Abstract
Objective
To reveal the characteristics and potential role of natural killer T-like cells (NKT-like cells) in the pathogenesis of primary Sjögren’s syndrome (pSS).
Methods
Forty-six patients with pSS and 30 healthy subjects were enrolled in the study. The frequencies and cell count of NKT-like cells as well as other lymphocyte subsets were analyzed by flow cytometry. The clinical and laboratory indicators of pSS patients were also collected. Then, the correlation between NKT-like cells and pSS patient manifestations was analyzed by Spearman’s rank test. In addition, NKT-like cells before and after therapy were also compared.
Results
Both the number and the frequencies of NKT-like cells were significantly decreased in pSS patients. The counts of NKT-like cells were positively correlated with CD4+ T cells (r = 0.464, P = 0.001), CD8+ T cells (r = 0.363, P = 0.013), NK cells (r = 0.488, P = 0.001), and IgM levels (r = 0.443, P = 0.002), while negatively correlated with the disease duration (r = − 0.33, P = 0.027). Moreover, after effective therapy, NKT-like cells were recovered both in the cell counts and frequencies.
Conclusion
In pSS, NKT-like cells were fundamentally decreased, potentially contributing to the disease pathogenesis. Modulating the status of NKT-like cells might provide a novel strategy for treating the disease.
Key Points
• NKT-like cells were significantly decreased in pSS patients.
• NKT-like cells were correlated with pSS patient manifestations.
• NKT-like cells might be serverd as a new marker for assessing the status of pSS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary Sjögren’s syndrome (pSS) is an autoimmune disease accompanied with dry mouth, dry eyes, and multiple organ and system damage. The pathogenesis of pSS is still not fully understood, but lymphocytes infiltrating into exocrine glands may play an important role in this disease. T cells are found to be a large part of the lymphocytes which infiltrate in salivary and lacrimal gland tissues, especially in the early stage of pSS[1]. In pSS, B cells are all known as an important element that participated in the activation of autoimmunity and the formation of autoantibodies [2]. NK cells are the first-line defense against exogenous and endogenous molecules, whose dysfunction also contributes to the initiation of the inflammatory process in exocrine glands of pSS patients [3].
Natural killer T-like cells (NKT-like cells), firstly named by Godfrey [4] in 2004, are a distinct lineage of lymphocytes which express the marker of both CD3 + T cells and CD56 + NK cells [5]. Different from classical NKT cells and non-classical NKT cells, NKT-like cells are a distinct lineage of NKT cells which are CD1d-independent and produce abundant IFN-γ [4].
NKT-like cells have the potential function of both T cells and NK cells [5, 6], including regulating the Th1 and Th2 balance and against infection and tumor, and serve as a bridge of innate immune and adaptive immune [7, 8]. Recently, dysfunction of NKT-like cells was reported in autoimmune diseases. In SLE, the number of circulating NKT-like cells was decreased and these cells maintained the inflammation in active SLE by upregulating granzyme B and TNF-α [9, 10]. NKT-like cells were also found to be able to produce cytokines that related to systemic sclerosis [11]. In pSS, the level and role of NKT-like cells remain inconsistent [12,13,14,15].
In this study, we detected both the proportion and cell count of NKT-like cells in pSS patients, and analyzed the correlation of these cells with clinical and laboratory features, trying to explore the characteristics and potential involvement of NKT-like cells in pSS.
Materials and methods
Study subject
Forty-six patients with primary Sjögren’s syndrome (pSS) were enrolled at the Department of Rheumatology and Immunology, Peking University People’s Hospital, during August to December in 2019. All patients fulfilled the 2016 American College of Rheumatology classification criteria for pSS [16] and had taken different corticosteroids immunosuppressants. Thirty healthy donors were selected as the healthy controls. All subjects were collected in accordance with a protocol approved by the ethical committee of Peking University People’s Hospital.
Sample preparation
Whole blood sample were collected from 46 pSS patients and 30 healthy donors for the flow cytometric analysis and routine blood test. Meanwhile, sera were collected from 46 pSS patients for other laboratory tests.
Flow cytometric analysis
According to the instruction, 50μL whole blood were added into two flow tubes, followed by staining with 10μL anti-CD3/-CD8/-CD45/-CD4 with different fluorescence and anti-CD3/-CD16/-CD56/-CD45/-CD19 antibodies, respectively. After 15 min, the mixture was incubated with 500μL hemolysin (purchased from BD company) for 15 min at room temperature. Finally, flow cytometry instrument (Mindray BriCyte E6 flow meter) was used to collect and analyze cells.
Other laboratory tests
The serum samples were collected for the following serologic parameters: C-reaction protein (CRP), immunoglobulins (IgG, IgA, IgM), complements (C3, C4), RF, D-dimer, erythrocyte sedimentation rate (ESR), ANA, SSA, SSB, and anti-α-fodrin. CRP, immunoglobulins, complements, and D-dimer were examined by immunonephelometry method. ESR and IgM-RF were measured by Westergren’s method and the rate nephelometry, respectively. ANA, anti-SSA/SSB, and anti-α-fodrin were detected by indirect immunofluorescence, western blotting, and enzyme-linked immunosorbent assay, respectively. While, white blood cells (WBC) and plates (PLT) were obtained from whole blood and detected by blood routine measurement.
Statistical analysis
SPSS 16.0 was applied to analyze the data. All data were analyzed by the test of normal distribution. For normally distributed data, results were expressed as mean ± SD. Meanwhile differences between groups were analyzed by Student t test. For nonparametric data, results were expressed as median (range 25 to 75%) values, and Mann–Whitney U test was used for comparison between the two groups. Spearman’s analysis was used for the correlation analysis. Moreover, multivariable analysis was then used to compare variables that showed p < 0.05 by single variable. For all statistical analyses, two-tailed p values less than 0.05 were considered statistically significant.
Results
Demographic and clinical features of the pSS patients
All the 46 pSS patients in the study were female (100%), with ages spanning from 29 to 77 years. The disease duration varied from 5 to 13 years (median, 7 years). These patients demonstrated different clinical manifestations, with 43 having dry mouth (93.48%), 40 having dry eyes (86.96%), 15 having salivary gland involvement (32.61%), 14 having arthritis (30.43%), 4 having liver disease (8.7%), and 19 having pulmonary fibrosis disease (41.3%). Different positive rates for ANA, anti-SSA, anti-SSB, and anti-α-fodrin were also shown in these patients. Approximately 36.9% of the patients were taking regular corticosteroids, while some were receiving methotrexate, azathioprine, or mycophenolate (Table 1).
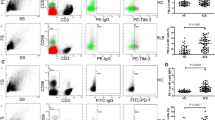
Reduced frequency and number of NKT-like cells in pSS patients
The number and frequency of NKT-like cells in pSS patients and healthy individuals were analyzed by detecting a group of triple-positive cells for anti-CD3/CD16/CD56 cells. As shown in Fig. 1, the frequency of NKT-like cells was significantly decreased in pSS patients (pSS vs. HC: 3.99% ± 0.37% vs 8.41% ± 1.10%, P < 0.001). Since the number of lymphocytes in pSS patients was significantly decreased (Supplementary Fig. 1), the number of NKT-like cells was further dampened. The mean number of NKT-like cells in the 46 pSS patients was 51 cells/μL, which was fundamentally lower than that in the 30 healthy controls (196 cells/μL, P < 0.001). All these indicate the diminished NKT-like cells in pSS patients.
Correlation of NKT-like cells with other lymphocytes
The correlation of NKT-like cells with other lymphocytes was then analyzed. The results showed that the cell count of NKT-like cells was positively correlated with that of CD4+ T cells (r = 0.464, P = 0.001), CD8+ T cells (r = 0.363, P = 0.013), and NK cells (r = 0.488, P = 0.001), respectively (Fig. 2). However, the correlation between the cell count of NKT-like cells and B cells was not statistically significant. Moreover, there was no significant correlation between NKT-like cells and other lymphocytes in the cell frequency.
Correlation of NKT-like cells with laboratory features of pSS patients
The correlation between NKT-like cells and the clinical and laboratory parameters of pSS patients was further analyzed. Detailed results are shown in Table 2. The results showed that the cell count of NKT-like cells was significantly negatively correlated with the disease duration (r = − 0.330, P = 0.027). Moreover, the cell count of NKT-like cells was positively related to IgM levels (r = 0.443, P = 0.002). In the cell proportion, there was no significant correlation between NKT-like cells and other laboratory features (data not shown).
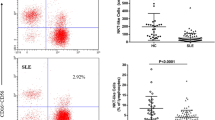
Recovery of NKT-like cells in pSS patients after therapy
We next sought to test whether NKT-like cells could be recovered in pSS patients after treatment. Whole blood was collected from 8 patients before and after medication for 3 to 6 months, and the proportion and cell number of NKT-like cells were analyzed. As shown in Fig. 3, in pSS patients, NKT-like cells were increased significantly in both the proportion and cell count after therapy, recovering to almost normal levels.
Discussion
In this study, we revealed that the frequencies of NKT-like cells were significantly decreased in pSS patients, which was in line with Zhao’s [14] and Sudzius’s studies [15]. Moreover, we also analyzed the cell count of NKT-like cells and achieved even more obvious findings. The cell count of NKT-like cells was significantly lower in pSS patients than that in the HC group, which was in agreement with previous reports [15, 17]. However, some research teams reported that NKT-like cells were increased in pSS patients [12, 13]. The discrepancies among these studies might be potentially due to the patients studied with different disease courses and background therapies. Notably, in this study, we revealed that the cell count of NKT-like cells was significantly negatively related to the disease course. This indicates that the number of NKT-like cells would be further significantly decreased with the progression of pSS.
The correlation of NKT-like cells with other lymphocytes was conducted in this research. The result showed that the cell count of NKT-like cells was positively related to CD4T cells, CD8T cells, and NK cells, respectively. Nevertheless, there was no relationship between the cell count of NKT-like cells and B cells. These results were in line with the previous report [15] and suggested that NKT-like cells may originate from both NK cells and T cells [4, 5]. In the cell proportion, we found no significant correlation between NKT-like cells and any subpopulation of lymphocytes, which was not completely consistent with Sudzius’s [15] study.
We observed that the cell count of NKT-like cells was significantly positively correlated with IgM. As well known, IgM is an indicator of recent infection and it participates in both innate immunity and adaptive immunity [18,19,20]. Besides the potential function of T cells and NK cells, NKT-like cells may serve as a bridge between the innate and adaptive immunity through IgM, which needs further study.
In addition, NKT-like cells were further studied after therapy in pSS patients. The results showed that NKT-like cells can increase significantly after drug treatment for 3 or 6 months both in the cell number and the cell proportion. This indicates that NKT-like cells may be a potential marker for assessing the status of pSS.
Several limitations may be in this study. The results were from a single-center study with relatively small sample sizes, which may lead to bias. Further large-scale, multicenter studies will be required to confirm the alteration of NKT-like cells in pSS. In addition, the results only focused on the number and frequency of NKT-like cells. In-depth functional analyses of these cells would further reveal their role in the pathogenesis of pSS.
In summary, we demonstrated that the number and frequencies of NKT-like cells were decreased significantly in patients with pSS. These cells were negatively correlated with the disease duration, and could be recovered after treatment in pSS. NKT-like cells might be served as a new marker for assessing the status of pSS.
References
Voulgarelis M, Tzioufas AG (2010) Pathogenetic mechanisms in the initiation and perpetuation of Sjogren’s syndrome. Nat Rev Rheumatol 6(9):529–37
Nocturne Gaëtane, Mariette X (2018) B cells in the pathogenesis of primary Sjögren syndrome. Nat Rev Rheumatol 14(3):133–45
Kiripolsky Jeremy, McCabe Liam G, Kramer Jill M (2017) Innate immunity in Sjögren’s syndrome. Clin Immunol 182:4–13
Godfrey Dale I, Robson MacDonald H, Kronenberg Mitchell, Smyth Mark J, Van Kaer Luc (2004) NKT cells: what’s in a name? Nat Rev Immunol. 4(3):231–7
Kronenberg Mitchell (2005) Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 23:877–900
Golden-Mason Lucy, Castelblanco Nicole, O’Farrelly Cliona, Rosen Hugo R (2007) Phenotypic and functional changes of cytotoxic CD56pos natural T cells determine outcome of acute hepatitis C virus infection. J Virol 81:9292–8
Kaer LV, Parekh VV, Wu L (2011) Invariant natural killer T cells: bridging innate and adaptive immunity. Cell & Tissue Research 343(1):43–55
Torina Alessandra, Guggino Giuliana, Manna Marco Pio La, Sireci Guido (2018) The Janus face of NKT cell function in autoimmunity and infectious diseases. International Journal of Molecular Sciences 19(2):440
Syh-Jae Lin, Ji-Yih Chen, Ming-Ling Kuo , Hsiu-Shan Hsiao, Pei-Tzu Lee, Jing-Long Huang (2016) Effect of interleukin-15 on CD11b, CD54, and CD62L expression on natural killer cell and natural killer T-like cells in systemic lupus erythematosus. Mediators of Inflammation 2016:9675861
Lin SJ, Kuo ML, Hsiao HS, Lee PT, Lee WI, Chen JY, Huang JL (2019) Cytotoxic function and cytokine production of natural killer cells and natural killer T-like cells in systemic lupus erythematosis regulation with interleukin-15. Mediators Inflamm 2019:4236562
Cossu Marta, van Bon Lenny, Nierkens Stefan, Bellocchi Chiara, Santaniello Alessandro, Dolstra Harry, Beretta Lorenzo, Radstake Timothy R D J (2016) The magnitude of cytokine production by stimulated CD56+ cells is associated with early stages of systemic sclerosis. Clin Immunol 173:76–80
Szodoray P, Papp G, Horvath IF, Barath S, Sipka S, Nakken B, Zeher M (2009) Cells with regulatory function of the innate and adaptive immune system in primary Sjögren’s syndrome. Clin Exp Immunol 157:343–9
Szodoray P, Gal I, Barath S, Aleksza M, Horvath IF, Gergely P Jr, Szegedi G, Nakken B, Zeher M (2008) Immunological alterations in newly diagnosed primary Sjögren’s syndrome characterized by skewed peripheral T-cell subsets and inflammatory cytokines. Scand J Rheumatol 37:205–12
Zhao Liling, Wenwen Xu, Chen Zhilei, Zhang Haoze, Zhang Shuo, Lian Chaofeng, Sun Jinlei, Chen Hua, Zhang Fengchun (2021) Aberrant distribution of CD3+CD56+ NKT-like cells in patients with primary Sjögren’s syndrome. Clin Exp Rheumatol 39(1):98–104
Sudzius Gintaras, Mieliauskaite Diana, Siaurys Almantas, Viliene Rita, Butrimiene Irena, Characiejus Dainius, Dumalakiene Irena (2015) Distribution of peripheral lymphocyte populations in primary Sjögren’s syndrome patients. J Immunol Res 2015:854706
Shiboski Caroline H, Shiboski Stephen C, Seror Raphaèle, Criswell Lindsey A, Labetoulle Marc, Lietman Thomas M, Rasmussen Astrid, Scofield Hal, Vitali Claudio, Bowman Simon J, Mariette Xavier, International Sjögren’s Syndrome Criteria Working Group (2016) American College of Rheumatology/European League Against Rheumatism Classifification Criteria for Primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017(69):35–45
Davies R, Hammenfors D, Bergum B, Jakobsen K, Solheim M, Vogelsang P, Brun JG, Bryceson Y, Jonsson R, Appel S (2017) Patients with primary Sjögren’s syndrome have alterations in absolute quantities of specific peripheral leucocyte populations. Scand J Immunol 86:491–502
Panda Saswati, Ding Jeak L (2015) Natural antibodies bridge innate and adaptive immunity. Immunol 194(1):13–20
Rapaka Rekha R, Ricks David M, Alcorn John F, Chen Kong, Khader Shabaana A, Zheng Mingquan, Plevy Scott, Bengtén Eva, Kolls Jay K (2010) Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med 207(13):2907–19
Liu Jun, Wang Ying, Xiong Ermeng, Hong Rongjian, Qing Lu, Ohno Hiroshi, Wang Ji-Yang (2019) Role of the IgM Fc receptor in immunity and tolerance. Front Immunol 10:529
Funding
This work was supported by grants from the Research and Development project of Peking University People’s Hospital (RDC2019-01 to Dr. Yingni Li), the National Natural Science Foundation of China (81971523 and 81671604 to Dr. F. Hu, 81871281 to Dr. Yuan Jia), the Beijing Nova Program (Z181100006218044 to Dr. F. Hu), and the Clinical Medicine Plus X-Young Scholars Project of Peking University (PKU2021LCXQ014 to Dr. F. Hu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures performed in studies involving human participants were in accordance with a protocol approved by the ethical committee of Peking University People’s Hospital. All the participants gave written informed consent.
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, X., Li, Q., Li, Y. et al. Diminished natural killer T-like cells correlates with aggravated primary Sjögren’s syndrome. Clin Rheumatol 41, 1163–1168 (2022). https://doi.org/10.1007/s10067-021-06011-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-06011-z