075-020-02249-1
Jariwala N, Benoit B, Kossenkov AV et al (2017) TIGIT and Helios are highly expressed on CD4+ T cells in Sézary syndrome patients. J Invest Dermatol 137:257–260. https://doi.org/10.1016/j.jid.2016.08.016
Joller N, Lozano E, Burkett PR et al (2014) Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity (Cambridge, Mass) 40:569–81. https://doi.org/10.1016/j.immuni.2014.02.012
Acknowledgements
We sincerely thank the platform provided by the Jiangsu Institute of Clinical Immunology and Jiangsu Key Laboratory of Clinical Immunology. We are grateful for the help provided by all departments of rheumatology in the First Affiliated Hospital of Soochow University.
Funding
The study was funded by the Science and Technology Programme of Suzhou (SLJ2021009), the Natural Science Key Project of Bengbu Medical College (2022byzd070), the National Natural Science Foundation of China (81873876), and the Gusu Talent Project of Suzhou (nos. GSWS2020011 and GSWS2020018).
Author information
Authors and Affiliations
Contributions
P.Z., CP.L., and X.C. designed the study, conducted the experiment, performed the data analysis, and wrote the manuscript. C.P., W.C., and XY.F. participated in the sample and clinical data collection. YH.Y. and C.S. participated in patient enrollment and disease activity evaluation. Y.S. assisted the experiment of this study. J.W. and CP.L helped optimize the research, proofread the paper, and revised the manuscript. All authors helped with the final approval of the version.
Corresponding authors
Ethics declarations
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jian Wu is the first corresponding author, and Cuiping Liu is the co-corresponding author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, P., Peng, C., Chang, X. et al. Decreased expression of TIGIT on CD14 + monocytes correlates with clinical features and laboratory parameters of patients with primary Sjögren’s syndrome. Clin Rheumatol 43, 297–306 (2024). https://doi.org/10.1007/s10067-023-06759-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06759-6