Abstract
Objectives
Antiphospholipid syndrome (APS) is multisystem autoimmune coagulopathy with antiphospholipid antibodies (aPL) in its ground, manifested as a primary disease (PAPS) or in the setting of other conditions, most commonly systemic lupus erythematosus. The objective of this cross-sectional study was to investigate various cardiac manifestations and their possible relation to aPL type and titer in a Serbian cohort of PAPS patients.
Methods
A total of 360 PAPS patients were analyzed and aPL analysis included detection of anticardiolipin antibodies (aCL: IgG/IgM), anti-ß2glycoprotein I (ß2GPI: IgG/IgM), and lupus anticoagulant (LA). Cardiac manifestations investigated were valvular lesions (comprehending valvular thickening and dysfunction not related to age and pseudoinfective endocarditis), coronary artery disease (CAD) with specific insight for myocardial infarction (MI), chronic cardiomyopathy (CMP), and acute decompensated heart failure (ADHF) as well as pulmonary hypertension (PH) and intracardiac thrombus presence.
Results
The prevalence of cardiac manifestations overall was 19.6%. There was a strong association between age and the majority of cardiac manifestations, as well as standard atherosclerotic risk factors. aCL IgG–positive patients had a higher prevalence of valvular lesions (p = 0.042). LA presence was significantly related to MI (p = 0.031) and PH (p = 0.044). CMP and ADHF were significantly related to higher titers of aCl IgG (p = 0.033, p = 0.025 respectively). Age and smoking were independent risk predictors for MI in PAPS with meaningful risk for LA positivity (OR 2.567 CI 0.671–9.820 p = 0.168).
Conclusions
Certain cardiac manifestations in PAPS were related to certain aPL type and/or titer levels, imposing confirmation in prospective studies. Preventive actions, comprehending proper anticoagulant/antithrombotic therapy, and intense action against standard atherosclerotic risk factors are of utmost importance in this group of patients.
Key Points • In Serbian patients with primary antiphospholipid syndrome (PAPS), prevalence of non-criteria cardiac manifestations was 19.6% and they were significantly related to certain antiphospholipid antibodies and titers. • Lupus anticoagulant was a meaningful predictor of myocardial infarction, enabling possible risk stratification and proper preventive and therapeutical strategies in this subgroup of PAPS patients. • Patients with high titers of aCL IgG are more prone to acute decompensated heart failure occurence, imposing careful follow-up of these patients • Based on the analysis of the Serbian PAPS cohort, even being non-criterial, cardiology manifestations are significantly present and inclusion of cardiologists in treatment and follow-up of these patients should be implied from the diagnosis establishment. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antiphospholipid syndrome (APS) or Hughes syndrome represents a systemic autoimmune disorder characterized by arterial and/or venous thrombosis, multiple and recurrent fetal losses, often accompanied by thrombocytopenia and elevated levels of antiphospholipid antibodies (aPL), such as lupus anticoagulant (LA), anticardiolipin antibodies (aCL), and anti- ß2GPI antibodies [1,2,3]. This syndrome is considered primary (PAPS) if unassociated with any other disease, or secondary APS, if presented in the setting of other diseases, most commonly systemic lupus erythematosus [4,5,6,7,8,9,10,11,12,13]. Considering its vascular pathogenesis, it may affect every organ and tissue. Cardiac manifestations are considerable, heterogeneous, and intriguing regarding early detection and treatment, especially in PAPS.
The objective of this cross-sectional study was to observe and investigate associations between various cardiac manifestations and aPL type, profile, and titer in a large cohort of Serbian PAPS patients.
Materials and methods
This study comprehends 360 PAPS patients (283 females and 77 males, mean age 44.0 ± 12.9) who were consecutively included in the national APS register, from the year 2000 in University Hospital Center Bezanijska kosa. All patients have met 2006 revised Sydney criteria for APS which comprises the persistent presence of aPL along with arterial/venous thrombosis (confirmed by peripheral vessels Doppler ultrasound, computed tomography, or echocardiography) and/or pregnancy morbidity (consisted of fetal losses, intrauterine growth restriction, early delivery, oligohydramnios, prematurity, fetal distress, fetal or neonatal thrombosis, pre-eclampsia/eclampsia, HELLP syndrome, placental insufficiency) [14]. One patient in our cohort was diagnosed with catastrophic antiphospholipid syndrome [15]. All APS patients were treated according to current guidelines [16]. The presence of comorbidities, such as arterial systemic hypertension (blood pressure ≥ 140 × 90 mmHg or use of anti-hypertensive drugs) and diabetes (glycated hemoglobin 7% or use of medication) as data on smoking habit, was also evaluated.
Diagnosis of cardiac manifestations
Transthoracic echocardiography (TTE) was performed in all patients using a standardized protocol that included M-mode, 2-dimensional (2-D), and Doppler recordings. Valvular lesions comprehended amplified valvular thickness and/or dysfunction and valvular vegetations, defined as precipitation of thrombus, not containing bacteria, on the valve cusps. The modified Duke criteria utilizing pathologic and clinical criteria were used to differentiate between true infective endocarditis and Libman-Sacks endocarditis [17]. A transesophageal echocardiographic (TEE) study has been performed in all patients with vegetations in order to confirm the diagnosis and establish the severity of the disease. TTE was also used as a diagnostic tool for assessment of left and right heart function, presence of segmental disorders in left ventricle kinetics due to coronary artery disease presence, and for the purpose of estimation of pressures in the pulmonary vasculature. The probability of pulmonary hypertension (PH) was derived comprehending data on tricuspid regurgitant velocity, right ventricular size, interventricular septal function, inferior vena cava diameter fluctuations with respiratory cycle, systolic right atrial area, the pattern of systolic flow velocity and early diastolic pulmonary regurgitant velocity, and diameter of the pulmonary artery [18, 19].
Chronic cardiomyopathy (CMP) was defined as the presence of a progressive disorder that impairs the structure and/or function of the muscles in the ventricles of the heart. Data on the history of previous cardiovascular events were collected from patients’ medical records. The diagnoses of coronary artery disease (CAD) and myocardial infarction (MI) were made by integrating the typical symptoms with electrocardiogram ST-T changes and with the crucial differentiation rendered by troponin I levels: elevated suggested myocardial necrosis, i.e., the establishment of a MI diagnosis. Acute decompensated heart failure (ADHF) was defined as a worsening of symptoms—typically shortness of breath (dyspnea), edema, and fatigue—in a patient with existing heart disease.
This study has been performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients have signed informed consent for participation in this study.
Laboratory tests
All patients were evaluated for the presence aPL, accompanied by routine biochemistry and complete blood cell counts. LA presence was based on the use of two different screening tests: diluted activated partial thromboplastin time and sensitive activated partial thromboplastin time according to ISHT recommendations [20]. aCL: IgG/IgM and aß2GPI: IgG/IgM antibodies were measured by an enzyme-linked immunosorbent assay (ELISA, Binding Site) and expressed in GPL or phospholipid (MPL) units (GPL-U and MPL-U). The level range was considered as low (11–40 PLU/ml), medium (41–99 PLU/ml), or high (> 100 PLU/ml). Following revised laboratory criteria for APS, patients were considered positive as having persistent medium or high titers of aPL on two or more occasions at least 12 weeks apart [21, 22]. Antinuclear antibodies (ANA) were determined by indirect immunofluorescence on mouse liver and HEp-2 cell substrate. Anti-double-stranded DNA (anti-dsDNA) antibodies were determined by ELISA, Binding Site. APS patients were classified into the following categories: category I, the presence of two or more laboratory criteria in combination; category IIa, where lupus anticoagulant is present alone; category IIb, where anticardiolipin antibodies are present alone; and category IIc, where anti-β2GPI are present alone [23].
Statistics
Results are presented as mean ± standard deviation or count (percent) depending on the data type. Groups were compared using t test, Mann–Whitney U test, Pearson chi-square test, or Fisher’s exact test, depending on data type and distribution (for continuous data). Multivariate logistic regression was used to explore the association between a dependent variable and independent predictors. All data were analyzed using SPSS 20.0 (IBM corp.) statistical software. All p-values less than 0.05 were considered significant.
Results
Structure of cardiac manifestations in PAPS and their relationship to standard atherosclerotic risk factors
Cardiac manifestations were observed in almost 20% of patients and the most frequent were valvular lesions and CAD (Table 1). Almost all cardiac manifestations analyzed increased their prevalence with age and the difference between different age groups is highly significant as presented in Table 2. Also, other standard atherosclerotic risk factors were significantly related to the majority of cardiac manifestations. Diabetic PAPS patients were more prone to CAD and CMP (p = 0.026, p = 0.020, respectively), smokers to MI, CAD, CMP, intracardiac thrombus presence, and overall cardiac manifestations (p = 0.018, p = 0.039, p = 0.016, p = 0.009, p = 0.005 respectively), hypertensive PAPS patients to CAD and CMP (p = 0.034, p = 0.022, respectively) while PAPS patients with high levels of serum lipids had significantly higher prevalence of MI and CAD (p = 0.029, p = 0.004, respectively). Valvular lesions were not related to standard atherosclerotic risk factors, except age.
aPL type and profile and their association to cardiac manifestations
The distribution of aPL types and their category in our PAPS cohort is presented in Table 1. LA was with the highest prevalence and the majority of patients were diagnosed with more than one laboratory criterion present (category I).
Furthermore, the association between aPL type presence and certain cardiac manifestations was analyzed. In our study group, aCL IgG–positive patients had a higher prevalence of valvular lesions (p = 0.042). LA presence was significantly related to MI (p = 0.031) and PH (p = 0.044). There was no significant relationship between aPL category and all cardiac manifestations analyzed. Results are presented in Table 3.
Relationship between aPL titers and cardiac manifestations
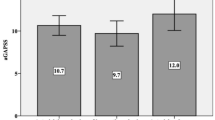
CMP was significantly positively related to aCl IgG titer (p = 0.033) as well as ADHF (p = 0.025) (Fig. 1), and intracardiac thrombus presence (p = 0.006). After distribution of PAPS patients into different groups of low, medium, and high levels of aPL titer, ADHF was significantly related to high titer of aCL IgG (p = 0.001). PAPS patients in this group were also at higher risk for intracardiac thrombus presence (p = 0.014) (Table 4).
Predictors of MI in PAPS
We sought possible predictors of MI development in PAPS. As presented in Table 5, after adjustment for age, gender, LA positivity, smoking, and hyperlipidemia (variables included in the model revealed p values of 0.05 and bellow in univariate analyses), age and smoking were independent predictors for MI presence in PAPS patients. However, LA positivity brought significant risk but without statistical significance in our multivariate model (OR 2.567 CI 0.671–9.820 p = 0.168).
Discussion
The field of PAPS seems to be still intriguing. The possibility that this syndrome might be a part of an autoimmune continuum as early presentation of another autoimmune disease is still under debate. Even without full knowledge of the real nature and course of this multisystem disorder, the presence of various cardiac manifestations is indisputable [24]. Though not fulfilling clinical diagnostic criteria, recently valvular lesions have been accepted as an integral part of PAPS [3]. Furthermore, evidence of CAD in APS is well known and, according to recent studies of Euro-Phospholipid Project Group on 1000 APS patients, the prevalence of acute MI in APS is ranging from 4 to 7%, usually associated with SLE [25].
In this cross-sectional study of a large cohort of Serbian PAPS patients, we analyzed cardiac manifestations and their possible relationship to aPL type, category, and titer. This is the continuation of our previous work, this time focused on PAPS only [26].
Our study revealed that almost one-fifth of all PAPS patients had some manifestation of cardiac involvement. Among them, CAD had the highest prevalence (10.2%), followed by valvular lesions (8.9%). We confirmed that age was significantly related to almost all cardiac manifestations analyzed and that standard atherosclerotic risk factors (smoking predominantly) had a significant impact on some cardiac manifestation in PAPS patients too.
Analyzing aPL profile of the Serbian PAPS population, we revealed that this syndrome is most commonly diagnosed with LA presence (55%) and in the setting of more than one laboratory criterion present (category I, 58.4% PAPS patients). Further analysis revealed that there is a significant relationship between LA presence and MI occurrence a well as PH presence (p = 0.031, p = 0.044 respectively) and a possible link between aCl IgG positivity and valvular lesions (p = 0.041).
According to available literature data, cardiac manifestations are associated with aPL, not fundamentally related to thrombosis, but mechanisms such as inflammation, complement pathway, and platelet activation as well. It is shown that IgG anti-CL/anti-β2GPI antibodies correlate with thrombosis and that there might be some relationship between different aPL types and manifestations [27,28,29,30]. Moreover, in a case–control RATIO study published by Urbanus R et al., aPL were analyzed in young women with different ischemic events. It was concluded that LA was a major risk factor for arterial thrombotic events and that presence of other cardiovascular risk factors increased the risk even further [31]. Indeed, LA represents the most controversial antibody population, illuminating well-known paradox of in vitro prolongation of clotting time connected to the risk of thrombosis rather than bleeding tendency [32]. In our study, age and smoking were significant independent risk factors for MI occurrence but LA positivity brought two and half times higher risk for its development (OR 2.567 CI 0.671–9.820 p = 0.168). Interestingly, different categories of PAPS patients according to Pengo et al. [23] based on laboratory criteria used for establishing APS diagnosis were not significantly related to any of the cardiac manifestations analyzed.
Data regarding the impact of titer levels on cardiac manifestations are scarce. Erkan D et al. analyzed the relevance of high aPL titer for non-criteria APS manifestation occurrence, concluding that patients with high aCL titers are more likely to have a higher prevalence of non-criteria manifestations [33]. This was confirmed in a recent prospective study, emphasizing the relationship of high aPL titers to the extra-criteria phenomenon of APS [34].
Our analysis showed that PAPS patients with CMP and ADHF were significantly positively related to titers of aCL-IgG, which, regarding ADHF, was confirmed after distribution of patients into groups with low, medium, and high aPL levels (p = 0.0001).
There are several limitations to the present study. This is a single-center, cross-sectional study which has no insight to the sequence of events and advanced causatives in the development of various cardiac manifestations. Nevertheless, the sample of APS patients studied is meaningful, giving more certainty to provided findings.
To conclude, cardiac manifestations in PAPS are various and meaningful. Some manifestations are related to certain aPL types and/or levels, which has to be confirmed in prospective studies. Our findings open the door for future PAPS patients’ randomization, regarding the risk of certain cardiac involvement development. At this very moment, proper anticoagulant/antithrombotic therapy, as well as intense action against standard atherosclerotic risk factors, is crucial.
References
Petri M (2000) Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun 15:145–151. https://doi.org/10.1006/jaut.2000.0409
Hughes GR (1993) The antiphospholipid syndrome: ten years on. Lancet 342:341–344. https://doi.org/10.1016/0140-6736(93)91477-4
Djokovic A, Stojanovich L, Stanisavljevic N, Banicevic S, Smiljanic D, Milovanovic B (2018) Relationship between cerebrovascular and valvular manifestations in a Serbian cohort of patients with antiphospholipid syndrome. Clin Exp Rheumatol 36(5):850–855
Kolitz T, Shiber S, Sharabi I, Winder A, Zandman-Goddard G (2019) Cardiac manifestations of antiphospholipid syndrome with focus on its primary form. Front Immunol 10:941. https://doi.org/10.3389/fimmu.2019.00941
Bertolaccini ML, Khamashta MA, Hughes GR (2005) Diagnosis of antiphospholipid syndrome. Nat Clin Pract Rheumatol 1:40–46. https://doi.org/10.1038/ncprheum0017
Denas G, Jose SP, Bracco A, Zoppellaro G, Pengo V (2015) Antiphospholipid syndrome and the heart: a case series and literature review. Autoimmun Rev 14(3):214–222. https://doi.org/10.1016/j.autrev.2014.11.003
de Souza AW, Silva NP, de Carvalho JF, D’Almeida V, Noguti MA, El S (2007) Impact of hypertension and hyperhomocystenemia on arterial thrombosis in primary antiphospholipid syndrome. Lupus 16:782–787. https://doi.org/10.1177/0961203307081847
Zuily S, Huttin O, Mohamed S, Marie PY, Selton-Suty C, Wahl D (2013) Valvular heart disease in antiphospholipid syndrome. Curr Rheumatol Rep 15:320. https://doi.org/10.1007/s11926-013-0320-8
Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT et al (2002) Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 46:1019–1027. https://doi.org/10.1002/art.10187
Erkan D, Asherson RA, Espinosa G (2003) Long term outcome of catastrophic antiphospholipid syndrome survivors. Ann Rheum Dis 62:530–533. https://doi.org/10.1136/ard.62.6.530
Narshi CB, Giles IP, Rahman A (2011) The endothelium: an interface between autoimmunity and atherosclerosis in systemic lupus erythematosus? Lupus 20:5–13. https://doi.org/10.1177/0961203310382429
Volkov I, Seguro L, Leon EP, Kovács L, Roggenbuck D, Schierack P et al (2020) Profiles of criteria and non-criteria anti-phospholipid autoantibodies are associated with clinical phenotypes of the antiphospholipid syndrome. Auto Immun Highlights 11(1):8. https://doi.org/10.1186/s13317-020-00131-3
Sciascia S, Amigo MC, Roccatello D, Khamashta M (2017) Diagnosing antiphospholipid syndrome: ‘extra-criteria’ manifestations and technical advances. Nat Rev Rheumatol 13(9):548–560. https://doi.org/10.1038/nrrheum.2017.124
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R et al (2006) International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4:295–306. https://doi.org/10.1111/j.1538-7836.2006.01753.x
Cervera R. CAPS Registry Project Group (2010) Catastrophic antiphospholipid syndrome (CAPS): update from the “CAPS Registry.” Lupus 19:412–418. https://doi.org/10.1177/0961203309361353
Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T et al (2000) Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633. https://doi.org/10.1086/313753
Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N et al (2019) EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 78(10):1296–1304. https://doi.org/10.1136/annrheumdis-2019-215213
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713. https://doi.org/10.1016/j.echo.2010.05.010
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A et al (2015) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 46(4):903–975. https://doi.org/10.1093/eurheartj/ehv317
Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M et al (2009) Subcommittee on lupus anticoagulant/antiphospholipid antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost 7:1737–1740. https://doi.org/10.1111/j.1538-7836.2009.03555.x
Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA (2010) Antiphospholipid syndrome. Lancet 376:1498–1509. https://doi.org/10.1016/S0140-6736(10)60709-X
Devreese KMJ (2020) How to interpret antiphospholipid laboratory tests. Curr Rheumatol Rep 22(8):38. https://doi.org/10.1007/s11926-020-00916-5
Pengo V, Biasiolo A, Pegoraro C, Cucchini U, Noventa F, Iliceto S (2005) Antibody profiles for the diagnosis of antiphospholipid syndrome. Thromb Haemost 93:1147–1152. https://doi.org/10.1160/TH04-12-0839
Font J, Cervera R (2006) Cardiac manifestations in antiphospholipid syndrome. In: Khamashta MA (ed) Hughes Syndrome. 2nd edn. Singapore Springer pp 41–53
Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramón E et al (2015) Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 74:1011–1018. https://doi.org/10.1136/annrheumdis-2013-204838
Djokovic A, Stojanovich L, Kontic M, Stanisavljevic N, Radovanovic S, Marisavljevic D (2014) Association between cardiac manifestations and antiphospholipid antibody type and level in a cohort of Serbian patients with primary and secondary antiphospholipid syndrome. Isr Med Assoc J 16:162–167
Pengo V, Ruffatti A, Legnani C, Gresele P, Barcellona D, Erba N et al (2010) Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 8(2):237–242. https://doi.org/10.1111/j.1538-7836.2009.03674.x
Stojanovich L, Kontic M, Smiljanic D, Djokovic A, Stamenkovic B, Marisavljevic D (2013) Association between non-thrombotic neurological and cardiac manifestations in patients with antiphospholipid syndrome. Clin Exp Rheumatol 31(2):234–242
Stojanovich L, Djokovic A, Kontic M (2015) Antiphospholipid-mediated thrombosis: interplay between type of antibodies and localisation of lung, and cardiovascular incidences in primary antiphospholipid syndrome. Clin Exp Rheumatol 33(4):531–536
Kelchtermans H, Chayouâ W, de Laat B (2018) The significance of antibodies against domain I of beta-2 glycoprotein I in antiphospholipid syndrome. Semin Thromb Hemost 44(05):458–465. https://doi.org/10.1055/s-0037-1601329
Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A (2009) Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol 8:998–1005. https://doi.org/10.1016/S1474-4422(09)70239-X
Molhoek JE, de Groot PG, Urbanus RT (2018) The lupus anticoagulant paradox. Semin Thromb Hemost 44(05):445–452. https://doi.org/10.1055/s-0037-1606190
Erkan D, Barbhaiya M, George D, Sammaritano L, Lockshin Md (2010) Moderate versus high-titer persistently anticardiolipin antibody positive patients: are they clinically different and does high-titer anti-beta 2-glycoprotein-I antibody positivity offer additional predictive information? Lupus 19(5):613–619. https://doi.org/10.1177/0961203309355300
Udry S, Latino JO, Belizna C, Perés Wingeyer S, Fernández Romero DS, de Larrañaga G (2019) A high-risk laboratory profile of antiphospholipid antibodies and thrombosis is associated with a large number of extra-criteria manifestations in obstetric antiphospholipid syndrome. Immunol Res 67(6):478–485. https://doi.org/10.1007/s12026-019-09110-x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Djokovic, A., Stojanovich, L., Stanisavljevic, N. et al. Cardiac manifestations in primary antiphospholipid syndrome and their association to antiphospholipid antibodies’ types and titers—cross-sectional study of Serbian cohort. Clin Rheumatol 41, 1447–1455 (2022). https://doi.org/10.1007/s10067-022-06056-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06056-8