Abstract
Extra-criteria manifestations such as thrombocytopenia and livedo are described associated with antiphospholipid syndrome (APS) but are not included in the current classification criteria. Their clinical expression might be important, as they may be associated with a high-risk profile of antiphospholipid antibodies (aPL) and thrombosis. We evaluated the association between the presence of extra-criteria manifestations in primary obstetric-APS (POAPS) and aPL profiles. We also evaluated whether the presence of extra-criteria manifestations in POAPS patients increases the risk of developing thrombosis during the follow-up period (median follow-up 5 years; range 3–9 years). We selected 79 women who were included in our study only if they were first diagnosed with POAPS (with no history of previous thrombosis) and reevaluated for the presence of thrombosis after the follow-up period. We evaluated the association between the aPL profile and extra-criteria manifestations. We also evaluated the relationship of thrombosis during the follow-up period with extra-criteria manifestations and other risk factors. Patients with three or more extra-criteria manifestations presented high rates of triple positivity for the aPL profile (75%) (p < 0.001). We also found a relationship between the presence of extra-criteria manifestations and the presence of high titers of aPL: 91.7% of patients with three or more extra-criteria manifestations had high titers of aPL (p < 0.01). We further evaluated the group of POAPS patients according to thrombotic events during the follow-up. Among these patients, 6 (7.6%) presented thrombosis. Notably, 100% of patients with a thrombotic event during the follow-up had more than three extra-criteria manifestations. POAPS patients with extra-criteria manifestations might have a high-risk aPL profile and a major risk of developing thrombosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antiphospholipid syndrome (APS) is an acquired autoimmune thrombophilia defined by the presence of thrombosis and/or pregnancy morbidity and is associated with antiphospholipid antibodies (aPL) [1]. Despite this definition, patients with APS may also have other clinical manifestations (referred to as extra-criteria manifestations) that are not included in the Sydney criteria, such as livedo reticularis and other cutaneous manifestations, neurological manifestations, heart valve disease, thrombocytopenia, and renal microangiopathy [2].
Although these extra-criteria manifestations are not specifically observed in APS patients, some studies suggest that their clinical expression could be associated with high-risk antibody profiles, such as high titers of aPL and/or the presence of more than one positive aPL [3,4,5], which defines the subjects as “increased risk” of developing clinical manifestations of APS. It is not completely clear whether a higher number of extra-criteria manifestations are associated with a defined laboratory profile. In fact, extra-criteria manifestations may not have any relevance at diagnosis. However, according to research and clinical studies on APS, some of these manifestations might be highly relevant regarding correlations with morbidities and mortality since they might be important for the risk of thrombosis [6,7,8,9].
The latest publications highlight the idea that thrombotic APS and obstetric APS might be two different clinical entities and should be separately studied in terms of risks and stratifications. Although these stratifications have been studied more extensively for thrombotic APS, there are no relevant studies on extra-criteria manifestations in obstetric APS [2, 3, 9, 10]. The first aim of our study was to evaluate the association between the presence of extra-criteria manifestations in primary obstetric-APS (POAPS) and aPL profiles. The second aim of our work was to conduct a thorough study on extra-criteria manifestation in a well-characterized POAPS population to determine a better method for the stratification of risks.
Materials and methods
Population study
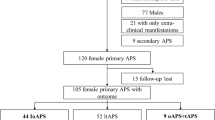
We carefully selected 79 women with complete data who attended our center (Acute Hospital “Dr. Carlos G. Durand,” Buenos Aires, Argentina) between April 2010 and July 2016 and who were diagnosed with POAPS based on international consensus classification criteria [1]. These women were included in our study only if they were first diagnosed with POAPS (with no history of previous thrombosis) and prospectively reevaluated for the presence of thrombosis after the follow-up period.
The presence of extra-criteria manifestations and the baseline clinical and laboratory features were evaluated at the time of diagnosis. We also evaluated the association between the aPL profile and extra-criteria manifestations.
The second part of the study consisted of the identification of patients who developed thrombosis during the follow-up period between 2010 and 2019 (median follow-up 5 years; range 3–9 years). The follow-up time was calculated from delivery. We evaluated the relationship of thrombosis during the follow-up period with extra-criteria manifestations and other risk factors. During the follow-up period, no patient received any treatment.
The strict exclusion criteria consisted of the following: other rheumatic disease, the presence of other thrombophilias (Factor V Leiden and/or prothrombin G20210A, hyperhomocysteinemia, and a deficit of protein C, protein S, and/or antithrombin), the administration of any additional treatment for POAPS and/or other rheumatic diseases, and a history of previous thrombosis at the time of diagnosis.
Laboratory tests
All patients were diagnosed with APS when their laboratory criteria were positive on two or more occasions at least 12 weeks after their first positive result. The following aPL tests were used: lupus anticoagulant (LA), anti-β2 glycoprotein I (aβ2GPI) antibodies IgG and/or IgM, and anticardiolipin antibodies (aCLs) IgG and/or IgM [1].
Blood samples
Blood was obtained by clean venipuncture (after an 8 h fast) and was collected into plastic tubes containing sodium citrate (ratio 9:1). After two centrifugation steps at 2500×g for 15 min, the platelet-poor plasma was immediately assayed for LA and then stored at − 40 °C. The blood was collected into tubes, allowed to clot at 37 °C, and then centrifuged at 1500×g for serum preparation. The serum was stored at − 40 °C until ready for use.
Serum
The aCL was measured using standardized enzyme immunoassays of IgG and IgM isotypes that were developed in-house as previously reported [11]. aβ2GPI was measured by a commercial enzyme immunoassay kit for IgG and IgM isotypes (BioSystems S.A., Barcelona, Spain). Serum levels of aCL or aβ2GPI at medium or high titers were considered a positive result. A medium titer was defined as > 99th percentile (greater than 40 GPL or MPL or greater than 40 UG or UM according to the international criteria). A high titer was arbitrarily defined as 80 GPL or MPL or as 80 UG or UM (according to previous publications) [12, 13].
Plasma citrate
The plasma samples were evaluated for the presence of LA activity using two tests: the dilute Russell viper venom time (TriniCLOT Lupus Screen and/or Confirm, Tcoag, Co. Wicklow, Ireland) and silica clotting time (Hemosil, Instrumentation Laboratory, Bedford, MA, USA). To identify the inhibitor, these clotting tests were conducted with a 1:1 mixture of the corresponding patient and normal plasma samples. As confirmatory tests, we used a phospholipid neutralization procedure. LA was diagnosed when at least one of the screening and one of the confirmatory procedures were positive according to the international criteria.
aPL profile
We classified the patients according to aPL positivity in the laboratory tests: triple-positive for aPL (LA+, aCL+, and aβ2GPI+), double-positive for aPL (LA+ and aCL+, LA and aβ2GPI+, or aCL+, and aβ2GPI+) and single-positive for aPL (LA+, aCL+, or aβ2GPI+).
We classified the patients according to serum levels of aCL IgG, and IgM or aβ2GPI IgG and IgM into low-, medium- or high-titer groups. A low titer was defined as a titer less than 39 GPL or MPL or less than 39 UG or UM. A medium titer was defined as 40 to 79 GPL or MPL or as 40 to 79 UG or UM. A high titer was arbitrarily defined as greater than or equal to 80 GPL or MPL or greater than or equal to 80 UG or UM (according to previous publications) [12, 13].
A high-risk laboratory profile was defined as patients having triple positivity for aPL and/or patients with high titers of aCL and/or aβ2GPI.
A low-risk laboratory profile was defined as having double or single positivity for aPL and/or low or medium serum levels of aCL and/or aβ2GPI.
Ethics
This study was approved by the ethics committee of the respective medical center and was performed according to the principles of the Declaration of Helsinki and the current national law. Informed consent was obtained from all the participants.
Extra-criteria manifestations of APS
Livedo reticularis, skin ulcerations, and other dermatological manifestations were diagnosed by a rheumatologist at the medical center. These conditions were also confirmed by skin biopsy when necessary.
Thrombocytopenia is defined as a platelet level < 100,000 mm3 (confirmed by at least two determinations and evaluations of the peripheral blood smear). We excluded patients with low platelet levels during pregnancy or who were undergoing treatment with drugs such as heparin. Other hematologic manifestations were evaluated by blood samples and confirmed by at least two determinations.
Hemolytic anemia was defined as a decline in hemoglobin accompanied by increased reticulocyte count, high serum lactate dehydrogenase, and reduced haptoglobin levels in the presence of a positive Coombs test.
Valvulopathy was confirmed by evaluation by a cardiologist at the medical center (confirmed with echocardiography).
aPL-related nephropathy was assessed by considering the kidney laboratory alterations and through a nephrologist’s evaluation (proteinuria and renal failure) [14]. The evaluation of these extra-criteria manifestations was limited by the lack of kidney biopsies from all patients.
Central nervous system extra-criteria manifestations (migraines, seizures, and chorea) were diagnosed by a clinician or neurologist. We excluded other etiologies, such as metabolic abnormalities, drugs or medication, hypertension, endocrine disorders, and/or other related disorders. Another limitation of this study was that we did not use the International Headache Society Criteria. Furthermore, the study did not include brain imaging for all patients.
We subdivided the patients into 3 groups according to the number of extra-criteria manifestations: group 1, no extra-criteria manifestations; group 2, one or two extra-criteria manifestations; and group 3, three or more extra-criteria manifestations.
Statistical analysis
The statistical analysis was performed using the SPSS statistical software package version 15.0 for Windows (SPSS, Chicago, IL, USA). Because the data were not normally distributed, they are presented as the median and percentile (25 and 75) or percentage. Nonparametric tests (Mann-Whitney U test) were used to compare the quantitative data, and the chi-squared test or Fisher’s exact test was used to compare proportions. p < 0.05 indicated statistical significance.
Results
This study included 79 nonconsecutive pregnant women diagnosed with POAPS (median age, 31 years; interquartile range, 28–36 years) who were treated at our clinical center between 2010 and 2016.
All patients had a history of pregnancy morbidity (Table 1): 30 (38.0%) experienced fetal loss, 57 (72.2%) experienced early miscarriage, 32 (40.5%) experienced late miscarriage, 20 (25.3%) experienced premature birth, 10 (12.7%) had a history of preeclampsia, and 12 (17.9%) had intrauterine growth restriction.
aCL was positive in 57 cases (72.1%) and presented with high titers in 15 cases (19.0%). aβ2GPI was positive in 43 cases (54.4%) and presented with high titers in 15 cases (19.0%). LA was positive in 26 cases (32.9%). High aPL titers (aCL and/or aβ2GPI) were present in 24 cases (30.4%).
A triple positivity profile for aPL was detected in 16 patients (20.3%), a double positivity profile for aPL was detected in 16 patients (20.3%), and a single positivity profile for aPL was detected in 47 patients (59.4%).
Extra-criteria manifestations of APS were present in 41 patients (51.8%): 20 (25.3%) had one extra-criteria manifestation, 9 (11.4%) had two extra-criteria manifestations, and 12 (15.2%) had three or more extra-criteria manifestations. A total of 27 patients (34.2%) had livedo reticularis, 9 patients (11.4%) had a history of confirmed thrombocytopenia, 6 patients (7.6%) had a history of confirmed hemolytic anemia, 2 patients (2.5%) had a history of valvulopathy, 1 patient (1.3%) had a history of nephropathy, 32 patients (40.5%) had a history of migraines (which was the most frequent extra-criteria manifestation), 2 patients had a history of chorea (2.5%), and 1 patient (1.3%) had a history of epilepsy. In addition, superficial vein thrombosis was present in 6 patients (7.6%) (Table 1).
Remarkably, patients with three or more extra-criteria manifestations presented high rates of triple positivity for the aPL profile (75%), whereas patients without extra-criteria manifestations presented high rates of single positivity for the aPL profile (81.6%). There was a close and statistically significant (p < 0.001) relationship between the number of extra-criteria manifestations and the aPL profile (Table 2) (Fig. 1).
We also found a relationship between the presence of extra-criteria manifestations and the presence of high titers of aPL: 91.7% (11/12) of patients with 3 or more extra-criteria manifestations had high titers of aPL, whereas only 13.2% of patients without extra-criteria manifestations had high titers (p < 0.01) (Table 3).
We further evaluated the group of POAPS patients according to thrombotic events during the follow-up period, and among these patients, 6 (7.6%) presented deep venous thrombosis of the lower limbs. All these events occurred away from the puerperium period. Notably, 100% of patients (6/6) with a thrombotic event during the follow-up period had more than 3 extra-criteria manifestations. Thrombocytopenia and livedo reticularis were present in 100% of the patients (6/6) with thrombotic manifestations + POAPS (Table 4). A triple positivity profile of aPL was present in 50.0% (3/6) of those patients. From another point of view, for those patients who presented 3 or more extra-criteria manifestations, 50% (6/12) developed thrombosis during the follow-up period.
Discussion
Extra-criteria manifestations are frequently observed in APS patients [6]. Although they are not included in the Sydney criteria for the definition of APS, recent studies have highlighted the importance of extra-criteria manifestations in the prognosis or morbidity of APS patients [2, 3, 10, 15,16,17]; however, there are few studies that focus on extra-criteria manifestations in POAPS patients. Therefore, we considered that the association between extra-criteria manifestations in POAPS and its association with aPL profile and thrombotic risk should be explored.
To increase the specificity of the extra-criteria manifestations of POAPS (considering that they are not exclusively observed in APS patients), we subdivided groups of patients according to the number of extra-criteria manifestations observed in each patient. Despite the distribution of aPL profiles which was heterogeneous among our group of POAPS patients, we found that it was directly associated with extra-criteria manifestations of APS. Specifically, patients with three or more extra-criteria manifestations more frequently presented a high-risk laboratory aPL profile. Furthermore, patients without extra-criteria manifestations more frequently presented a low-risk laboratory aPL profile. These findings are in agreement with the previous results from different groups worldwide who reported that extra-criteria manifestations of APS would be associated with high titers of antibodies [4, 5] and/or with positive results for more than one type of aPL antibody [3], even though most of these studies were conducted in patients with thrombotic APS. Hence, we hypothesized that extra-criteria manifestations might not occur incidentally in POAPS patients, since their onset might be linked to complex mechanisms directly related to the presence of aPL. Therefore, extra-criteria manifestations could be interpreted as markers of autoimmunity associated with POAPS [6, 18, 19]. Thus, those patients with high-risk laboratory profiles could more frequently develop extra-criteria manifestations.
The second aim of our study was to evaluate the impact of extra-criteria manifestations of APS on the risk of developing thrombosis during a follow-up period. We found an association between the presence of extra-criteria manifestations and the risk of developing thrombosis and that this risk might increase according to the number of extra-criteria manifestations of APS. Remarkably, extra-criteria manifestations of APS seemed to better predict thrombotic events than triple positivity profiles in our cohort POAPS patients [16].
Kato et al. proposed in an elegant and well written review to include thrombocytopenia in the thrombotic risk assessment of APS patients based on the idea that thrombocytopenia in the presence of aPL may unexpectedly increase the risk of thrombosis in patients with APS [10, 20]. This was based on a publication by Hisada et al. [21], who demonstrated that a low platelet count in patients with aPL is associated with a high risk of developing thrombosis. In our study, 100% of patients who developed thrombosis during the follow-up period presented thrombocytopenia, and strikingly, 66% of the patients who manifested thrombocytopenia developed thrombosis. This finding is also reinforced by the observations of Potara et al. [9], who showed that low platelet counts occurred early in patients with a risk of developing catastrophic APS, and this was considered a warning signal for disease activity.
Kato et al. also proposed that other extra-criteria manifestations, such as livedo reticularis, heart valve disease, superficial vein thrombosis, and renal microangiopathy, should be included in the thrombotic risk assessment of APS patients since different studies suggested their association with thrombosis [8, 10, 22,23,24,25]. We consider that this proposal could be reinforced by our findings that all patients who presented thrombosis had several extra-criteria manifestations in their clinical features.
The latest publications highlight the idea that there are two main distinct clinical variants of APS: obstetric and vascular [26]. According to recent data from the EUROAPS project, POAPS is associated with a low frequency of thrombotic events and requires less aggressive treatment for vascular events than thrombotic APS [26, 27]. The identification of risk factors associated with thrombosis in POAPS patients, such as triple positivity of antibodies and the presence of a high number of extra-criteria manifestations, could be a tool to predict thrombotic events in POAPS patients and consequently to identify patients who should receive a thromboprophylactic treatment beyond pregnancy [6,7,8, 21, 22, 28,29,30]. Indeed, in our study, those patients who did not present extra-criteria manifestations and had “low-risk” laboratory aPL profiles did not seem to display an elevated risk of developing thrombosis.
In our opinion, one of the main strengths of our study arises from the selection of our group of POAPS patients. In previous studies, there were included series of patients with all kinds of APS without discriminating between them as obstetric or thrombotic APS patients. Besides, we consider that another robust point of our study is that we performed a comprehensive analysis of the global distribution of extra-criteria manifestations according to aPL and the associated risk of developing thrombosis. However, we must acknowledge that one of the limitations of our study is its small sample size but, unfortunately, the limited number of patients with extra-criteria manifestations is a crucial problem suffered from of all studies performed on this subject.
In conclusion, our findings suggest that POAPS patients with extra-criteria manifestations typically display a high-risk aPL profile and a high risk of developing thrombosis, especially when they have a large number of clinical manifestations.
References
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemostas : JTH. 2006;4(2):295–306. https://doi.org/10.1111/j.1538-7836.2006.01753.x.
Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, et al. The relevance of “non-criteria” clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun Rev. 2015;14(5):401–14. https://doi.org/10.1016/j.autrev.2015.01.002.
Radin M, Ugolini-Lopes MR, Sciascia S, Andrade D. Extra-criteria manifestations of antiphospholipid syndrome: risk assessment and management. Semin Arthritis Rheum. 2018;48(1):117–20. https://doi.org/10.1016/j.semarthrit.2017.12.006.
Stojanovich L, Kontic M, Djokovic A, Marisavljevic D, Ilijevski N, Stanisavljevic N, et al. Association between systemic non-criteria APS manifestations and antibody type and level: results from the Serbian national cohort study. Clin Exp Rheumatol. 2013;31(2):234–42.
Erkan D, Barbhaiya M, George D, Sammaritano L, Lockshin M. Moderate versus high-titer persistently anticardiolipin antibody positive patients: are they clinically different and does high-titer anti-beta 2-glycoprotein-I antibody positivity offer additional predictive information? Lupus. 2010;19(5):613–9. https://doi.org/10.1177/0961203309355300.
Sciascia S, Amigo MC, Roccatello D, Khamashta M. Diagnosing antiphospholipid syndrome: ‘extra-criteria’ manifestations and technical advances. Nat Rev Rheumatol. 2017;13(9):548–60. https://doi.org/10.1038/nrrheum.2017.124.
de Jesus GR, Sciascia S, Andrade D, Barbhaiya M, Tektonidou M, Banzato A, et al. Factors associated with first thrombosis in patients presenting with obstetric antiphospholipid syndrome (APS) in the APS Alliance for Clinical Trials and International Networking Clinical Database and Repository: a retrospective study. BJOG: an international journal of obstetrics and gynaecology. 2019;126(5):656–61. https://doi.org/10.1111/1471-0528.15469.
Kontic M, Stojanovich L, Mijailovic-Ivkovic M, Velinovic M, Srnka J, Zdravkovic M. Are the cutaneous manifestations in patients with primary antiphospholipid syndrome a marker for predicting lung manifestations? Clin Exp Rheumatol. 2018;36(1):56–61.
Pontara E, Banzato A, Bison E, Cattini MG, Baroni G, Denas G, et al. Thrombocytopenia in high-risk patients with antiphospholipid syndrome. Journal of thrombosis and haemostasis : JTH. 2018;16(3):529–32. https://doi.org/10.1111/jth.13947.
Kato M, Hisada R, Atsumi T. Clinical profiles and risk assessment in patients with antiphospholipid antibodies. Expert Rev Clin Immunol. 2019;15(1):73–81. https://doi.org/10.1080/1744666X.2019.1543025.
de Larranaga GF, Forastiero RR, Carreras LO, Alonso BS. Different types of antiphospholipid antibodies in AIDS: a comparison with syphilis and the antiphospholipid syndrome. Thromb Res. 1999;96(1):19–25. https://doi.org/10.1016/s0049-3848(99)00059-6.
Latino JO, Udry S, Aranda FM, Peres Wingeyer SDA, Fernandez Romero DS, de Larranaga GF. Pregnancy failure in patients with obstetric antiphospholipid syndrome with conventional treatment: the influence of a triple positive antibody profile. Lupus. 2017;26(9):983–8. https://doi.org/10.1177/0961203317692432.
Latino JO, Udry S, Wingeyer SP, Romero DF, Micone P, de Larranaga G. What is the best time to assess the antiphospholipid antibodies (aPL) profile to better predict the obstetric outcome in antiphospholipid syndrome (APS) patients? Immunol Res. 2018;66(5):577–83. https://doi.org/10.1007/s12026-018-9024-5.
Amigo MC, Garcia-Torres R, Robles M, Bochicchio T, Reyes PA. Renal involvement in primary antiphospholipid syndrome. J Rheumatol. 1992;19(8):1181–5.
Zigon P, Podovsovnik A, Ambrozic A, Tomsic M, Hocevar A, Gaspersic N, et al. Added value of non-criteria antiphospholipid antibodies for antiphospholipid syndrome: lessons learned from year-long routine measurements. Clin Rheumatol. 2019;38(2):371–8. https://doi.org/10.1007/s10067-018-4251-7.
Martinez-Valle F, Ordi-Ros J, Selva-O’Callaghan A, Balada E, Solans-Laque R, Vilardell-Tarres M. Livedo racemosa as a marker of increased risk of recurrent thrombosis in patients with negative anti-phospholipid antibodies. Med Clin. 2009;132(20):767–71. https://doi.org/10.1016/j.medcli.2008.09.044.
Toubi E, Krause I, Fraser A, Lev S, Stojanovich L, Rovensky J, et al. Livedo reticularis is a marker for predicting multi-system thrombosis in antiphospholipid syndrome. Clin Exp Rheumatol. 2005;23(4):499–504.
Zuily S, Huttin O, Mohamed S, Marie PY, Selton-Suty C, Wahl D. Valvular heart disease in antiphospholipid syndrome. Curr Rheumatol Rep. 2013;15(4):320. https://doi.org/10.1007/s11926-013-0320-8.
Weinstein C, Miller MH, Axtens R, Buchanan R, Littlejohn GO. Livedo reticularis associated with increased titers of anticardiolipin antibodies in systemic lupus erythematosus. Arch Dermatol. 1987;123(5):596–600.
Atsumi T, Furukawa S, Amengual O, Koike T. Antiphospholipid antibody associated thrombocytopenia and the paradoxical risk of thrombosis. Lupus. 2005;14(7):499–504. https://doi.org/10.1191/0961203305lu2145rr.
Hisada R, Kato M, Sugawara E, Fujieda Y, Oku K, Bohgaki T, et al. Thrombotic risk stratification by platelet count in patients with antiphospholipid antibodies: a longitudinal study. Journal of thrombosis and haemostasis : JTH. 2017;15(9):1782–7. https://doi.org/10.1111/jth.13763.
Frances C, Niang S, Laffitte E, Pelletier F, Costedoat N, Piette JC. Dermatologic manifestations of the antiphospholipid syndrome: two hundred consecutive cases. Arthritis Rheum. 2005;52(6):1785–93. https://doi.org/10.1002/art.21041.
Krause I, Lev S, Fraser A, Blank M, Lorber M, Stojanovich L, et al. Close association between valvar heart disease and central nervous system manifestations in the antiphospholipid syndrome. Ann Rheum Dis. 2005;64(10):1490–3. https://doi.org/10.1136/ard.2004.032813.
Shen Y, Chen XW, Sun CY, Dai M, Yan YC, Yang CD. Association between anti-beta2 glycoprotein I antibodies and renal glomerular C4d deposition in lupus nephritis patients with glomerular microthrombosis: a prospective study of 155 cases. Lupus. 2010;19(10):1195–203. https://doi.org/10.1177/0961203310368409.
Zuily S, Regnault V, Guillemin F, Kaminsky P, Rat AC, Lecompte T, et al. Superficial vein thrombosis, thrombin generation and activated protein C resistance as predictors of thromboembolic events in lupus and antiphospholipid patients. A prospective cohort study. Thromb Res. 2013;132(1):e1–7. https://doi.org/10.1016/j.thromres.2013.04.012.
Meroni PL, Borghi MO, Grossi C, Chighizola CB, Durigutto P, Tedesco F. Obstetric and vascular antiphospholipid syndrome: same antibodies but different diseases? Nat Rev Rheumatol. 2018;14(7):433–40. https://doi.org/10.1038/s41584-018-0032-6.
Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Saez-Comet L, Lefkou E, Mekinian A, et al. The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): a survey of 1000 consecutive cases. Autoimmun Rev. 2019;18(4):406–14. https://doi.org/10.1016/j.autrev.2018.12.006.
Drozdinsky G, Hadar E, Shmueli A, Gabbay-Benziv R, Shiber S. Obstetric antiphospholipid syndrome and long term arterial thrombosis risk. J Thromb Thrombolysis. 2017;44(3):371–5. https://doi.org/10.1007/s11239-017-1526-9.
Gris JC, Bouvier S, Molinari N, Galanaud JP, Cochery-Nouvellon E, Mercier E, et al. Comparative incidence of a first thrombotic event in purely obstetric antiphospholipid syndrome with pregnancy loss: the NOH-APS observational study. Blood. 2012;119(11):2624–32. https://doi.org/10.1182/blood-2011-09-381913.
Erkan D, Merrill JT, Yazici Y, Sammaritano L, Buyon JP, Lockshin MD. High thrombosis rate after fetal loss in antiphospholipid syndrome: effective prophylaxis with aspirin. Arthritis Rheum. 2001;44(6):1466–7. https://doi.org/10.1002/1529-0131(200106)44:6<1466::AID-ART242>3.0.CO;2-C.
Acknowledgments
We would like to thank Mrs. Analía Lucero and Valentina Lara for their excellent technical support.
Funding
Funding was provided through the “Scholarship for research in medicine to a young physician” from the Foundation Florencio Fiorini 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the ethics committee of the respective medical center and was performed according to the principles of the Declaration of Helsinki and the current national law. Informed consent was obtained from all the participants.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Udry, S., Latino, J.O., Belizna, C. et al. A high-risk laboratory profile of antiphospholipid antibodies and thrombosis is associated with a large number of extra-criteria manifestations in obstetric antiphospholipid syndrome. Immunol Res 67, 478–485 (2019). https://doi.org/10.1007/s12026-019-09110-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-019-09110-x