Abstract
Eosinophilic granulomatosis with polyangiitis (EGPA) is characterized by necrotizing vasculitis of small-sized vessels with extravascular granulomas and eosinophilic infiltration. The case of a 48-year-old Japanese woman with EGPA, who presented concurrently with subarachnoid hemorrhage (SAH) and coronary vasculitis, is reported. She initially presented with bronchial asthma, and then 8 months later she developed various symptoms caused by systemic eosinophilic vasculitis and was admitted to our hospital. Three days after admission, she started oral corticosteroid therapy, and her 2009 Five-Factor Score (FFS) was 0. However, she developed an SAH, followed by coronary vasculitis 1 day later. With extensive treatment with a combination of betamethasone, cyclophosphamide, intravenous immunoglobulin, and rituximab, her systemic vasculitis improved dramatically. This seems to be the first case of EGPA with SAH and coronary vasculitis. In previous reports of EGPA with SAH, 4 of 11 cases developed SAH as an exacerbation of systemic vasculitis during remission induction therapy. The present patient also had SAH during remission induction therapy. However, the period between bronchial asthma and SAH was only 8 months. This is the shortest among case reports of EGPA with SAH. In addition, the present patient rapidly developed coronary vasculitis. These findings suggest that EGPA causes SAH and coronary vasculitis as early complications of systemic vasculitis. In EGPA, it is necessary to pay careful attention to rapid changes of disease activity, even when the FFS indicates a good prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA) was defined by the Chapel Hill Consensus Conference as an eosinophil-rich and necrotizing granulomatous inflammation often involving the respiratory tract and necrotizing vasculitis affecting small- to medium-sized vessels [1]. EGPA is characterized clinically by the appearance of bronchial asthma and eosinophilia [1]. EGPA is known to target small-sized vessels more frequently than medium-sized vessels [2]. EGPA typically presents with bronchial asthma, allergic rhinitis, nasal and sinus polyposis, and mononeuropathy multiplex. The clinical course of EGPA is classified into three sequential phases. The first is the prodromal phase, presenting with asthma, rhinosinusitis, and nasal polyposis. The second is the eosinophilic phase, characterized by peripheral blood and tissue eosinophilia. The third is the vasculitic phase, with purpura, peripheral neuropathy, and constitutional symptoms. The prodromal phase often persists for many years, and the period between the prodromal phase and the vasculitic phase is known to be an average of 3 years [3]. The present report describes an EGPA patient who concurrently had SAH and coronary vasculitis early during steroid-based remission-induction therapy. The vasculitic involvements in the central nervous system and coronary arteries are discussed along with a review of previous case reports.
Methods
The review was based on a literature search for articles concerning SAH in EGPA published from 1951 through May 2015. Using the Medline database (PubMed, National Library of Medicine, Bethesda, MD; keywords: Churg-Strauss syndrome, eosinophilic granulomatosis with polyangiitis, and subarachnoid hemorrhage), 11 cases of EGPA with SAH were identified. In this report, a total of 12 cases, including previous cases and the present one, were reviewed.
Case report
A 48-year-old Japanese woman with cough and paroxysmal dyspnea was clinically diagnosed with bronchial asthma. She had no history of hypertension, heart disease, diabetes, dyslipidemia, or smoking before the onset of bronchial asthma. Two months later, she also developed paranasal sinusitis. Although she was treated with inhaled and nasal corticosteroids, her symptoms failed to improve. Five months later, she complained of arthralgias in her hands, followed by fever, fatigue, and paralysis of both feet. Seven months later, she had purpura with a bullous eruption on the right foot. She was admitted to our hospital with persistent fever, arthralgias, and purpura on both legs 8 months after the diagnosis of bronchial asthma.
Peripheral blood tests showed a remarkable increase in the eosinophil count to 11,960/µL. Antinuclear antibody was negative, and myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA) was slightly increased to 4.1 U/mL (< 3.5 U/mL). Urinalysis showed no microscopic hematuria. Nerve conduction studies showed a decrease in the amplitude of the sensory nerve action potentials in bilateral sural nerves. Computed tomography (CT) of the paranasal sinuses showed that the left maxillary sinus was filled with fluid. Chest CT showed ground-glass opacity in the left upper lobe. A skin biopsy of the purpuric lesion of the lower limb was performed, and the pathological examination showed necrotizing small-vessel vasculitis with perivascular eosinophilic infiltration without granulomas in the dermis and subcutaneous fat tissue (Fig. 1).
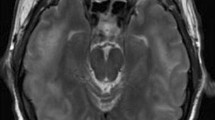
Given the asthmatic symptoms, peripheral eosinophilia, mononeuropathy multiplex, pulmonary infiltration, paranasal sinus abnormality, and extravascular eosinophils, the diagnosis of EGPA was made according to the American College of Rheumatology criteria [4]. On admission, the electrocardiogram was normal, and the echocardiogram showed no abnormality in wall motion. Brain MRI and MRA showed no aneurysms and no stenoses of the main intracranial arteries. Her 2009 Five-Factor Score (FFS) was 0 [5]. Three days after admission, treatment with oral prednisolone was started with 55 mg daily. The fever, arthralgias, and paresthesiae improved, and the percentage of eosinophils in white blood cell decreased from 76% (11,960/µL) to 5% (345/µL) 6 days after admission.
Nine days after admission, she suddenly complained of a strong headache with nausea and vomiting. Brain CT showed widespread high density in the subarachnoid space, indicating SAH (Fig. 2). Neurosurgeons immediately performed intracranial angiography, but no aneurysms were found. The MPO-ANCA titer was increased from 4.1 U/mL to 6.6 U/mL. These findings suggested that the SAH was caused by central nervous system vasculitis. The patient was given intravenous (IV) cyclophosphamide 750 mg/day (500 mg/m2). Treatment was changed from prednisolone to betamethasone because of the brain edema. Her diastolic blood pressure was controlled at around 80 mmHg with intravenous antihypertensive treatment. The next day, she showed sinus bradycardia, and her electrocardiogram showed negative T waves in leads I, aVL, and V5-6 (Fig. 3). She had no pain or clinical manifestation of heart failure. However, creatinine phosphokinase and brain natriuretic peptide (BNP) were increased to 328 U/L (< 200 U/L) and 1141 pg/mL, respectively. An echocardiogram showed that the inferoposterior wall of the right coronary artery area was hypokinetic, and her left ventricular ejection fraction (LVEF) was decreased to 40% (Fig. 4a), unlike Takotsubo cardiomyopathy which shows ballooning of the left ventricular apex [6]. However, the severity of her illness precluded coronary angiography. Although the possibility that these cardiac symptoms were caused by myocardial infarction based on atherosclerosis could not be excluded, the increase in the MPO-ANCA titer suggested that they were caused by coronary vasculitis [7]. In addition to IV cyclophosphamide, intravenous immunoglobulin (IVIG, 0.4 g/kg/day for 5 days) was given [8]. Also, we used vasodilatory drug, nicorandil, for the treatment of cardiac ischemia.
The patient recovered with no neurological symptoms. Fifteen days after admission, the hypokinesia in the inferoposterior wall disappeared. Her LVEF was improved to about 60% at 20 days after admission (Fig. 4b). BNP values were decreased to 69 pg/mL. Then, IV infusions of 750 mg cyclophosphamide were performed three times every 2 weeks. The dose of betamethasone was gradually decreased, while azathioprine was added at 27 days after admission.
At 44 days after admission, her eosinophilic cell counts had increased, although she took betamethasone 4 mg daily and azathioprine 50 mg daily. Rituximab 375 mg/m2 was given weekly for 4 weeks. After treatment, the MPO-ANCA titer decreased to 1.5 U/mL, and there were no symptoms due to vasculitis. A follow-up CT scan showed disappearance of the sinusitis, ground-glass opacity, and SAH (Fig. 5). She was discharged from the hospital. After discharge from the hospital, negative T waves disappeared and her electrocardiogram became normal.
Discussion
The case of an EGPA patient with SAH and coronary vasculitis early during steroid-based remission induction therapy was presented. This seems to be the first reported case of EGPA with SAH and coronary vasculitis concurrently. In addition, the period between the appearance of bronchial asthma and SAH was only 8 months. This is the shortest among the case reports of EGPA with SAH.
As shown in the Table 1, radiographic abnormalities of the lung were present in 5 of 12 patients with EGPA and SAH [9,10,11,12,13,14,15,16,17,18,19]. These patients showed various abnormalities, such as Loeffler’s pneumonia, ground glass opacity, migratory infiltrates, peripheral consolidation, and interstitial pulmonary infiltrate [9, 10, 13, 17]. The occurrence of these pulmonary involvements is less frequent than that of bronchial asthma [20]. It seemed that there was no remarkable difference in radiographic abnormalities of the lung among EGPA cases with or without SAH [21].
Neurologic complications
Peripheral neuropathy is the most common manifestation of neurologic involvement in EGPA: 75% of patients with EGPA show peripheral neuropathy [22], while only 8% of patients show central nervous system (CNS) involvement [2]. Most cases of CNS involvement had cerebral infarction. On the other hand, only 12 cases of SAH have been reported in the literature, including the present one [9,10,11,12,13,14,15,16,17,18,19]. The occurrence of SAH is rare. SAH in EGPA patients is thought to be caused by the rupture of multiple small aneurysms, which are formed by vasculitis-mediated vulnerability of vascular walls [17]. In Table 1, the clinical information of all EGPA cases with SAH is summarized [9,10,11,12,13,14,15,16,17,18,19]. The proportion of males is 25% and the age range at SAH onset in these patients was from 23 to 64 years. When SAH occurred, 9 of 12 patients also had systemic vasculitis, such as cutaneous vasculitis and peripheral neuropathy.
The findings of cerebral angiography showed an aneurysm, arterial dissection, or vascular irregularity in six patients. On the other hand, four patients showed no abnormalities. Two patients lacked angiography results. The present patient was included among the cases without abnormalities. When the treatment for EGPA in these patients who had already been treated with remission induction therapy was compared, five patients had SAH during the initial remission induction therapy [13,14,15,16]. Three patients developed SAH at least 3 years after finishing the initial remission induction therapy [17,18,19]. The present patient corresponded to the former group. As mentioned above, the onset of SAH varies. The present patient showed rapid deterioration of disease activity soon after occurrence of systemic vasculitis. In addition, only the present patient showed coronary vasculitis with SAH. Five of 12 patients [9, 13, 14, 16, 17] died due to SAH; three of these patients [13, 14, 16] were resistant to combination therapy with glucocorticoids and cyclophosphamide. The occurrence of SAH is very rare, but it is necessary to keep it in mind as one of the fatal complications of EGPA.
Cardiac complications
Cardiac involvement in EGPA is the major cause of death, accounting for 48% of deaths [22]. EGPA involves various cardiac disorders, including cardiomyopathy, myocardial infarction, valvular heart disease, congestive heart failure, pericardial effusion, and acute or chronic constrictive pericarditis [23]. Pericardial effusion and cardiomyopathy are the most common cardiac complications of EGPA. Myocardial damage is reported to be caused by the release of major basic protein from the activated eosinophils. Myocardial ischemia is also related to small-vessel distal coronary vasculitis [24]. On the other hand, medium-vessel coronary vasculitis is less frequent than small-vessel vasculitis [25]. Cottin et al. reported that the frequency of myocardial infarction due to coronary vasculitis was 6% (3 in 48 cases) in ANCA-positive EGPA patients [26]. Although the mechanism of vasculitic lesions in medium-vessel arteries is unclear, the coronary vasculitis causes acute coronary syndrome and can be fatal.
Postmortem examinations have shown that coronary vasculitis is seen in 6 of 10 patients with EGPA, though ante mortem diagnosis of cardiac vasculitis is rare [25]. In the present patient, coronary angiography could not be performed because of the severity of her illness. However, the electrocardiogram and echocardiogram examinations were useful for the clinical diagnosis of coronary vasculitis. This implies the importance of early diagnosis to prevent fatal cardiac damage. Treatment with corticosteroids and cyclophosphamide is reported to be effective in EGPA patients with cardiac involvement [27]. In addition to these treatments, the present patient was treated with IVIG, and the systemic manifestations improved. If the manifestations of systemic vasculitis deteriorate rapidly and severely, it is necessary to treat with such aggressive immunosuppressant therapy.
Utility of FFS
The 2009 FFS is frequently used as a tool to assess the prognosis of patients with EGPA [5]. The FFS score is evaluated based on five points, factoring in age (≥ 65 years), renal insufficiency, gastrointestinal signs, cardiac insufficiency, and otolaryngeal manifestations. This score represents the severity of systemic small-vessel vasculitis. An FFS score > 2 indicates a poor prognosis. However, in all seven EGPA patients with SAH, the FFS score was 0 or 1 [11,12,13,14,15,16]. This shows that the FFS score does not predict the possibility of severe involvement by medium-vessel vasculitis in the central nervous system. However, whether the occurrence of medium-vessel vasculitis such as SAH and coronary vasculitis is related to the severity of systemic small-vessel vasculitis is unknown. This problem should be further investigated in more EGPA cases with SAH. Because SAH and coronary vasculitis are potentially fatal, it is necessary to pay careful attention to these complications in those with a low FFS score.
EGPA is included in ANCA-associated vasculitis, which predominantly affects small-sized vessels [1]. Vasculitis of medium-sized vessels, such as subarachnoid hemorrhage and epicardial coronary vasculitis, occurs less frequently than vasculitis of small-sized vessels in EGPA [9,10,11,12,13,14,15,16,17,18,19, 24]. However, these complications are exceptionally fatal, and it is important to perform proper treatment immediately. The FFS is often used to evaluate the severity of systemic small-vessel vasculitis, and the occurrence of medium-vessel vasculitis cannot be evaluated by the FFS. Fatal medium-vessel vasculitis, such as SAH and coronary vasculitis, could occur even when the FFS score indicates a good prognosis. We should consider the possibility of medium-vessel vasculitis occurring during treatment and pay careful attention to rapid changes in disease activity.
References
Jennette JC, Falk RJ, Bacon PA et al (2013) 2012 revised International Chapel Hill Consensus Conference nomenclature of Vasculitides. Arthritis Rheum 65(1):1–11
Guillevin L, Cohen P, Gayraud Met al (1999) Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine (Baltimore) 78(1):26–37
Lhote F, Cohen P, Guillevin L (1998) Polyarteritis nodosa, microscopic polyangiitis and Churg–Strauss syndrome. Lupus 7(4):238–258
Masi AT, Hunder GG, Lie JT et al (1990) The American College of Rheumatology 1990 criteria for the classification of Churg–Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 33(8):1094–1100
Guillevin L, Pagnoux C, Seror R et al (2011) The Five Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 90(1):19–27
Dote K, Sato H, Tateishi H et al (1991) Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases (article in Japanese). J Cardiol 21(2):203–214
Weiner M, Segelmark M (2016) The clinical presentation and therapy of diseases related to anti-neutrophil cytoplasmic antibodies (ANCA). Autoimmun Rev 15(10):978–982
Tsurikisawa N, Taniguchi M, Saito H et al (2004) Treatment of Churg-Strauss syndrome with high-dose intravenous immunoglobulin. Ann Allergy Asthma Immunol 92(1):80–87
Strauss L, Churg S (1951) Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol 27(2):277–301
Taormina G, Andolina G, Banco MA et al (2014) An uncommon presentation of eosinophilic granulomatosis with polyangiitis: a case report. J Med Case Rep 8:190
Calvo-Romero JM, del Carmen Bonilla-Gracia M, Bureo-Dacal P (2002) Churg-Strauss syndrome presenting as spontaneous subarachnoid haemorrhage. Clin Rheumatol 21(3):261–263
Sheerin UM, Barreto J, Brown MM et al (2008) Subarachnoid haemorrhage as the first clinical manifestation of Churg–Strauss syndrome. J Neurol 255(4):607–608
Chang Y, Kargas SA, Goates JJ et al (1993) Intraventricular and subarachnoid hemorrhage resulting from necrotizing vasculitis of the choroid plexus in a patient with Churg–Strauss syndrome. Clin Neuropathol 12(2):84–87
Go MH, Park JU, Kang JG et al (2012) Subarachnoid and intracerebral hemorrhage in patients with churg-strauss syndrome: two case reports. J Cerebrovasc Endovasc Neurosurg 14(3):255–261
Diamanti L, Berzero G, Bini P et al (2014) Spinal hemorrhage in eosinophilic granulomatosis with polyangiitis (Churg-Strauss). J Neurol 261(2):438–440
Lee MX, Teng GG, Raju GC et al (2015) Catastrophic subarachnoid hemorrhage in eosinophilic granulomatosis with polyangiitis without asthma. Int J Rheum Dis doi. https://doi.org/10.1111/1756-185X.12594 (Epub ahead of print)
Maloon A, Fritz VU, Kaplan CL (1985) Neurological complications of systemic vasculitis. A report of 2 cases. S Afr Med J 68(8):603–605
Sakamoto S, Ohba S, Eguchi K et al (2005) Churg–Strauss syndrome presenting with subarachnoid hemorrhage from ruptured dissecting aneurysm of the intracranial vertebral artery. Clin Neurol Neurosurg 107(5):428–431
Menditto VG, Di Rienzo A, De Nicola M et al (2013) Subarachnoid haemorrhage from PICA aneurysm rupture in a Churg-Strauss patient: a case report and a review of the literature. Clin Neurol Neurosurg 115(2):197–199
Solans-Laqué R, Fraile G, Rodriguez-Carballeira M et al (2017) Clinical characteristics and outcome of Spanish patients with ANCA-associated vasculitides: Impact of the vasculitis type, ANCA specificity, and treatment on mortality and morbidity. Medicine (Baltimore) 96(8):e6083
Cottin V, Bel E, Bottero P et al (2016) Respiratory manifestations of eosinophilic granulomatosis with polyangiitis (Churg–Strauss). Eur Respir J 48(5):1429–1441
Lanham JG, Elkon KB, Pusey CD et al (1984) Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg–Strauss syndrome. Medicine (Baltimore) 63(2):65–81
Pagnoux C, Guillevin L (2005) Cardiac involvement in small and medium-sized vessel vasculitides. Lupus 14(9):718–722
Kane GC, Keogh KA (2009) Involvement of the heart by small and medium vessel vasculitis. Curr Opin Rheumatol 21(1):29–34
Hellemans S, Dens J, Knockaert D (1997) Coronary involvement in the Churg-Strauss syndrome. Heart 77(6):576–578
Cottin V, Bel E, Bottero P et al (2017) Revisiting the systemic vasculitis in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): a study of 157 patients by the Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires and the European Respiratory Society Taskforce on eosinophilic granulomatosis with polyangiitis (Churg-Strauss). Autoimmun Rev 16(1):1–9
Cohen P, Pagnoux C, Mahr Aet al (2007) Churg-Strauss syndrome with poor-prognosis factors: a prospective multicenter trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in forty-eight patients. Arthritis Rheum 57(4):686–693
Author information
Authors and Affiliations
Contributions
Study conception and design: SM. Analysis of skin biopsy: HS and YH.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
External editing support
The author declares that he has no external editing support.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video. An echocardiogram showed that the inferoposterior wall of the right coronary artery area was hypokinetic (AVI 14120 KB)
Rights and permissions
About this article
Cite this article
Matsuda, S., Yoshida, S., Fujiki, Y. et al. Eosinophilic granulomatosis with polyangiitis complicated by subarachnoid hemorrhage and coronary vasculitis: a case report and review of the literature. Rheumatol Int 38, 689–696 (2018). https://doi.org/10.1007/s00296-017-3875-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3875-2