Abstract
Objectives
To assess the efficacy and safety of the commonly used urate-lowering therapies (ULTs): febuxostat, allopurinol, and lesinurad in hyperuricemic patients with gout.
Methods
We included all randomized controlled trials (RCTs) that compared ULTs with placebo or head to head. The primary efficacy endpoint was the proportion of subjects achieving the target serum urate (SU) level at month 6. Safety outcomes included total adverse events (AEs), serious AEs, withdrawals due to AEs, and AEs per organ system. A Bayesian network model was used to compare all ULTs with placebo and among themselves.
Results
Fifteen RCTs were included for the analysis, in which 7968 patients were randomly assigned to take either placebo or one of 11 ULTs: allopurinol, febuxostat 40/80/120/240 mg/day, lesinurad 400 mg/day, lesinurad 200/400/600 mg/day plus allopurinol, and lesinurad 200/400 mg/day plus febuxostat. All ULTs were effective in achieving the target SU level at month 6 compared with placebo (ORs between 26.81 and 1928). Febuxostat 80/120/240 mg/day was superior to allopurinol and well tolerated for urate reduction. And as febuxostat dosage increased, more patients achieved the target SU level. Furthermore, the lesinurad combination with xanthine oxidase inhibitor (XOI) groups had a higher proportion of patients achieving the target SU level than the febuxostat 40 mg/day group (ORs between 2.89 and 9.17), the allopurinol group (ORs between 3.56 and 11.27), or the lesinurad 400 mg/day monotherapy group (ORs between 12.30 and 39.17) but might have a high risk of AEs.
Conclusions
All ULTs are effective in achieving the target SU level compared with placebo in hyperuricemic patients with gout. Lesinurad in combination with febuxostat or allopurinol is effective in urate lowering, especially for patients with inadequate response to XOI monotherapy.
Key Points • All urate-lowering therapies (ULTs) were effective in achieving the target serum urate (SU) level at month 6 compared with placebo in hyperuricemic patients with gout. • Febuxostat 80/120/240 mg/day was superior to allopurinol and well tolerated for urate reduction. And as febuxostat dosage increased, more patients achieved the target SU level. • Lesinurad in combination with febuxostat or allopurinol was effective in urate lowering, especially for patients with inadequate response to xanthine oxidase inhibitor monotherapy, but might have a high risk of AEs. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gout is an inflammatory arthritis resulting from elevated body uric acid pools. The serum urate (SU) level is the single most important risk factor for developing gout [1]. The SU level is elevated when it exceeds 6.8 mg/dl, the limit of solubility of monosodium urate (MSU) crystals in serum at 37 °C. This leads to deposition of MSU crystals, mainly in joints, and causes extremely painful flares [2, 3]. Most individuals with hyperuricemia are asymptomatic, but in some, intra-articular deposition of MSU crystals may occur [4]. Without adequate treatment, gouty arthritis can progress into a chronic, deforming, and physically disabling disease through the development of tophi, joint destruction, and persistent pain. Furthermore, hyperuricemic patients with gout frequently suffer from comorbidities including hypertension, diabetes mellitus, impaired renal function, and cardiovascular disease. And increasing SU may be an independent risk factor for these commonly associated comorbidities [5,6,7].
Long-term maintenance of SU levels below its saturation threshold can reduce the frequency of acute gout flares, decrease the uric acid pool, and resolve the existing tophi [8]. According to the current guidelines for management of gout, the goal of ULTs is to achieve and maintain a SU level of < 6.0 mg/dl or < 5.0 mg/dl in patients with severe gout [9, 10].
Currently, the most commonly prescribed urate-lowering therapies (ULTs) involve reducing urate production using xanthine oxidase inhibitors (XOIs) and increasing renal uric acid excretion using uricosurics. Allopurinol is the most commonly used XOI for urate reduction. However, patients taking allopurinol have a high risk, although rare, of severe allopurinol hypersensitivity syndrome (AHS), e.g., Stevens-Johnson syndrome [11]. Risk factors for serious adverse events of allopurinol include the recent intake of allopurinol, the positive HLA-B*58:01 allele, commonly seen in Asians, and factors influencing agent concentration, such as the prolonged half-life of major allopurinol oxidation product, oxypurinol, in patients with renal dysfunction [12]. Febuxostat, another XOI, has been available for the management of hyperuricemia in patients with gout for some years. It inhibits both oxidized and reduced forms of xanthine oxidase [13] and can be prescribed for patients with mild to moderate renal impairment at unchanged doses [14, 15], though lacking data for patients with severe renal impairment. Regarding uricosuric drugs, benzbromarone is the commonly employed agent in clinical practice in Asia, which acts by preventing the tubular reabsorption of urate, but not available in the USA due to severe hepatic adverse events. Lesinurad, a new uricosuric drug, is commonly used in combination with an XOI [16, 17]. It decreases the reabsorption of urate and promotes the renal excretion of uric acid by inhibiting the urate transporter 1 (URAT1).
To date, there are several RCTs comparing allopurinol, febuxostat, benzbromarone, and lesinurad with placebo or head to head. Only one previous pairwise meta-analysis revealed that lesinurad in combination with an XOI was effective in urate lowering compared with XOI monotherapy [18]. The available clinical evidence can be better examined with Bayesian network meta-analysis, which incorporates both direct and indirect comparisons of treatments [19,20,21]. The purpose of this study was to conduct a systematic review and meta-analysis of published RCTs comparing the efficacy and safety of ULTs for the treatment of hyperuricemic patients with gout.
Methods
Literature search
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline [22]. The PubMed, Embase, and Cochrane Library databases (up to October 31, 2019) were searched without restrictions on language or publication date. The following terms were searched in the title/abstract: (“gout” OR “hyperuricemia”) AND (“xanthine oxidase inhibitor” OR “allopurinol” OR “febuxostat” OR “uricosuric” OR “benzbromarone” OR “probenecid” OR “lesinurad” OR “pegloticase”) AND “random*”. In addition, we performed manual searches of references of relevant reviews on hyperuricemia or gout.
We included all RCTs that enrolled hyperuricemic patients with gout and compared the ULT with placebo or head to head. Patients with renal impairment were also included. Studies were excluded if none of the primary or secondary outcomes of interest (see below) was reported. The concomitant use of medications for gout flare prophylaxis such as non-steroidal anti-inflammatory drugs (NSAIDs) or colchicine was allowed when initiating ULTs.
Data extraction and quality assessment
Two researchers (M.D.F and J.L) independently selected the studies and extracted data from the included studies. The following information was extracted from each eligible RCT: first author, publication year, country, interventions, treatment duration, outcomes, patients’ characteristics, baseline SU level, and renal function. Data was entered into a standardized form using MS Excel 2010. Any disagreement was resolved by discussion between the two authors. Our analysis was based on intention-to-treat principle.
The methodological quality of the included studies was assessed separately by two reviewers (M.D.F and J.L) using the risk of bias tool of the Cochrane Collaboration [23], which consists of seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other bias. Any discrepancy was resolved by consensus between the two reviewers.
Outcomes of interest
The primary efficacy endpoint was the proportion of subjects achieving the target SU level (defined as < 6.0 mg/dl or < 5.0 mg/dl for patients with severe gout) at month 6 [10]. Secondary efficacy endpoints were the proportions of subjects achieving the target SU level at month 1 and month 12. Safety outcomes included total adverse events (AEs), serious AEs, withdrawals due to AEs, and AEs per organ system (e.g., skin-related, hepatic, and cardiovascular) during the study.
Statistical analysis
We performed Bayesian network meta-analysis using the random-effects binomial likelihood model for multiarm trials for efficacy outcomes and safety outcome of total AEs with WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK) [24]. The odds ratios (ORs) were reported from the median of the posterior distribution and the accompanying 95% credible intervals (CrIs). And we estimated the probability of being the best treatment (Pbest) for each therapy [25]. Heterogeneity between studies was estimated from the posterior median between-study variance τ2, with τ2 < 0.04 indicating a low level of heterogeneity and τ2 > 0.40 a high level. Furthermore, we performed sensitivity analysis by excluding trials with high risk of bias, trials enrolling patients with severe renal impairment, or trials with the target SU level of < 5.0 mg/dl.
Furthermore, we performed traditional pairwise meta-analysis using Review Manager 5.3.3 (Cochrane Collaboration, Denmark) for the outcomes of serious AEs, withdrawals due to AEs, and AEs per organ system. As these outcomes were rare events, the Peto ORs with correspondent 95% confidence intervals (CIs) were reported.
Results
Selected studies and characteristics of the included
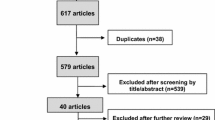
The details of the selection process of trials are shown in Fig. 1. The literature search yielded 962 manuscripts, of which 902 were ineligible after screening the titles and abstracts. The remaining 60 manuscripts were selected for a full-text review. Finally, 15 RCTs [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] were included for the analysis, in which 7968 patients were randomly assigned to take either placebo or one of 11 ULTs: allopurinol, febuxostat 40/80/120/240 mg/day, lesinurad 400 mg/day, lesinurad 200/400/600 mg/day plus allopurinol, and lesinurad 200/400 mg/day plus febuxostat. The network diagram of direct treatment comparisons is described in Fig. 2.
The characteristics of the included trials are presented in Table 1. The treatment duration ranged from 1 to 24 months. The baseline SU level was more than 6.0 mg/dl for all trial subjects. Only one trial enrolled patients with severe renal impairment (glomerular filtration rate < 30 ml/min/1.73m2) [34]. Febuxostat was given at a dosage of 40 to 240 mg/day, allopurinol at 100 to 900 mg/day, and lesinurad at 200 to 600 mg/day. Allocation concealment or blinding of outcome assessment was not clear in majority of trials. Two trials were of high risk of bias due to the lack of blinding of participants [37, 39].
Efficacy of urate-lowering therapies
Of the 15 trials included in the analysis, 11 reported the proportions of patients achieving the target SU level at month 6 [26, 29,30,31, 33,34,35,36,37,38, 40]. Network meta-analysis revealed that all ULTs were more effective in achieving the target SU level at month 6 than placebo (ORs between 26.81 and 1928) (Table 2). Febuxostat ranging from 80 to 240 mg/day had a significantly higher likelihood of achieving the target SU level than allopurinol, febuxostat 40 mg/day, or lesinurad 400 mg/day monotherapy. Patients receiving febuxostat 120/240 mg/day were more likely to achieve the target SU level than those receiving febuxostat 80 mg/day or lesinurad 200 mg/day plus allopurinol therapies. And febuxostat 240 mg/day appeared superior to lesinurad plus allopurinol and lesinurad 200 mg/day plus febuxostat therapies at achieving the target SU level. In addition, the lesinurad combination with allopurinol or febuxostat groups had a higher proportion of patients achieving the target SU level than the febuxostat 40 mg/day group (ORs between 2.89 and 9.17), the allopurinol group (ORs between 3.56 and 11.27), or the lesinurad 400 mg/day monotherapy group (ORs between 12.30 and 39.17), respectively. And lesinurad 400 mg/day plus febuxostat was more effective in achieving the target SU level at month 6 than lesinurad 200 mg/day plus allopurinol/febuxostat or febuxostat 80 mg/day monotherapy. The probability analysis showed that febuxostat 240 mg/day had the greatest probability of achieving the target SU level at month 6 (Pbest 79.9%), followed by lesinurad 400 mg/day plus febuxostat, febuxostat 120 mg/day, lesinurad 400 mg/day plus allopurinol, lesinurad 200 mg/day plus febuxostat, lesinurad 200 mg/day plus allopurinol, febuxostat 80 mg/day, febuxostat 40 mg/day, allopurinol, and lesinurad 400 mg/day monotherapy, respectively. No significant heterogeneity was found among studies for the target SU level at month 6.
For secondary endpoints, 6 trials [28, 31, 32, 36, 37, 39] reported the proportions of patients achieving the target SU level at month 1, focusing on 8 ULTs: allopurinol, febuxostat 40/80/120 mg/day, lesinurad 400 mg/day, and lesinurad 200/400/600 mg/day plus allopurinol. All ULTs were efficacious compared with placebo (ORs between 465.8 and 1.34 × 104) except lesinurad 400 mg/day monotherapy (not shown). However, no significant differences were found among ULTs. The ranking probabilities indicated that febuxostat 120 mg/day had the greatest probability of achieving the target SU level at month 1 (Pbest 53.6%), followed by lesinurad 200/400/600 mg/day plus allopurinol, febuxostat 80 mg/day, febuxostat 40 mg/day, allopurinol, and lesinurad 400 mg/day monotherapy, respectively.
And 6 trials [26, 27, 30, 31, 33, 34] reported the proportions of patients with the target SU level at month 12, focusing on 8 ULTs: allopurinol, febuxostat 40/80/120 mg/day, lesinurad 200/400 mg/day plus allopurinol, and lesinurad 200/400 mg/day plus febuxostat. All ULTs were more effective in achieving the target SU level than placebo (ORs between 104.9 and 8.37 × 106) (not shown). Additionally, febuxostat 120 mg/day appeared superior to allopurinol at achieving the target SU level (OR 6.32, 95% CrI 1.06–38.08). But there were no significant differences among other ULTs. The probability analysis showed that lesinurad 400 mg/day plus febuxostat had the greatest probability of achieving the target SU level at month 12 (Pbest 53.4%), followed in order by lesinurad 200 mg/day plus febuxostat, febuxostat 120 mg/day, febuxostat 80 mg/day, lesinurad 200/400 mg/day plus allopurinol, allopurinol, and febuxostat 40 mg/day, respectively.
For sensitivity analysis, there was no significant change of the proportion of patients achieving the target SU level at month 6 after excluding trials with high risk of bias [37, 39], trials enrolling patients with severe renal impairment [34], or trials with the target SU level of < 5.0 mg/dl [30]. And febuxostat 240 mg/day remained the best therapy for achieving the target SU level at month 6 (Pbest 95.7%).
Safety of urate-lowering therapies
Fifteen RCTs [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] reported the incidence of total AEs, focusing on 11 ULTs: allopurinol, febuxostat 40/80/120/240 mg/day, lesinurad 400 mg/day, lesinurad 200/400/600 mg/day plus allopurinol, and lesinurad 200/400 mg/day plus febuxostat. Network meta-analysis demonstrated no statistically significant differences between ULTs and placebo for the risk of total AEs, except that lesinurad 400 mg/day plus allopurinol had a higher risk of AEs than placebo (OR 1.95, 95% CrI 1.23–3.03) (Table 3). And the lesinurad 400 mg/day plus allopurinol group had a higher risk of AEs than the allopurinol monotherapy and febuxostat 40/80/120 mg/day groups (ORs between 1.53 and 2.20). Furthermore, patients taking lesinurad combination with allopurinol/febuxostat had a higher risk of AEs than those with febuxostat 120 mg/day (ORs between 1.69 and 2.33). Notably, the febuxostat 120 mg/day group had a lower risk of AEs than the allopurinol and febuxostat 40 mg/day groups (OR 0.70, 95% CrI 0.53–0.91 and OR 0.70, 95% CrI 0.52–0.94, respectively). The analysis of probabilities showed that febuxostat 120 mg/day had the greatest probability of being the safest treatment (Pbest 65.3%). There was no significant heterogeneity among studies for total AEs.
Fourteen RCTs [26,27,28,29,30,31,32,33,34,35,36, 38,39,40] reported serious AEs and withdrawals due to AEs. For serious AEs, there were no statistically significant differences among ULTs and placebo, except that lesinurad 400 mg/day plus allopurinol had a higher risk of serious AEs than lesinurad 200 mg/day plus allopurinol and allopurinol monotherapy (Peto OR 2.01, 95% CI 1.15–3.50 and Peto OR 1.91, 95% CI 1.10–3.32, respectively) (Table S1). For withdrawals due to AEs, no significant differences were found among ULTs and placebo, except that the febuxostat 120 mg/day group had a higher risk of withdrawals due to AEs than the allopurinol group (Peto OR 1.66, 95% CI 1.01–2.71) (Table S2).
Regarding skin-related AEs, patients taking allopurinol had a higher risk of skin-related AEs than those with febuxostat 80 mg/day (Peto OR 1.43, 95% CI 1.03–1.96) (Table S3). But there were more hepatic AEs in patients taking febuxostat 80 mg/day compared with placebo (Peto OR 2.95, 95% CI 1.25–6.96) (Table S4). Notably, the allopurinol group had a lower risk of cardiovascular AEs than the placebo and febuxostat 80/240 mg/day groups (Peto ORs between 0.19 and 0.47) (Table S5).
Sensitivity analysis was performed by excluding trials with high risk of bias [37, 39], subjects with severe renal impairment [34], or trials with the target SU level of < 5.0 mg/dl [30]. The results of total AEs changed little (data not shown). And the febuxostat 120 mg/day group remained the safest therapy for lowering the SU level (Pbest 70.3%). Similarly, results were unchanged for serious AEs, withdrawals due to AEs, and AEs per organ system.
Discussion
This meta-analysis provides comparative review on the efficacy and safety of ULTs in the treatment of hyperuricemic patients with gout. Our analysis confirmed that all ULTs were effective in achieving the target SU level compared with placebo at months 6 and 12. Lesinurad in combination with febuxostat or allopurinol was superior to allopurinol, lesinurad, or febuxostat 40 mg/day monotherapy in achieving the target SU level at month 6, which is consistent with the recent meta-analysis and clinical trials [18, 26, 30, 32, 33]. Thus, if patients respond inadequately to XOI monotherapy, the addition of lesinurad is recommended, which not only reduces urate production but also increases renal excretion of urate.
In our analysis, febuxostat ranging from 80 to 240 mg/day had a significantly greater proportion of patients with the target SU level than allopurinol, lesinurad, or febuxostat 40 mg/day monotherapy. To date, several reviews and meta-analyses have demonstrated that febuxostat was effective in lowering the SU level compared with allopurinol or placebo [41,42,43,44]. And several RCTs have revealed that febuxostat reduced SU levels in a dose-dependent manner [28, 29, 35, 38], which is in accordance with our results.
With regard to safety, there were no significant differences between ULTs and placebo for total AEs, serious AEs, or withdrawals due to AEs, except that lesinurad 400 mg/day plus allopurinol had a higher risk of total AEs than placebo. In addition, significantly more serious AEs were found in the lesinurad 400 mg/day plus allopurinol group than the allopurinol and lesinurad 200 mg/day plus allopurinol groups. These results are in agreement with the recent RCTs [26, 33]. Of note, febuxostat 120 mg/day had a lower risk of total AEs than allopurinol, febuxostat 40 mg/day, or lesinurad plus XOI therapy. Similarly, the recent network meta-analysis of 15 RCTs also demonstrated that febuxostat 120 mg/day was effective and well tolerated for lowering the SU level [45]. Notably, our analysis showed febuxostat 120 mg/day was associated with a high risk of withdrawals due to AEs than allopurinol. Becker et al. reported more withdrawals due to AEs in the febuxostat 120 mg/day group than the allopurinol group; the most common reasons were abnormal liver function and skin rashes [27]. Conversely, Schumacher et al. revealed that withdrawals due to AEs were similar among different doses of febuxostat and allopurinol [35]. For AEs per organ system, patients taking febuxostat 80 mg/day had less skin-related AEs than allopurinol but with more hepatic AEs than placebo. Additionally, the allopurinol group had less cardiovascular AEs than the placebo and febuxostat 80/240 mg/day groups. In line with this, Zhang et al. showed that long-term allopurinol treatment might decrease cardiovascular events [46]. However, the conclusion should be made with caution due to the paucity of data. And a recent meta-analysis revealed that febuxostat neither increased nor decreased cardiovascular events [47]. More long-term RCTs are needed to evaluate the safety of ULTs in hyperuricemic patients with gout.
Our study also has several limitations. Firstly, most trials enrolled hyperuricemic patients with gout who were not taking any ULT at screening, while 4 trials enrolled patients whose target SU level was not achieved after taking a urate-lowering drug [26, 30, 32, 33]. This might have introduced heterogeneity to the analysis. Secondly, most trials estimated the proportion of patients with the target SU level of < 6.0 mg/dl. But Dalbeth et al. reported the proportion of subjects achieving the SU level of < 5.0 mg/dl because all eligible patients in the study had more than 1 measurable tophus [30]. Nevertheless, sensitivity analysis did not show significant change of the results. Thirdly, the dose of allopurinol ranged from 100 to 900 mg/day between different trials, which might have influenced the efficacy of allopurinol. Finally, due to data limitations, we could not evaluate the rates of gout flares and resolution of tophi among ULTs. Further long-term prospective trials are warranted.
Conclusions
All ULTs are effective in achieving the target SU level compared with placebo in hyperuricemic patients with gout. Lesinurad in combination with febuxostat or allopurinol is effective in urate lowering, especially for patients with inadequate response to XOI monotherapy, but may have a high risk of AEs.
References
Hamburger M, Baraf HS, Adamson TC 3rd, Basile J, Bass L, Cole B, Doghramji PP, Guadagnoli GA, Hamburger F, Harford R, Lieberman JA 3rd, Mandel DR, Mandelbrot DA, McClain BP, Mizuno E, Morton AH, Mount DB, Pope RS, Rosenthal KG, Setoodeh K, Skosey JL, Edwards NL (2011) 2011 recommendations for the diagnosis and management of gout and hyperuricemia. Phys Sportsmed 39(4):98–123
Jia E, Zhu J, Huang W, Chen X, Li J (2018) Dual-energy computed tomography has limited diagnostic sensitivity for short-term gout. Clin Rheumatol 37(3):773–777
Xu L, Liu S, Guan M, Xue Y (2016) Comparison of prednisolone, etoricoxib, and indomethacin in treatment of acute gouty arthritis: an open-label, randomized, controlled trial. Med Sci Monit 22:810–817
Puig JG, de Miguel E, Castillo MC, Rocha AL, Martinez MA, Torres RJ (2008) Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides Nucleotides Nucleic Acids 27(6):592–595
Ioachimescu AG, Brennan DM, Hoar BM, Hazen SL, Hoogwerf BJ (2008) Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum 58(2):623–630
Rock KL, Kataoka H, Lai JJ (2013) Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol 9(1):13–23
Wu J, Zhang YP, Qu Y, Jie LG, Deng JX, Yu QH (2019) Efficacy of uric acid-lowering therapy on hypercholesterolemia and hypertriglyceridemia in gouty patients. Int J Rheum Dis 22(8):1445–1451
Perez-Ruiz F, Liote F (2007) Lowering serum uric acid levels: what is the optimal target for improving clinical outcomes in gout? Arthritis Rheum 57(7):1324–1328
Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, Kaldas M, Gogia M, Perez-Ruiz F, Taylor W, Liote F, Choi H, Singh JA, Dalbeth N, Kaplan S, Niyyar V, Jones D, Yarows SA, Roessler B, Kerr G, King C, Levy G, Furst DE, Edwards NL, Mandell B, Schumacher HR, Robbins M, Wenger N, Terkeltaub R, American College of R (2012) 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 64(10):1431–1446
Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda-Sanabria J, Coyfish M, Guillo S, Jansen TL, Janssens H, Liote F, Mallen C, Nuki G, Perez-Ruiz F, Pimentao J, Punzi L, Pywell T, So A, Tausche AK, Uhlig T, Zavada J, Zhang W, Tubach F, Bardin T (2017) 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 76(1):29–42
Arellano F, Sacristan JA (1993) Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother 27(3):337–343
Stamp LK, Day RO, Yun J (2016) Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol 12(4):235–242
Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, Becker MA (2005) Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci 76(16):1835–1847
Hoshide S, Takahashi Y, Ishikawa T, Kubo J, Tsuchimoto M, Komoriya K, Ohno I, Hosoya T (2004) PK/PD and safety of a single dose of TMX-67 (febuxostat) in subjects with mild and moderate renal impairment. Nucleosides Nucleotides Nucleic Acids 23(8–9):1117–1118
Mayer MD, Khosravan R, Vernillet L, Wu JT, Joseph-Ridge N, Mulford DJ (2005) Pharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairment. Am J Ther 12(1):22–34
Deeks ED (2017) Lesinurad: a review in hyperuricaemia of gout. Drugs Aging 34(5):401–410
Huneycutt E, Board C, Clements JN (2018) Lesinurad, a selective URAT-1 inhibitor with a novel mechanism in combination with a xanthine oxidase inhibitor, for hyperuricemia associated with gout. J Pharm Pract 31(6):670–677
Wu JY, Chang YT, Lin YC, Lee CH, Loh EW, Wu MY, Chang YS, Tam KW (2018) Efficacy and safety of lesinurad in patients with hyperuricemia associated with gout: a systematic review and meta-analysis of randomized controlled trials. PLoS One 38(11):1106–1119
Sutton AJ, Abrams KR (2001) Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res 10(4):277–303
Caldwell DM, Ades AE, Higgins JP (2005) Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331(7521):897–900
Lu G, Ades AE (2004) Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 23(20):3105–3124
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784
Higgins J, Green S (2011) Cochrane handbook of systematic reviews of interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from http://www.cochrane-handbook.org/. Accessed 31 May 2020
Zhao BC, Huang TY, Deng QW, Liu WF, Liu J, Deng WT, Liu KX, Li C (2017) Prophylaxis against atrial fibrillation after general thoracic surgery: trial sequential analysis and network meta-analysis. Chest 151(1):149–159
Salanti G, Ades AE, Ioannidis JP (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64(2):163–171
Bardin T, Keenan RT, Khanna PP, Kopicko J, Fung M, Bhakta N, Adler S, Storgard C, Baumgartner S, So A (2017) Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann Rheum Dis 76(5):811–820
Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N (2005) Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 353(23):2450–2461
Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, Vernillet L, Joseph-Ridge N (2005) Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum 52(3):916–923
Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, Lademacher C (2010) The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 12(2):R63
Dalbeth N, Jones G, Terkeltaub R, Khanna D, Kopicko J, Bhakta N, Adler S, Fung M, Storgard C, Baumgartner S, Perez-Ruiz F (2017) Lesinurad, a selective uric acid reabsorption inhibitor, in combination with febuxostat in patients with tophaceous gout: findings of a phase III clinical trial. Arthritis Rheumatol 69(9):1903–1913
Dalbeth N, Saag KG, Palmer WE, Choi HK, Hunt B, MacDonald PA, Thienel U, Gunawardhana L (2017) Effects of febuxostat in early gout: a randomized, double-blind, placebo-controlled study. Arthritis Rheumatol 69(12):2386–2395
Perez-Ruiz F, Sundy JS, Miner JN, Cravets M, Storgard C (2016) Lesinurad in combination with allopurinol: results of a phase 2, randomised, double-blind study in patients with gout with an inadequate response to allopurinol. Ann Rheum Dis 75(6):1074–1080
Saag KG, Fitz-Patrick D, Kopicko J, Fung M, Bhakta N, Adler S, Storgard C, Baumgartner S, Becker MA (2017) Lesinurad combined with allopurinol: a randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study). Arthritis Rheumatol 69(1):203–212
Saag KG, Whelton A, Becker MA, MacDonald P, Hunt B, Gunawardhana L (2016) Impact of febuxostat on renal function in gout patients with moderate-to-severe renal impairment. Arthritis Rheumatol 68(8):2035–2043
Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, Lademacher C, Joseph-Ridge N (2008) Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 59(11):1540–1548
Tausche AK, Alten R, Dalbeth N, Kopicko J, Fung M, Adler S, Bhakta N, Storgard C, Baumgartner S, Saag K (2017) Lesinurad monotherapy in gout patients intolerant to a xanthine oxidase inhibitor: a 6 month phase 3 clinical trial and extension study. Rheumatology (Oxford) 56(12):2170–2178
Wang S (2018) The efficacy of febuxostat and allopurinol in the treatment of gout with hyperuricemia. Pharmacotherapy 31(4(special)):1623–1627
Xu S, Liu X, Ming J, Chen S, Wang Y, Liu X, Liu H, Peng Y, Wang J, Lin J, Ji H, Liu B, Lu Y, Liu P, Zhang Y, Ji Q (2015) A phase 3, multicenter, randomized, allopurinol-controlled study assessing the safety and efficacy of oral febuxostat in Chinese gout patients with hyperuricemia. Int J Rheum Dis 18(6):669–678
Yu KH, Lai JH, Hsu PN, Chen DY, Chen CJ, Lin HY (2016) Safety and efficacy of oral febuxostat for treatment of HLA-B*5801-negative gout: a randomized, open-label, multicentre, allopurinol-controlled study. Scand J Rheumatol 45(4):304–311
Zhang F, Liu Z, Jiang L, Zhang H, Zhao D, Li Y, Zou H, Wang X, Li X, Shi B, Xu J, Yang H, Hu S, Qu S (2019) A randomized, double-blind, non-inferiority study of febuxostat versus allopurinol in hyperuricemic Chinese subjects with or without gout. Rheumatol Ther 6(4):543–557
Ye P, Yang S, Zhang W, Lv Q, Cheng Q, Mei M, Luo T, Liu L, Chen S, Li Q (2013) Efficacy and tolerability of febuxostat in hyperuricemic patients with or without gout: a systematic review and meta-analysis. Clin Ther 35(2):180–189
Faruque LI, Ehteshami-Afshar A, Wiebe N, Tjosvold L, Homik J, Tonelli M (2013) A systematic review and meta-analysis on the safety and efficacy of febuxostat versus allopurinol in chronic gout. Semin Arthritis Rheum 43(3):367–375
Frampton JE (2015) Febuxostat: a review of its use in the treatment of hyperuricaemia in patients with gout. Drugs 75(4):427–438
Tayar JH, Lopez-Olivo MA, Suarez-Almazor ME (2012) Febuxostat for treating chronic gout. Cochrane Database Syst Rev 11:CD008653
Li S, Yang H, Guo Y, Wei F, Yang X, Li D, Li M, Xu W, Li W, Sun L, Gao Y, Wang Y (2016) Comparative efficacy and safety of urate-lowering therapy for the treatment of hyperuricemia: a systematic review and network meta-analysis. Sci Rep 6:33082
Zhang T, Pope JE (2017) Cardiovascular effects of urate-lowering therapies in patients with chronic gout: a systematic review and meta-analysis. Rheumatology (Oxford) 56:1144–1153
Cuenca JA, Balda J, Palacio A, Young L, Pillinger MH, Tamariz L (2019) Febuxostat and cardiovascular events: a systematic review and meta-analysis. Int J Rheumatol 2019:1076189
Funding
This work was supported by the National Science Foundation of China (grant nos. 81871294, 81972204, and 81702327), the Natural Science Foundation of Guangdong Province (grant no. 2019A1515011097), the China Postdoctoral Science Foundation (grant nos. 2018M640834 and 2019T120756), the Science and Technology Planning Project of Guangzhou (grant no. 201904010089), the Innovation Program of Shenzhen (grant no. JCYJ20180508165208399), and the 111 Project from the Ministry of Education of China (grant no. D18010).
Author information
Authors and Affiliations
Contributions
J.R.G and N.S conceived the study. J.R.G, M.D.F, J.L, and X.F.L designed the study. M.D.F, J.L, and X.Y.W conducted the literature search and data extraction. M.D.F, B.C.Z, and X.F.L performed the network meta-analysis. M.D.F, J.L, and X.Y.W interpreted the data. M.D.F and J.L wrote the first draft of the manuscript. J.R.G, X.F.L, and N.S critically revised the manuscript and provided final approval of the manuscript.
Corresponding authors
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 120 kb).
Rights and permissions
About this article
Cite this article
Fan, M., Liu, J., Zhao, B. et al. Comparison of efficacy and safety of urate-lowering therapies for hyperuricemic patients with gout: a meta-analysis of randomized, controlled trials. Clin Rheumatol 40, 683–692 (2021). https://doi.org/10.1007/s10067-020-05272-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05272-4