Abstract
Microscopic polyangiitis (MPA) is a systemic small-vessel vasculitis associated with anti-neutrophil cytoplasmic antibody (ANCA) and predominantly causes kidney and pulmonary injuries. Subarachnoid hemorrhage, a life-threatening manifestation of the central nervous system (CNS), rarely occurs in patients with ANCA-associated vasculitis (AAV). We report the case of a young man with spontaneous SAH recurrence and active nephritis. The patient was treated with a glucocorticoid pulse and intravenous cyclophosphamide (CTX) in combination with decreasing cerebral perfusion pressure and analgesic therapy. All the patients’ symptoms except the proteinuria resolved. We reviewed the clinical characteristics of 34 previously reported cases of SAH with AAV, comprising six cases of MPA, eight cases of granulomatosis with polyangiitis (GPA), and 19 cases of eosinophilic granulomatosis with polyangiitis (EGPA), and one case of unclassified AAV. All the cases showed features of active vasculitis. Concomitant nephritis and peripheral neuropathy were found in the MPA and EGPA cases with SAH, respectively. Renal and pulmonary manifestations were predominant in the patients with GPA and SAH. Ten patients had aneurysmal abnormalities, and six patients had cardiac abnormalities. Thirty-one patients were treated with glucocorticoids, and 18 patients received concurrent immunosuppressants. Patients with SAH had a mortality rate of 38.2%. The presence of cerebrovascular events or cardiac involvement in patients with AAV and SAH is associated with increased mortality of 64.3%. Our study indicates that SAH should be cautioned as a disease occurring in patients with AAV. Early diagnosis with aggressive immunosuppressive therapy can help improve the prognosis of patients with SAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) are a group of systemic necrotizing vasculitides that affect predominantly small vessels such as capillaries, venules, and arterioles. AAV includes granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). It is more common in those aged over 60 years males, especially in East Asiaz [1]. The clinical manifestations of AAV largely depend on the affected vasculature. The lungs, kidneys, and skin are typically affected organs [2]. CNS manifestations have rarely been reported [3] in patients with AAV. A few cases have demonstrated stroke, hypertrophic pachymeningitis, massive intracerebral hemorrhage (ICH), SAH, and spinal SAH [4, 5] in MPA. EGPA presents with four distinct neurological characteristics, including cerebral ischemic lesions, ICHs, cranial nerve palsies, and loss of visual acuity [6]. CNS involvement is characterized by pachymeningitis, cerebral ischemic lesions, hemorrhagic lesions, and hypophyseal lesions in patients with GPA [7]. Here, we report a patient who presented with SAH as the initial symptom of MPA and who experienced a relapse of SAH. We also reviewed the clinical characteristics of 34 previously reported cases of AAV with SAH.
Case presentation
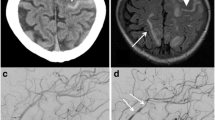
A 31-year-old male with a 2-day history of acute headache and fever was admitted to our department. He complained of “tearing” back pain following a sudden sneeze, neck rigidity, and diffuse back pain, preventing him from lying down. The patient had no history of trauma or hypertension. Physical examination revealed blood pressure of 123/83 mmHg and a regular pulse rate of 82 beats/min. The patient had nuchal rigidity with positive Kernig’s and Brudzinski’s signs. Laboratory examinations revealed a white blood cell (WBC) count of 13.22 × 109/L (normal range: 3.5–9.5 × 109/L), a hemoglobin level of 151 g/L, and a platelet count of 293 × 1012/L. Urinalysis showed proteinuria (24-h urine protein 5.9 g), microscopic hematuria (urine sediment red blood cells 254.0/μL), and cast (pathological renal tubules of 1.0/μL). The creatinine level was 115.0 μmol/L (normal range: 57–97 μmol/L), eGFR level was 72.6 mL/min, with an increased erythrocyte sedimentation rate (ESR) of 34 mm/H (normal range: 0–20 mm/H) and a slightly increased C-reactive protein (CRP) of 12.7 mg/L (normal range: 0–10 mg/L). The tests revealed a positive p-ANCA and an elevated myeloperoxidase-ANCA (anti-MPO) level of 3.26 (normal range: normal < 1). Pulmonary computed tomography (CT) scan revealed multiple focal emphysema in bilateral lungs, bullae, and tiny ground-glass nodules in the lower lobe of the right lung. Brain CT and magnetic resonance imaging/magnetic resonance angiogram did not show bleeding, aneurysm, or malformation. A lumbar puncture revealed bloody cerebrospinal fluid (CSF) (Fig. 1a), with a pressure of 180 mmH2O, elevated protein of 2708.8 mg/L, a glucose level of 0.79 mmol/L, whereas blood glucose level of 4.1 mmol/L, chloride level of 121.0 mmol/L, red blood cell of 52,200 × 106/L, and normal WBC count. Cerebrospinal fluid x-pert and metagenomic next-generation sequencing (mNGS) were negative, ruling out the presence of CNS infection. He was diagnosed with SAH.
Seven years earlier, the patient presented with a severe headache and vomiting. On examination, his blood pressure was 135/75 mmHg, and his pulse rate was 78 beats/min. Laboratory testing showed an increased CRP of 53.9 mg/dL and an ESR of 70 mm/h. Urinalysis showed microscopic haematuria (2 +) and proteinuria (1.78 g/24 h). Emergent cranial CT revealed SAH, and cerebral digital subtraction angiography was performed, which did not reveal any aneurysms or arteriovenous malformations. Based on his positive MPO-ANCA and renal biopsy findings with pauci-immune necrotizing glomerulonephritis and tubulointerstitial inflammation (Fig. 2), a diagnosis of MPA was made, in accordance with 2012 revised International Chapel Hill Consensus Conference Nomenclature of vasculitides [8]. The patients’ spontaneous intracranial SAH was attributed to MPA. He was administered prednisone (60 mg/day) combined with intravenous pulse CTX administration for 6 months and then switched to mycophenolate mofetil (MMF 1.0 g/day) for maintenance immunosuppression. The patient achieved complete remission in 12 months with normal urinalysis and serum creatinine level without neurologic sequelae.
In conjunction with the medical history of the patient, the recurring symptoms of fever and an increase in the urine protein, ESR, CRP, and MPO-ANCA were attributed to active vasculitis. The patient was given intravenous methylprednisolone (0.5 g) daily for 3 days and then tapered to oral prednisone at a dose of 1 mg/kg/day combined with nimodipine and analgesic therapy, followed by an intravenous injection of 0.8 g CTX. His symptoms were relieved within 2 weeks. The second lumbar puncture showed yellow cerebrospinal fluid (Fig. 1b), a pressure of 120 mmH2O, protein of 1056.3 mg/L, glucose level of 3.47 mmol/L, chloride level of 111.0 mmol/L, and normal WBC count. The patient was discharged from the hospital with no neurological symptoms. During the 6-month follow-up, the patient was treated with intravenous CTX every month with prednisone tapering to 10 mg/day. The patient was in good condition, and all symptoms except proteinuria had resolved.
Literature review
The review is based on a literature search of PubMed, Web of Science, and Embase databases up to December 2021. The following MeSH terms or keywords were used: “microscopic polyangiitis,” “granulomatosis with polyangiitis,” “Churg-Strauss syndrome,” “eosinophilic granulomatosis with polyangiitis,” “anti-neutrophil cytoplasmic antibody-associated vasculitis,” and “subarachnoid hemorrhage” without language restrictions. Case reports and case series of patients with the diagnoses of AAV and SAH were eligible for inclusion. Publications were excluded if they did not meet the above criteria, if AAV overlapped other connective tissue diseases, or if they were review articles with no clinical case reports. Our literature review identified 143 citations, 73 were not relevant, and 37 were duplicate records. Ultimately, we included 33 reports with 34 cases.
Discussion
The clinical presentation of AAV depends on the affected vessels, with mostly kidney and lung involvement. Mononeuritis multiplex [1] is the most frequent neurological manifestation of AAV. In contrast, CNS involvement is uncommon, with 5–15% in AAV [9] and 2–8% in MPA [10], including cerebrovascular events, such as hypophysitis, posterior reversible encephalopathy syndrome, isolated mass lesions, hypertrophic pachymeninges, and spinal cord lesions [11]. A retrospective study found that cerebral ischemic lesions were the main manifestations in Chinese patients with AAV [12]. The co-occurrence of AAV and SAH is uncommon and has not been fully elucidated. We describe a rare manifestation of MPA in a young man who presented with relapsing SAH. The patient had no history of hypertension, aneurysm, or arteriovenous malformations, without an increased risk of SAH. SAH was considered to be due to active vasculitis. He received a glucocorticoid pulse and intravenous CTX in combination with decreasing intracerebral hemorrhage (ICH) therapy, achieving remission at follow-up.
A noncontrast CT scan is a sensitive method to identify patients with subarachnoid hemorrhage. But CT imaging depends on patients presenting within 6 h of onset of acute headache and exhibits inadequate sensitivity to detect spontaneous SAH [13]. Lumbar puncture has been found to show evidence of hemorrhage in 3% of patients with a normal head CT [14]. Four of the 34 cases with negative CT were confirmed to have SAH using a lumbar puncture.
Interestingly, the patient showed lower glucose levels in CSF, which frequently accompany intracranial infection. However, an extensive evaluation, including mNGS of CSF, excluded the diagnoses of infection. Hypoglycorrhachia in CSF following SAH has seldom been reported and is associated with multiple reasons [15]. Alterations in the carrier transport system of glucose in and out of the CSF, caused by diffuse meningeal inflammation, increase anaerobic glycolysis. Vasospasm accounts for a decrease in CSF glucose levels.
We reviewed the literature and summarized the clinical characteristics and treatment of 34 cases with SAH (Table 1). Among the 34 cases, six were attributable to MPA, eight to GPA, 19 to EGPA, and one to unclassified AAV. Their ages ranged from 17–85 years, and 55.9% of them were women. The disease duration was up to 20 years. Three patients presented with SAH as the initial symptom of AAV. Three patients experienced a recurrence of SAH. Nephritis was the major non-CNS system disorder in MPA. EGPA was associated with more concomitant peripheral neuropathy. Renal and pulmonary manifestations were more common in patients with GPA and SAH. Ruptured saccular aneurysms are the main cause of nontraumatic SAHs [12]. As illustrated by the cases, only ten patients with aneurysmal SAH and two patients with intracranial artery dissection had similar incidences in different types of AAV. All the cases appeared to have evidence of active vasculitis, organ or life-threatening features, including active glomerulonephritis, progressive peripheral or cranial neuropathy, and gastrointestinal and cardiac disease due to vasculitis. Other manifestations included arthritis, myalgia, rhinosinusitis, skin vasculitis, pulmonary nodules, and asthma. They were accompanied by enhanced high-titer ANCA, elevated inflammatory factors, or increased eosinophilic granulocytes.
Patients with concomitant other CNS manifestations or cardiac abnormalities contributed substantially to the overall mortality. Ten patients had one or more cerebrovascular events, one with combined idiopathic late-onset cerebellar ataxia, two with cerebral infarction, six with ventricular hemorrhage, two with occipital hematoma, and spinal epidural hematoma in one patient. Cardiac abnormalities were observed in six patients with AAV and SAH, with four cases causing lethality.
SAH is often associated with a poor outcome, with a mortality rate of over 50%, irrespective of treatment [12]. In the case series, all patients with SAH had a mortality rate of 38.2%. Thirty-one patients were treated with glucocorticoids, and 18 patients also received immunosuppressive therapy. CTX was the most commonly used immunosuppressant. Three patients received rituximab (RTX) treatment and achieved remission. Patients with SAH benefited from combined therapy with corticosteroids and immunosuppressants. All cases of AAV with SAH had a mortality rate of 38.2% and benefited from combined therapy with corticosteroids and immunosuppressants. However, the data demonstrated that concomitant cerebrovascular events or cardiac involvement in patients with AAV and SAH could progressively deteriorate the prognosis with a mortality rate of 64.3%.
Conclusion
Our study suggests that SAH is a rare severe manifestation and associated with active AAV, which should be considered in patients with AAV due to the high rate of fatality, even in patients with a negative CT scan. Early diagnosis and immunosuppressive therapy are crucial to achieving a favorable prognosis.
References
Almaani S, Fussner LA, Brodsky S, Meara AS, Jayne D (2021) ANCA-associated vasculitis: an update. J Clin Med 10(7)
Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Charles Jennette J, Nachman PH (2005) Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody–associated small-vessel vasculitis. Ann Intern Med 143:621–631
Pancharovski T, Patibandla S, Lin AN, Liu YX, Green S, Pendharkar SS (2019) Recognizing central nervous system involvement as a progressive feature of microscopic polyangiitis: a diagnostic dilemma. Am J Med 132(9):e673–e676
Andre R, Cottin V, Saraux JL, Blaison G, Bienvenu B, Cathebras P et al (2017) Central nervous system involvement in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): report of 26 patients and review of the literature. Autoimmun Rev 16(9):963–969
De Luna G, Terrier B, Kaminsky P, Le Quellec A, Maurier F, Solans R et al (2015) Central nervous system involvement of granulomatosis with polyangiitis: clinical-radiological presentation distinguishes different outcomes. Rheumatol (Oxford) 54(3):424–432
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F et al (2013) 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 65(1):1–11
Wludarczyk A, Szczeklik W (2016) Neurological manifestations in ANCA-associated vasculitis- assessment and treatment. Expert Rev Neurother 16(8):861–863
Izgi E, Gedikli Y, Ogul H (2019) Granulomatosis with polyangiitis presents with sinus changes and brainstem lesions. Br J Hospital Med (Lond England : 2005) 80(9):i
Zheng Y, Zhang Y, Cai M, Lai N, Chen Z, Ding M (2018) Central nervous system involvement in ANCA-associated vasculitis: what neurologists need to know. Front Neurol 9:1166
Ma TT, Li ZY, Geng YS, Chen M, Zhao MH (2020) Central nervous system involvement in patients with antineutrophil cytoplasmic antibody-associated vasculitis: a study of 29 cases in a single Chinese center. Clin Rheumatol 39(7):2185–2193
Hutchinson PJ, Kirkpatrick PJ (2012) Headache. Diagnosing subarachnoid hemorrhage: are CT scans enough? Nat Rev Neurol 8(3):126–127
Kairys N, J MD, Garg M (2022) Acute subarachnoid hemorrhage. StatPearls Publishing, Treasure Island (FL)
Hutchinson PJ, Kirkpatrick PJ (2012) Headache. Diagnosing subarachnoid hemorrhage: are CT scans enough? Nature reviews Neurology 8(3):126–127
Sassi SB, Ghorbel IB, Mizouni H, Houman MH, Hentati F (2011) Microscopic polyangiitis presenting with peripheral and central neurological manifestations. Neurol Sci 32(4):727–729
Frederick M, Vincet MD (1981) Hypoglycorrhachia after subarachnoid hemorrhage. Neurosurgery 8(1):7–9
Aratani S, Sakai Y, Tsuruoka S (2017) A Case of Microscopic Polyangiitis with Subarachnoid Hemorrhage and Cardiovascular Complications. J Nippon Med Sch 84:251–254
Wang X, Wang J (2015) Microscopic polyangiitis presenting as spontaneous subarachnoid haemorrhage. Nephrology (Carlton) 20(2):110
Kimura H, Akutsu N, Shiomi R, Kohmura E (2012) Subarachnoid hemorrhage caused by ruptured intracranial fusiform aneurysm associated with microscopic polyangiitis. Neurol Med Chir (Tokyo) 7(52):495–498
Baldwin L, Poller D, Ellison D (2001) February 2001: A 74 year old man with a history over 3 months of increasing dyspnea and malaise. Brain Pathology 11(3):389–390, 393
Katsuhito Ihara MK, Yamamuro Megumi, Inoshita Seiji (2019) Microscopic polyangiitis associated with subarachnoid hemorrhag. J Rural Med 14(1):125–131
Sakura M, Takahashi S, Urabe A, Hosokawa R (2016) An autopsy of microscopic polyangiitis with death from subarachnoid hemorrhage. Nephrology 21:279
Marnet D, Ginguene C, Marcos A, Cahen R, Mac Gregor B, Turjman F et al (2010) Wegener granulomatosis and aneurysmal subarachnoid hemorrhage: an insignificant association? Neurochirurgie 56(4):331–336
Venning MC, Burn DJ, Bashir SH, Deopujari CE, Mendelow AD (1991) Subarachnoid haemorrhage in Wegener’s granulomatosis, with negative four vessel angiography. Br J Neurosurg 5:195–198
Cruz DN, Segal AS (1997) A patient with Wegener’s granulomatosis presenting with a subarachnoid hemorrhage: case report and review of CNS disease associated with Wegener’s granulomatosis. Am J Nephrol 17(2):181–186
Fomin S, Patel S, Alcasid N, Tang X, Frank E (2006) Recurrent subarachnoid hemorrhage in a 17 year old with wegenergranulomatosis. J Clin Rheumatol 12(4):212–213
Nardone R, Lochner P, Tezzon F (2004) Wegener’s granulomatosis presenting with intracerebral hemorrhages. Cerebrovasc Dis (Basel, Switzerland) 17(1):81–82
Miles JD, McWilliams L, Liu W, Preston DC (2011) Subarachnoid hemorrhage in Wegener granulomatosis: a case report and review of the literature. CNS Spectr 16(6):121–126
Takei H, Komaba Y, Kitamura H, Hayama N, Osawa H, Furukawa T et al (2004) Aneurysmal subarachnoid hemorrhage in a patient with Wegener’s granulomatosis. Clin Exp Nephrol 8(3):274–278
Lee MXW, Teng GG, Raju GC, Lim AYN (2017) Catastrophic subarachnoid hemorrhage in eosinophilic granulomatosis with polyangiitis without asthma. Int J Rheum Dis 20(12):2127–2131
Calvo-Romero JM, del Carmen Bonilla-Gracia M, Bureo-Dacal P (2002) Churg-Strauss Syndrome Presenting as Spontaneous Subarachnoid Haemorrhage. Clin Rheumatol 21(3):261–263
Sakamoto S, Ohba S, Eguchi K, Shibukawa M, Kiura Y, Okazaki T et al (2005) Churg-Strauss syndrome presenting with subarachnoid hemorrhage from ruptured dissecting aneurysm of the intracranial vertebral artery. Clin Neurol Neurosurg 107(5):428–431
Matsuda S, Yoshida S, Fujiki Y, Satomi H, Takeuchi T, Hirose Y et al (2018) Eosinophilic granulomatosis with polyangiitis complicated by subarachnoid hemorrhage and coronary vasculitis: a case report and review of the literature. Rheumatol Int 38(4):689–696
Southam C, Hahn C (2019) Intracerebral and spinal subarachnoid hemorrhage in eosinophilic polyangiitis. The Canadian Journal of Neurological Sciences Le Journal Canadien des Sciences Neurologiques 46(4):475–476
Maloon A, Fritz VU, Kaplan CL (1985) Neurological complications of systemic vasculitis. A report of 2 cases. S Afr Med J 68(8):603–605
Shimizu K, Ohoba H, Shimada H, Inoue Y, Jinn Y, Yoshimura N (2011) A case of Churg-Strauss syndrome with subarachnoid hemorrhage and left phrenic nerve paralysis. Nihon Kokyuki Gakkai Zasshi 49(9):642–646
Tyvaert L, Devos P, Deloizy M, Belhadia A, Stekelorom T (2004) Manifestations neurologiques périphériques et centrales révélatrices d’un syndrome de Churg et Strauss. Revue Neurologique 160(1):89–92
Diamanti L, Berzero G, Bini P, Ravaglia S, Rognone E, Cavagna L et al (2014) Spinal hemorrhage in eosinophilic granulomatosis with polyangiitis (Churg-Strauss). J Neurol 261(2):438–440
Go MH, Park JU, Kang JG, Lim YC (2012) Subarachnoid and intracerebral hemorrhage in patients with churg-strauss syndrome: two case reports. J Cerebrovasc Endovasc Neurosurg 14(3):255–261
Sheerin UM, Barreto J, Brown MM, Brew S, Losseff NA (2008) Subarachnoid haemorrhage as the first clinical manifestation of Churg-Strauss syndrome. J Neurol 255(4):607–608
Menditto VG, Di Rienzo A, De Nicola M, Balzano L, Polonara S (2013) Subarachnoid haemorrhage from PICA aneurysm rupture in a Churg-Strauss patient: a case report and a review of the literature. Clin Neurol Neurosurg 115(2):197–199
Chang Y, Kargas SA, Goates JJ, Horoupian DS (1993) Intraventricular and subarachnoid hemorrhage resulting from necrotizing vasculitis of the choroid plexus in a patient with Churg-Strauss syndrome. Clin Neuropathol 12(2):84–87
Ito M, Kato N, Ching-Chan S, Kayama T (2014) A case of Churg-Strauss syndrome with subarachnoid hemorrhage. Brain Nerve 66(3):283–288
Taormina G, Andolina G, Banco MA, Costanza-Gaglio EJ, Bonura A, Buscemi S (2014) An uncommon presentation of eosinophilic granulomatosis with polyangiitis: a case report. Journal of Medical Case Reports 13(9):190
Muraishi K, Sugita K, Fujiwara S, Suzuki J, Izumiyama T, Okazaki T (1988) Allergic granulomatous angiitis with subarachnoid hemorrhage–a case report. No Shinkei Geka Neurological Surgery 16(5 Suppl):463–467
Lazaro Romero A, Carilla Sanroman A, Horna Canete L, Serrano Ponz M (2021) Spontaneous spinal epidural haematoma and nonaneurysmal subarachnoid haemorrhage in a patient with eosinophilic granulomatosis with polyangiitis. Neurologia (Engl Ed) 36(9):723–725
Mrackova J, Holeckova I, Rohan V, Tupy R, Mracek J, Geier P (2020) Eosinophilic granulomatosis with polyangiitis-an uncommon cause of intracerebral and subarachnoid hemorrhage: a case report. Int J Stroke 15(1 SUPPL):670
Lescuyer Sylvain R-LM, Martinez C, Rakotoarivelo H-N, Weber J-C (2016) Subarachnoid haemorrhage in patient with eosinophilic granulomatosis with polyangiitis (Churg and Strauss) A probable central nervous localisation of vasculitis. International Journal of Brain Disorders and Treatment 7(5):273–275
Harland TA, Seinfeld J, Cava LF, Neumann RT, Roark C, Kumpe D et al (2019) Anti-neutrophil cytoplasmic antibody associated central nervous system vasculitis with brain and spinal cord subarachnoid hemorrhage: a rare case report and review of the literature. J Clin Neurosci 62:253–255
Funding
This study was funded by the Sanming Project of Medicine in Shenzhen (SZSM201612080).
Author information
Authors and Affiliations
Contributions
The case was diagnosed and followed up by Jingjing Xie, Suli Wang, Ye Yu, and Jia Li. Jingjing Xie conceived and planned the case report. Ertao Jia, Zhiling Li, and Jianyong Zhang performed material preparation, data collection, and analysis. Jingjing Xie wrote the initial draft of the manuscript. Jia Li revised and edited the manuscript. The final version was read, corrected, and approved by both authors, and both agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Consent to participate
The patient signed a written informed consent form for the publication of the results of this case study.
Conflict of interest
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, J., Jia, E., Wang, S. et al. Relapsing subarachnoid hemorrhage as a clinical manifestation in microscopic polyangiitis: a case report and literature review. Clin Rheumatol 41, 3227–3235 (2022). https://doi.org/10.1007/s10067-022-06163-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06163-6