Abstract
Objective
Hyperuricemia is a strong precursor of gout, which deteriorates patients’ health and quality of life. Sustained adherence to urate-lowering therapies (ULTs) is crucial for efficacy and therapeutic cost-effectiveness. Recently, several new ULTs have been proposed. We performed a systematic review and meta-analysis of randomized controlled trials (RCTs) to reassess the efficacy and safety of the current ULTs, focusing on adherence attrition-related adverse event reporting.
Method
The Bayesian network meta-analysis was applied to compare ULTs. Drug efficacy and safety were measured by whether the target level of serum urate acid was achieved and whether any adverse events occurred. The results were summarized using the pooled estimates of effect sizes (odds ratios), their precisions (95% credible interval), and the ranking probabilities.
Results and Conclusions
Thirty-nine RCTs were identified, accumulating 19,401 patients. Consistent with previous studies, febuxostat (≥ 40 mg/day) was superior to other monoagent ULTs. The new findings were as follows: (i) dual-agent ULTs were superior to febuxostat alone, and further surveillance on the adverse effects when lesinurad is uptitrated is needed, and (ii) terminalia bellerica 500 mg/day, a novel xanthine oxidase inhibitor (XOI) made of natural fruit extracts, and topiroxostat ≥ 80 mg/day, an XOI used mostly in Japan, could be new effective options for lowering the occurrence of adherence attrition events. Evidence from RCTs regarding second-line agents, such as probenecid and pegloticase, remains insufficient for clinical decision-making.
Key Points • Dual-agent ULTs were superior to febuxostat alone, and further surveillance on the adverse-effects when lesinurad is uptitrated is needed. • Terminalia bellerica 500 mg/day, a novel xanthine oxidase inhibitor (XOI) made of natural fruit extracts, and topiroxostat 80 mg/day, an XOI used mostly in Japan, could be new effective options for lowering the occurrence of adherence attrition events. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperuricemia is a physiological abnormality of increased serum uric acid (sUA) concentration owing to urate under-excretion, overproduction, or both [1,2,3,4]. This precipitates the deposition of monosodium urate crystals in the joints and generates tophi, leading to inflammatory reactions manifesting as stabbing pain, swelling, and limb deformation [3,4,5,6,7]. In addition to being an independent precursor of gout [8], hyperuricemia also promotes the incidence or progression of metabolic disorders in various organs [9,10,11,12], impairing somatic, mental, and social well-being [13, 14]. Its incidence is increasing across subgroups of age, sex, socioeconomic levels, and geographic areas [14,15,16,17]. This underlines the need for efficacious and quality hyperuricemia management worldwide in both public health and clinical medicine settings.
At present, hyperuricemia is managed using urate-lowering therapies (ULTs) to decrease the sUA level [1,2,3,4,5,6], and they are usually prescribed lifelong [18]. Acute gout flares (GFs), induced by rapidly lowering the sUA, are commonly encountered on initiation or dose up-titration of ULTs [19]. Accordingly, adherence attrition of ULT has been a long-standing issue and remains challenging. Since poor adherence dilutes the therapeutic effectiveness [20,21,22], it is essential for clinical decision-makers to understand whether any agents are themselves, other than patient attributes or administration modalities, more likely to give rise to adverse events (AEs).
Overall, the most substantial impact of low efficacy of or poor adherence to ULTs has been a long-term burden on health care cost and manpower [23, 24]. Evidence accumulated from 18 observational studies from 1974 to 2016 reported non-adherence rates of 21.5–82.6%, non-persistence rates (temporarily suspending for at least 30 days during therapy) of 54–87%, and post-discontinuation gouty arthritis relapse rates of 36.4–81% with higher likelihood of relapse in patients with poor pre-discontinuation sUA management [20, 25]. Irrespective of the extent of medical resources allocated to non-adherence patients, poor efficacy and low cost-effectiveness continue to be the concerns. Therefore, the current study assessed the efficacy and safety of ULTs with a focus on the occurrence of adherence attrition by type-specific AEs. In addition, a re-verification of ULT efficacy is necessary because several ULTs were approved or left out in the previous meta-analyses [26,27,28] (e.g., arhalofenate, lesinurad, topiroxostat, Terminalia, and dual agents). Hence, using the Bayesian network meta-analysis, we aimed to comprehensively compare all the market approved ULTs for the treatment of hyperuricemia.
Materials and methods
The operational hypothesis and outcomes
This meta-analysis study focused on three outcomes: (i) ULT efficacy, measured with the proportion of patients achieving the therapeutic target level of sUA (≤ 6 or 5 mg/dL in severe gout patients); (ii) safety of ULT, measured with the proportion of patients reported AEs of overall, serious AEs (SAE), and death; and (iii) adherence attrition events (AAEs) occurrence, measured with the proportion of patients reported discontinuation study medication owing to AEs (DCE), gout flare attacks (GFs), drug-related AEs (dAEs), and skin-related AEs (skAEs). Reports of withdrawals with no definite statements regarding the reasons were not included in DCE. The last part was set based on a postulate that a ULT associated with a higher occurrence of AAEs in the contexture of RCT implementation would be associated with lower adherence in a realized clinical circumstance, provided equivalent dosage titration was administered.
Searching logics and selection criteria
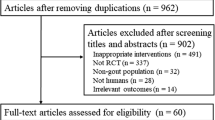
This meta-analysis included only peer-reviewed RCTs. We searched for studies in the following databases: PubMed, EMBASE, and Medline. For unpublished trials, trials published in conference abstracts, or protocol-only studies, we retrieved the most updated progress through the websites of trial registry systems (Appendix 1) for their peer-reviewed publications. The trial registry systems, logic, and searching and screening processes are portrayed in Fig. 1 and Appendix 1. Extended searching based on those eligible articles was performed by examining their “related articles” shown in the side-menu of PubMed and Google Scholar.
Studies were eligible for inclusion if (i) the publication year was from inception of databases to February 28, 2019; (ii) the study was conducted in line with RCT design with random allocation implemented at the individual patient level; (iii) the study had at least one arm adopted to the currently on-market or newly announced agents (as listed in Appendix 2); (iv) the study population was patients of primary hyperuricemia, gout, or both; and (v) the study endpoints included the proportion of patients whose sUAs were controlled under the target level by ULT. We excluded studies that (i) had no arms identical to the arms of other trials, (ii) were duplicated or non-relevant studies (e.g., RCT extension studies or governmental reports based on RCT results), (iii) were designed to evaluate only acute symptom alleviation (e.g., pain score), (iv) were implemented on patients of non-human, no-adults, healthy, or secondary hyperuricemia (e.g., tumor lysis syndrome, and pyrazinamide-induced hyperuricemia), (v) left the efficacy assessment using the aforementioned international consensus standard for effective therapy unpublished, (vi) performed an efficacy assessment using a standard apart from the consensus [1,2,3,4,5], or (vii) were non-English publication with full-text inaccessible. Eventually, 39 articles were left for subsequent analyses [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67].
Data extraction and quality assessment
Data assembling and methodological quality assessment were performed by one reviewer (Y-JL) and were double-checked by another reviewer (S-SC). Any inconsistency was resolved through discussion following the ad hoc guidelines for assessing the risk of bias in RCTs. A template of data extraction was established based on the extraction over the first 10 eligible articles. After data extraction of all 39 articles, a repeat extraction was performed in an order distinct from the initial extraction to make the extraction rules over all 39 articles as consistent as possible. Finally, a round of visual inspection was performed for confirmation (by reviewer YJL). Then, another reviewer (SSC) provided the final confirmation and consulted all other authors to integrate and resolve inconsistencies. Quality assessments of all the eligible studies were carried out using the Cochrane Collaboration’s tool for assessing the risk of bias [68].
Statistical analysis
RevMan 5.3 (Cochrane Review Manager, Cochrane Collaboration, Oxford, UK) was used to visualize the quality assessment results. The quantitative synthesis analysis was performed by Bayesian network meta-analysis with a random-effect model using the software R (version 3.5.2). The number of studies and patients contributing to head-to-head comparisons were visualized using network geometric plots. Pooled odds ratio (OR) and its 95% credible interval (CrI) were reported as the effect size estimates and the associated effect size precisions for comparison of efficacy, safety, and AAEs. Consistency between direct and indirect comparisons was tested using a node-splitting method and summarized with forest plots by direct and indirect evidence.
Of the final included 39 articles, four trials evaluated dual-agent ULTs; all others evaluated single prescriptions. We first evaluated the efficacy of ULTs under a network meta-analysis model incorporating all 39 articles as a pooled model (model MP) upon the assumption that the controlled groups in studies with the dual-agent regimens allopurinol + placebo and febuxostat + placebo were equivalent to the active-controlled groups for single prescriptions: allopurinol and febuxostat, respectively. Sensitivity analyses were then performed under three different scenarios of separated synthesis: (i) evaluation based on evidence solely from trials for single prescriptions (model MS) and those for dual-agent prescriptions (model MC); (ii) evaluation based on the aforementioned pooled model assumptions could be altered against the separate module evaluations; and (iii) since the estimates for the efficacy of ULT pegloticase were not stability in the assessment, another analysis based on evidence without this agent (model MP1) was performed to assess the influence of pegloticase in MP.
Results
The 39 eligible RCTs comprised a total of 19,401 patients. The characteristics of the study population are summarized in Table 1 and Appendix 3. The included studies were published from 1999 through 2019, and consisted of placebo and 14 ULTs (allopurinol, febuxostat, febuxostat immediate release (IR) formulation, febuxostat extended release (XR) formulation, Terminalia bellerica, Terminalia chebula, topiroxostat, arhalofenate, benzbromarone, lesinurad, probenecid, pegloticase, lesinurad + allopurinol, and lesinurad + febuxostat), and derived 33 active arms by varying formulations and dosages.
Characteristics of studies and quality assessment
In general, more male patients were enrolled in all the studies, with male patients making up > 80% of the total patient population. Of all studies, 83.8% had participants with a mean age of ≥ 50 years, and 21.6% had participants with a mean age of ≥ 60 years. Almost all studies had an average BMI of > 25 kg/m2 and a basal sUA level of > 6 mg/dL, except for one study [67]. Four studies [64,65,66,67] enrolled patients using dual-agent ULTs, and patients in these dual-agent ULTs trial had a lower basal sUA. Of the 39 studies, 8 (20.5%) studies were multinational trials, 12 (30.8%) studies were conducted solely in North America, 17 (43.6%) studies were conducted in Asia (of which Japan by itself contributed 12 studies (30.8%)), and 2 studies were (5.1%) in Australia. Over half of the RCTs (64.1% = 25/39) performed ex ante registration on a public website. It was also observed that trials conducted in Europe and North America enrolled more obese patients than those conducted in Asia.
Open-label, single blinding, and unclear blinding trials were rated as high risk in either performance bias or detection bias, or both (Appendix 4). Most studies explained the blinding maneuvers in patient groups and investigators, but few explained blinding of assessors. The reporting bias mainly lacked complete AE reports. Only a few trials explicitly stated that they did not receive any funding from pharmaceutical industries and reported ex ante registries, and were therefore rated as low risk in other bias.
Arms administering allopurinol, febuxostat, and placebo contributed the most majority of evidence for direct comparison: 21 (55.3%) trials had at least one arm using allopurinol (Al and Alc in Appendix 5) [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44, 46, 47, 64,65,66], and 22 (57.9%) trials used febuxostat [29,30,31,32, 35,36,37,38,39, 43, 44, 46,47,48,49,50,51,52,53,54,55,56, 67]. The other ULTs contributed less evidence. The study by White WB enrolled the most number of patients, and comprised of nearly 32% (= 6190/19,401) of the total sample size [45]. However, the White WB study was included only in efficacy analyses and not the other analyses, because the White WB study reported AEs only concerning cardiovascular events.
Network meta-analyses of efficacy, safety, and AAE occurrence
The network geometry plots for evaluating efficacy and safety with MP and for evaluating DCEs, GFs, dAEs, and skAEs are displayed in Fig. 2. Of the included trials, 32 (84.2%) trials reported 670 (5.6% in 11,887) DCEs, 27 (71.1%) trials reported 1781 (20.5% in 8677) GFs, 17 (44.7%) trials reported 1245 (17.7% in 7040) dAEs, and 16 (42.1%) trials reported 307 (4.7% in 6480) skAEs. AAEs occurrence was observed to differ substantially over the time of study publication year. Classifying studies by the publication year (1999–2009, vs. 2010–2019), reporting rates declined in GFs (6/7 = 85.7% vs. 21/31 = 67.7%) and skAEs (6/7 = 85.7% vs. 10/31 = 32.3%), but an increased in dAEs (1/7 = 14.3% vs. 16/31 = 51.6%) was observed.
Network geometry plots of the assessments analyzed under a pooled model (Mp) in this study. (1) The edges connecting the nodes indicate head-to-head comparisons. The thicker the edges, the higher the number of studies contributing to the evidence. The sizes of nodes were proportional to the sample sizes of the administered ULT in this meta-analysis. Code 911 indicates placebo. The other codes of ULTs are noted below by pharmacologic attributes. (2) Xanthine oxidase inhibitors (XOIs): 111 = Allopurinol, 121 = Febuxostat 20 mg/day, 122 = Febuxostat 40 mg/day, 123 = Febuxostat 60 mg/day, 124 = Febuxostat 80 mg/day, 125 = Febuxostat 120 mg/day, 126 = Febuxostat 240 mg/day, 127 = Febuxostat 40/80 mg/day, 128 = Febuxostat XR 40 mg/day, 129 = Febuxostat XR 80 mg/day, 131 = Terminalia bellerica 250 mg/day, 132 = Terminalia bellerica 500 mg/day, 133 = Terminalia chebula 500 mg/day, 141 = Topiroxostat 40 mg/day, 142 = Topiroxostat 60 mg/day, 143 = Topiroxostat 80 mg/day, 144 = Topiroxostat 120 mg/day, 145 = Topiroxostat 160 mg/day, 211 = allopurinol + colchicine, 221 = allopurinol + placebo, 231 = Febuxostat 80 mg/day + placebo. (3) Uricosuric drugs: 311 = Arhalofenate 600 mg/day, 312 = Arhalofenate 800 mg/day, 321 = Benzbromarone, 331 = Lesinurad 400 mg/day, 341 = Probenecid 2 g/day. (4) Recombinant porcine-like uricase drugs: 411 = Pegloticase 4 mg/2 weeks, 412 = Pegloticase 8 mg/2 weeks, 413 = Pegloticase 8 mg/4 weeks, 414 = Pegloticase 12 mg/4 weeks. (5) Uricosuric combined with XOI prescription: 811 = Lesinurad 200 mg/day + allopurinol, 812 = Lesinurad 400 mg/day + allopurinol, 813 = Lesinurad 600 mg/day + allopurinol, 821 = Lesinurad 200 mg/day + febuxostat 80 mg/day, 822 = Lesinurad 400 mg/day + febuxostat 80 mg/day
The pooled OR estimates and the ranking probabilities of efficacy assessments are displayed in Tables 2, 3, and 4 (the analysis results for overall AEs and AAEs assessments are listed in Appendices 6 and 7; the corresponding 95% CrIs and forest plots with selected reference ULTs in Fig. 3). All active agents exhibited significantly favorable efficacy than placebo (see the last row of Table 2 and Fig. 3a), except those extracted from Terminalia. bellerica 250 mg/day and T. chebula 500 mg/day (pooled ORs [95% CrI] were 0.79 [0.1, 8.6] and 0.33 [0.1, 2.2], respectively). Of the whole estimation sampling history, there was a 54% probability for placebo to be ranked as the least efficient agent and 99.9% to be ranked as one of the lowest three (Table 3).
Figure 3 b shows the forest plot for comparing the efficacy of different ULTs as compared to allopurinol. Among xanthine oxidase inhibitors (XOIs), febuxostat was found to be significantly superior to allopurinol (OR estimates: 1.97–20.41), except for the lowest dosage and the varying dosage (20 mg/day and mixed-40/80 mg/day in an arm). The two newly launched formulations (XR, 40 and 80 mg/day) were equivalent to the corresponding standard formulations (OR for XR vs. IR: 1.57 [0.9, 2.9] and 1.09 [0.6, 2] for febuxostat 40 and 80 mg/day). Topiroxostat was non-inferior to allopurinol, had a lower efficacy than febuxostat 120–240 mg/day, and was superior to T. bellerica 250 mg/day or T. chebula 500 mg/day. Among uricosuric agents, benzbromarone was superior to all of the other four but was inferior to febuxostat 120 mg/day (0.24 [0.07,0.83]) and 240 mg/day (0.09 [0.02,0.37]). All dual-agent ULTs had a superior efficacy to monotherapy of lesinurad (ORs: 10.66–39.78), allopurinol (ORs: 4.7–15.47), and febuxostat (ORs: 2.09–7.84). In general, the dual agents had a superior efficacy to XOI agents and uricosuric agents (rankings: 6–14 vs. 5–34 and 18–31), and most XOI agents had a superior efficacy to the uricosuric agents (Table 3).
Patients receiving placebo had lower overall AEs than those receiving probenecid 2 g/day, pegloticase 4 mg/2 weeks and 8 mg/4 weeks, and lesinurad 400 mg/day + allopurinol (see the last column of Table 2; Fig. 3c).
Of the 38 eligible RCTs, 29 (76.3%) reported 239 (1.85%) SAEs and 34 (89.5%) reported 11 (0.085%) cases of death in 12,900 patients (see Appendix 9). Given that the number of SAEs and deaths was scarce, the data are insufficient for definitive conclusions. However, it was observed that dual-agent regimens have substantially higher risk of SAE (lesinurad 400/day + allopurinol vs. placebo, allopurinol, febuxostat 40 mg/day, and lesinurad 200/day + allopurinol: 3.2 [1.4, 7.5], 1.97 [1, 4], 2.56 [1.1, 6.1], and 2.08 [1.1, 4.2]) and the dual-agents had higher risk of all-cause AEs than most XOIs (rankings: 7–15 vs. 6–29; Appendix 7 (a)).
The top three single agents with the most frequent DCEs were pegloticase, probenecid, and lesinurad, respectively (ranking: 2–6). Compared with allopurinol, febuxostat, topiroxostat, arhalofenate, or benzbromarone monotherapy (ranking: 7–29), and dual agents appeared to have more DCEs. The occurrence rates of DCE were also observed to increase with the doses of lesinurad. The Fx8 (febuxostat of 80 mg/day XR formulations) tended to be associated with fewer DCEs than febuxostat of IR formulations (rankings: 25 vs. 15). This could result from the extended releasing pharmacological characteristics of Fx8. It was also observed that DCEs increased for febuxostat ≥ 40 mg/day with an increase in dosage (trend in ORs: 0.91~1.86, in rankings: 20~10). The detailed assessment results of DCEs are in Appendix 6 (a), Appendix 7 (b), and Appendix 8 (b) and (f).
An increased in risk of GFs were observed in three comparison modes (see Appendix 6 (a), Appendix 7 (c), and Appendix 8 (b) and (g) for detailed results): (1) febuxostat > 80 mg/day and dual-agent vs. placebo (OR < 1 in the last column of Appendix 6 (a); rankings: 4–12 and 5–10 vs. 18); (2) febuxostat ≥ 120 mg/day compared with topiroxostat (OR: 0.05–0.77; rankings: 7–12 vs. 6–26); (3) topiroxostat > 80 mg/day vs. ≤ 60 mg/day (OR: 8.29 [1.1–242.4] and 8.2 [1.1–229.2]).
Compared to placebo, a higher risk of dAEs was observed in dual-agent ULTs, lesinurad, and febuxostat of 40, 80, 40/80, 80 XR, and 120 mg/day (rankings 14 vs. 2–6, 1, 11, 2–14, and 15–18). The data on skAEs were too scarce to make a conclusion. The detailed assessment results of dAEs are in Appendix 6 (b), Appendix 7 (d), and Appendix 8 (c) and (h).
Heterogeneity and inconsistency
Substantial heterogeneity were observed for the following assessments: (i) efficacy assessment in comparison of febuxostat 60 mg/day and topiroxostat 160 mg/day vs. allopurinol (p = 0.049 and < 0.001) and of topiroxostat 160 mg/day and placebo vs. topiroxostat 120 mg/day (p = 0.00025 and 0.00025); (ii) all-cause AE assessments, topiroxostat 120 mg/day vs. allopurinol (p = 0.00175) and placebo vs. topiroxostat 120 mg/day (p = 0.001); for DCEs, febuxostat 80 mg/day vs. febuxostat 40 mg/day (p = 0.035) and topiroxostat 160 mg/day vs. topiroxostat 120 mg/day (p = 0.004); and (iii) dAE assessments, febuxostat 40 mg/day vs. allopurinol (p = 0.028), placebo vs. febuxostat 40 mg/day and topiroxostat 120 mg/day (p = 0.013 and 0.029) (see Appendix 10).
Sensitivity analysis
The discrepancy between the full-pooled model (MP) and the separated models (MS and MC, Appendix 11.A) were negligible for most comparisons except for pegloticase. Because these estimates were derived from fragile evidence, broad variation among the iterated estimates contributed to such vast discrepancy. Nevertheless, most differences between the estimates of the model with and without pegloticase (MP and MP1; Appendix 11.B) were very minor. Moreover, incorporating pegloticase into the full model did not alter the findings for other agents; therefore, the results based on model MP are retained in our main text, and the rest are attached as Supplementary Information.
Discussions
In general, the main result of this study is in line with the previous meta-analysis comparing the efficacy of different ULTs. Similar to the network meta-analysis published by Li S in 2016 [26], it was found that febuxostat was associated with the best urate-lowering efficacy among all the monoagent ULTs investigated. In clinical practice, both allopurinol and febuxostat are recommended as first-line drugs, but febuxostat is only prescribed when allopurinol is contraindicated or not tolerated. This is because febuxostat is far more expensive than allopurinol [69], and the AE profile of febuxostat is less well characterized than allopurinol. Allopurinol was approved by the US Food and Drug Administration (FDA) in 1965, but febuxostat was only approved by US-FDA in 2009. Hence, febuxostat has a much shorter period of post-marketing surveillance than allopurinol, and some rare AE might not be reported with the smaller patient population. In fact, a recent study by White WB [45], which has a sample size of 6190 patients, suggested that cardiovascular mortalities were observed to be higher with febuxostat than with allopurinol. Therefore, the routine clinical practice of first prescribing allopurinol when it is not contraindicated for patients newly diagnosed with hyperuricemia should be continued.
However, this study has several improvements in study design, when compared to previous reviews [20, 25,26,27,28]. First, the Bayesian network meta-analysis was used to facilitate exhaustive mutual comparison, and this allows the incorporation of zero-event observations. Second, the analysis was based on data only from RCTs, where potential confounding factors could be controlled as much as possible. Third, the synthesis analysis included newly launched [27, 64,65,66,67], innovative [56], new formulations [48, 49], and agent used only in Japan [33, 34, 57,58,59]. Fourth, since poor adherence is related to worse sUA control [21, 22] and null efficacy, adherence attrition-related AEs were also evaluated to elucidate the necessity and direction of future cost-effectiveness analysis for ULTs.
Profile of patients with gout have sex discrepancy: women of both prevalent and incident cases were approximately 6–10 years older at initial diagnosis [70], had a higher burden of comorbidities, had different comorbidity profiles, were more obese [71], had fewer dietary triggers (seafood, red meat, hard liquor, wine, and beer) [71], and had more diuretic-triggered gout flares [70, 71]. Nevertheless, current evidence dominantly based on male individuals.
The reporting of AAEs was a little inadequate. First, the disclosure rate of dAEs was < 50% and that of GFs was merely 71%, both events considerably influence patient adherence to therapy [72]. Second, the reporting rate by publication years (1999–2009 vs. 2010–2019): declined in GFs and skAEs and increased in dAEs. This lower reporting could be attributed to several reasons, including, null finding, favorable-outcome selection, or changing viewpoints. The lower reporting of AAEs made evidence retrieval and clinical decision-making difficult. To make future comprehensive utility and cost-effectiveness analysis for ULTs more feasible, emphasis should be put on sophisticated AE reporting (e.g., frequency and time of GFs, time to DCEs), especially for RCTs involving chronic diseases that necessitate long-term medication use, where interferences can be controlled.
The results of our study should be interpreted in light of both strengths and weaknesses. The main strength of this study is including only peer-reviewed RCTs to reduce the effects of confounding factors as low as possible. Apart from the sUA lowering induced GF [19], intolerance, or allergy [17, 73, 74], the interference toward ULT adherence could come from the following sources: heterogeneous demographics [18]; socioeconomic level, health care capability, and health literacy [75]; physical disability [25, 76,77,78,79]; strategies on patient management [75]; physicians’ prescription habits, specialties, and competence [17, 18, 76, 77, 80, 81]; and information accessibility [80]. Thus, based on the study design, differences in adherence attrition occurrence observed here was likely owing to the ULT agents.
In addition, the Bayesian methods applied here, in contrast to the Frequentist method (e.g., computation in STATA), facilitated the incorporation of zero-event observations without requiring a technical correction of data, while imposed more variation on pooled estimates. The included evidence incorporated direct comparison to active controls (allopurinol or febuxostat) for every ULTs, except for pegloticase. However, the unique bridge connecting pegloticase and other agents presented a zero-event observation, i.e., no patients achieved the target therapeutic effect in the placebo arm [63]. These resulted in poor precision of the assessment for pegloticase. The zero-event observations were found in 11 out of the 39 included RCTs. This introduced a discrepancy in study estimates between this and a previous review [26].
There are several important limitations to this study. First, non-pharmacological hyperuricemia management interventions were excluded in this study, for example, weight loss, economic and fundamental disease risks modification, lifestyle modification [7], and healthier diet [82]. These interventions were excluded for lacking comparability, and patient adherence remains the key issue for their realistic effectiveness. Second, despite trying to be as compressive as possible to investigate all the trials on ULTs, some ULTs (e.g., azapropazone, benziodarone, sulfinpyrazone, ethebencid, zoxazolamine, and ticrynafen) were not included due to the evidence scarcity. Reasons for the limited available evidence include side-effects (e.g., ticrynafen and benzbromarone users are prone to blood pressure disorder, hepatotoxicity, and nephrotoxicity), relatively newly approved drugs (e.g., arhalofenate), unfavorable mode of administration (e.g., pegloticase, intravenous infusion administered), factors related to cost or standard practices of the regional physician community, and market factors such as profitability by patent in-force (e.g., azapropazone and benzbromarone). Third, the RCTs that have been published were predominantly conducted in economically developed areas, where citizens are more susceptible to hyperuricemia [15, 24]. It is unclear if the same result will be obtained on patients from the developing countries. Fourth, treatment options are not equally available in different parts of the world, and it is unclear if the same efficacy/AE profile will be obtained from a different ethnic group. For example, in the US, trials on ULTs are limited to febuxostat, allopurinol, probenecid, and pegloticase, while trials on Topiroxostat can only be found in Japan. Finally, pharmaceutical companies supported most RCTs, and it is hard to completely get rid of profit-counting in the design of the trial [83, 84].
To conclude, evidence of RCTs regarding the second-line agents and the XOIs launched after febuxostat is scarce and uneven across nations. We cannot overemphasize the need for more sophisticated reporting of adherence attrition AEs in order to allow cost-effectiveness analysis. Comparisons on the efficacy, safety, and adherence attrition occurrence over various ULTs revealed the following conclusions: (i) febuxostat (≥ 40 mg/day) and the dual regimens (XOIs + uricosuric agents) were superior to others in efficacy, but lesinurad-based dual regimens require further surveillance on their AE pattern when lesinurad is up-titrated; (ii) evaluation on long-existed second-line agents (probenecid and pegloticase) remains insufficient; (iii) T. bellerica 500 mg/day, a novel natural fruit extract–based XOI, could be a cost-effective alternative for superior efficacy to placebo and lower AE occurrence; (iv) topiroxostat ≥ 80 mg/day could be equivalent to febuxostat, although the evidence is largely dependent on a single nation (Japan); and (v) more evidence is required for arhalofenate.
References
Richette P et al (2017) 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 76(1):29–42
Khanna D et al (2012) American college of rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res 64(10):1431–1446
Hui M, Carr A, Cameron S, Davenport G, Doherty M, Forrester H, Jenkins W, Jordan KM, Mallen CD, McDonald T, Nuki G, Pywell A, Zhang W, Roddy E, British Society for Rheumatology Standards, Audit and Guidelines Working Group (2017) The British Society for Rheumatology guideline for the management of gout. Rheumatology 56(7):e1–e20
Yu KH, Chen DY, Chen JH, Chen SY, Chen SM, Cheng TT et al (2018) Management of gout and hyperuricemia: Multidisciplinary consensus in Taiwan. Int J Rheum Dis. 21(4):772–787
Shekelle PG, Newberry SJ, FitzGerald J, Motala A, O'Hanlon CE, Tariq A, Okunogbe A, Han D, Shanman R (2017) Management of gout: a systematic review in support of an American college of physicians clinical practice guideline. Ann Intern Med 166(1):37–51
Ashiq K et al (2018) A systematic review on the prevalence, pathophysiology, diagnosis, management and treatment of gout (2007-2018). GSC Biol Pharm Sci 5(1):050–055
Ahmed S et al (2018) Pathophysiology, clinical consequences, epidemiology and treatment of hyperurecemic gout. RADS J Pharm Pharm Sci 6(1):88–93
Stamp L, Morillon MB, Taylor WJ, Dalbeth N, Singh JA, Lassere M et al (2018) Serum urate as surrogate endpoint for flares in people with gout: A systematic review and meta-regression analysis. Semin Arthritis Rheum. 48(2):293–301
Luo Q, Xia X, Li B, Lin Z, Yu X, Huang F (2019) Serum uric acid and cardiovascular mortality in chronic kidney disease: a meta-analysis. BMC Nephrol. 20(1):18
Liu J, Tao L, Zhao Z, Mu Y, Zou D, Zhang J et al (2018) Two-Year Changes in Hyperuricemia and Risk of Diabetes: A Five-Year Prospective Cohort Study. J Diabetes Res. 2018:6905720
Zheng X, Gong L, Luo R, Chen H, Peng B, Ren W et al (2017) Serum uric acid and non-alcoholic fatty liver disease in non-obesity Chinese adults. Lipids Health Dis. 16(1):202
Zhou F et al (2019) Association of serum uric acid levels with the incident of kidney disease and rapid eGFR decline in Chinese individuals with eGFR > 60 mL/min/1.73 m2 and negative proteinuria. Clin Exp Nephrol 23(7):871–879
Fu T et al (2018) Depression and anxiety correlate with disease-related characteristics and quality of life in Chinese patients with gout: a case-control study. Psychol Health Med 23(4):400–410
Kiadaliri AA, Englund M, Uhlig T (2018) Burden of gout in the Nordic region, 1990–2015: findings from the Global Burden of Disease Study 2015. Scand J Rheumatol 47(5):410–417
Song P, Wang H, Xia W, Chang X, Wang M, An L (2018) Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci Rep. 8(1):4314
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M (2015) Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 74(4):661–667
McGowan B, Bennett K, Silke C, Whelan B (2016) Adherence and persistence to urate-lowering therapies in the Irish setting. Clin Rheumatol. 35(3):715–721
Janssen CA, Oude Voshaar MAH, Vonkeman HE, Krol M, van de Laar M (2018) A retrospective analysis of medication prescription records for determining the levels of compliance and persistence to urate-lowering therapy for the treatment of gout and hyperuricemia in The Netherlands. Clin Rheumatol. 37(8):2291–2296
Yamanaka H, Togashi R, Hakoda M, Terai C, Kashiwazaki S, Dan T, Kamatani N (1998) Optimal range of serum urate concentrations to minimize risk of gouty attacks during anti-hyperuricemic treatment. Adv Exp Med Biol 431:13–18
Beslon V, Moreau P, Maruani A, Maisonneuve H, Giraudeau B, Fournier JP (2018) Effects of Discontinuation of Urate-Lowering Therapy: A Systematic Review. J Gen Intern Med. 33(3):358–366
Mikuls TR, Cheetham TC, Levy GD, Rashid N, Kerimian A, Low KJ, Coburn BW, Redden DT, Saag KG, Foster PJ, Chen L, Curtis JR (2019) Adherence and outcomes with urate-lowering therapy: a site-randomized trial. Am J Med 132(3):354–361
Hill-McManus D, Soto E, Marshall S, Lane S, Hughes D (2018) Impact of non-adherence on the safety and efficacy of uric acid-lowering therapies in the treatment of gout. Br J Clin Pharmacol. 84(1):142–152
Shields G, Beard SM (2015) A systematic review of the economic and humanistic burden of gout. PharmacoEconomics 33(10):1029–1047
Zhu Y, Choi HK, Pandya BJ (2011) Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum 63(10):3136–3141
Scheepers LEJM et al (2018 Apr) (2018) Medication adherence among patients with gout: a systematic review and meta-analysis. Semin Arthritis Rheum. 47(5):689–702
Li S, Yang H, Guo Y, Wei F, Yang X, Li D et al (2016) Comparative efficacy and safety of urate-lowering therapy for the treatment of hyperuricemia: a systematic review and network meta-analysis. Sci Rep. 6:33082
Wu J-Y, Chang YT, Lin YC, Lee CH, Loh EW, Wu MY, Chang YS, Tam KW (2018) Efficacy and safety of lesinurad in patients with hyperuricemia associated with gout: a systematic review and meta-analysis of randomized controlled trials. Pharmacotherapy 38(11):1106–1119
Franca Gois PH, de Moraes Souza ER (2017) Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane Database Syst Rev 4(4):CD008652. Published 2017 Apr 13. https://doi.org/10.1002/14651858.CD008652.pub3
Becker MA et al (2005) Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 353(23):2450–2461
Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E et al (2010) The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 12(2):R63
Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J et al (2015) Effects of Xanthine Oxidase Inhibition in Hyperuricemic Heart Failure Patients: The Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) Study. Circulation. 131(20):1763–1771
Goldfarb DS, MacDonald PA, Gunawardhana L, Chefo S, McLean L (2013) Randomized controlled trial of febuxostat versus allopurinol or placebo in individuals with higher urinary uric acid excretion and calcium stones. Clin J Am Soc Nephrol. 8(11):1960–1967
Hosoya T, Ogawa Y, Hashimoto H, Ohashi T, Sakamoto R (2016) Comparison of topiroxostat and allopurinol in Japanese hyperuricemic patients with or without gout: a phase 3, multicentre, randomized, double-blind, double-dummy, active-controlled, parallel-group study. J Clin Pharm Ther. 41(3):290–297
Hosoya T, Sasaki T, Ohashi T (2017) Clinical efficacy and safety of topiroxostat in Japanese hyperuricemic patients with or without gout: a randomized, double-blinded, controlled phase 2b study. Clin Rheumatol. 36(3):649–656
Huang X, Du H, Gu J, Zhao D, Jiang L, Li X et al (2014) An allopurinol-controlled, multicenter, randomized, double-blind, parallel between-group, comparative study of febuxostat in Chinese patients with gout and hyperuricemia. Int J Rheum Dis. 17(6):679–686
Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, Ueda T, Yamamoto T, Yamanaka H, Matsuzawa Y (2011) An allopurinol-controlled, randomized, double-dummy, double-blind, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: phase 3 clinical study. J Clin Rheumatol 17(4 Suppl 2):S13–S18
Naoyuki K et al (2011) An allopurinol-controlled, multicenter, randomized, open-label, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: phase 2 exploratory clinical study. J Clin Rheumatol 17(4 Suppl 2):S44–S49
Kumar B, Agarwal PK (2013) Comparative evaluation of efficacy and safety profile of febuxostat with allopurinol in patients with hyperuricemia and gout. Int J Pharm Med Biol Sci 2(4):52–56
Nakagomi A et al (2015) Effects of febuxostat and allopurinol on the inflammation and cardiac function in chronic heart failure patients with hyperuricemia. IJC Metab Endocrine 8:46–55
Perez-Ruiz F, Calabozo M, Fernandez-Lopez MJ, Herrero-Beites A, Ruiz-Lucea E, Garcia-Erauskin G et al (1999) Treatment of chronic gout in patients with renal function impairment: an open, randomized, actively controlled study. J Clin Rheumatol. 5(2):49–55
Poiley J, Steinberg AS, Choi YJ, Davis CS, Martin RL, McWherter CA et al (2016) A randomized, double-blind, active- and placebo-controlled efficacy and safety study of arhalofenate for reducing flare in patients with gout. Arthritis Rheumatol. 68(8):2027–2034
Reinders MK, Haagsma C, Jansen TL, van Roon EN, Delsing J, van de Laar MA et al (2009) A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300-600 mg/day versus benzbromarone 100-200 mg/day in patients with gout. Ann Rheum Dis. 68(6):892–897
Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J et al (2008) Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 59(11):1540–1548
Sezai A, Soma M (2013) Nakata K-i, Hata M, Yoshitake I, Wakui S, et al. Comparison of Febuxostat and Allopurinol for Hyperuricemia in Cardiac Surgery Patients (NU-FLASH Trial). Circulation Journal. 77(8):2043–2049
White WB et al (2018) Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 378(13):1200–1210
Xu S, Liu X, Ming J, Chen S, Wang Y, Liu X et al (2015) A phase 3, multicenter, randomized, allopurinol-controlled study assessing the safety and efficacy of oral febuxostat in Chinese gout patients with hyperuricemia. Int J Rheum Dis. 18(6):669–678
Yu KH, Lai JH, Hsu PN, Chen DY, Chen CJ, Lin HY (2016) Safety and efficacy of oral febuxostat for treatment of HLA-B*5801-negative gout: a randomized, open-label, multicentre, allopurinol-controlled study. Scand J Rheumatol 45(4):304–311
Gunawardhana L, Becker MA, Whelton A, Hunt B, Castillo M, Saag K (2018) Efficacy and safety of febuxostat extended release and immediate release in patients with gout and moderate renal impairment: phase II placebo-controlled study. Arthritis Res Ther. 20(1):99
Saag KG, Becker MA, Whelton A, Hunt B, Castillo M, Kisfalvi K, Gunawardhana L (2019) Efficacy and safety of febuxostat extended and immediate release in patients with gout and renal impairment: a phase III placebo-controlled study. Arthritis Rheumatol 71(1):143–153
Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald P, Palo WA, Eustace D, Vernillet L, Joseph-Ridge N (2005) Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum 52(3):916–923
Naoyuki K et al (2011) Placebo-controlled, double-blind study of the non-purine-selective xanthine oxidase inhibitor Febuxostat (TMX-67) in patients with hyperuricemia including those with gout in Japan: phase 3 clinical study. J Clin Rheumatol 17(4 Suppl 2):S19–S26
Naoyuki K et al (2011) Placebo-controlled double-blind dose-response study of the non-purine-selective xanthine oxidase inhibitor febuxostat (TMX-67) in patients with hyperuricemia (including gout patients) in Japan: late phase 2 clinical study. J Clin Rheumatol 17(4 Suppl 2):S35–S43
Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, Ito S, Yamamoto T, Tomino Y, Ohno I, Shibagaki Y, Iimuro S, Imai N, Kuwabara M, Hayakawa H, Ohtsu H, Ohashi Y, FEATHER Study Investigators (2018) Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis 72(6):798–810
Dalbeth N, Saag KG, Palmer WE, Choi HK, Hunt B, MacDonald P, Thienel U, Gunawardhana L (2017) Effects of febuxostat in early gout: a randomized, double-blind, placebo-controlled study. Arthritis Rheumatol 69(12):2386–2395
Saag KG, Whelton A, Becker MA, MacDonald P, Hunt B, Gunawardhana L (2016) Impact of febuxostat on renal function in gout patients with moderate-to-severe renal impairment. Arthritis Rheumatol 68(8):2035–2043
Usharani P, Nutalapati C, Pokuri VK, Kumar CU, Taduri G (2016) A randomized, double-blind, placebo-, and positive-controlled clinical pilot study to evaluate the efficacy and tolerability of standardized aqueous extracts of Terminalia chebula and Terminalia bellerica in subjects with hyperuricemia. Clin Pharmacol Adv Appl 8:51–59
Hosoya T, Ohno I, Nomura S, Hisatome I, Uchida S, Fujimori S, Yamamoto T, Hara S (2014) Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin Exp Nephrol 18(6):876–884
Hosoya T, Sasaki T, Hashimoto H, Sakamoto R, Ohashi T (2016) Clinical efficacy and safety of topiroxostat in Japanese male hyperuricemic patients with or without gout: an exploratory, phase 2a, multicentre, randomized, double-blind, placebo-controlled study. J Clin Pharm Ther 41(3):298–305
Wada T, Hosoya T, Honda D, Sakamoto R, Narita K, Sasaki T, Okui D, Kimura K (2018) Uric acid-lowering and renoprotective effects of topiroxostat, a selective xanthine oxidoreductase inhibitor, in patients with diabetic nephropathy and hyperuricemia: a randomized, double-blind, placebo-controlled, parallel-group study (UPWARD study). Clin Exp Nephrol 22(4):860–870
Reinders MK, van Roon E, Jansen TL, Delsing J, Griep EN, Hoekstra M, van de Laar M, Brouwers JR (2009) Efficacy and tolerability of urate-lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Ann Rheum Dis 68(1):51–56
Tausche AK et al (2017) Lesinurad monotherapy in gout patients intolerant to a xanthine oxidase inhibitor: a 6 month phase 3 clinical trial and extension study. Rheumatology (United Kingdom) 56(12):2170–2178
Sundy JS, Becker MA, Baraf HS, Barkhuizen A, Moreland LW, Huang W, Waltrip RW 2nd, Maroli AN, Horowitz Z, Pegloticase Phase 2 Study Investigators (2008) Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol-conjugated uricase) in patients with treatment-failure gout: results of a phase II randomized study. Arthritis Rheum 58(9):2882–2891
Sundy JS et al (2011) Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 306(7):711–720
Bardin T, Keenan RT, Khanna PP, Kopicko J, Fung M, Bhakta N, Adler S, Storgard C, Baumgartner S, So A (2017) Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann Rheum Dis 76(5):811–820
Perez-Ruiz F, Sundy JS, Miner JN, Cravets M, Storgard C, RDEA594-203 Study Group (2016) Lesinurad in combination with allopurinol: results of a phase 2, randomised, double-blind study in patients with gout with an inadequate response to allopurinol. Ann Rheum Dis 75(6):1074–1080
Saag KG, Fitz-Patrick D, Kopicko J, Fung M, Bhakta N, Adler S, Storgard C, Baumgartner S, Becker MA (2017) Lesinurad combined with allopurinol: a randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study). Arthritis Rheumatol 69(1):203–212
Dalbeth N et al (2017) Lesinurad, a selective uric acid reabsorption inhibitor, in combination with febuxostat in patients with tophaceous gout: findings of a phase III clinical trial. Arthritis Rheumatol 69(9):1903–1913
Deeks JJ, Higgins JPT, Altman DG (2011) Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, ed. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration
Stamp LK, Chapman PT (2014) Urate-lowering therapy: current options and future prospects for elderly patients with gout. Drugs Aging 31(11):777–786
Drivelegka P, Sigurdardottir V, Svard A, Jacobsson LTH, Dehlin M (2018) Comorbidity in gout at the time of first diagnosis: sex differences that may have implications for dosing of urate lowering therapy. Arthritis Res Ther. 20(1):108
Harrold LR, Etzel CJ, Gibofsky A, Kremer JM, Pillinger MH, Saag KG et al (2017) Sex differences in gout characteristics: tailoring care for women and men. BMC Musculoskelet Disord. 18(1):108
Singh JA (2018) Goals of gout treatment: a patient perspective. Clin Rheumatol 37(9):2557–2566
Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS et al (2015) Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. 351:h4848
Ramasamy SN, Korb-Wells CS, Kannangara DR, Smith MW, Wang N, Roberts DM, Graham GG, Williams KM, Day RO (2013) Allopurinol hypersensitivity: a systematic review of all published cases, 1950-2012. Drug Saf 36(10):953–980
Latif Z, Abhishek A (2018) Are doctors the best people to manage gout? Is there a role for nurses and pharmacists? Curr Rheumatol Rep 20(3):14
Solomon DH et al (2008) Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis. 67(5):609–613
Doherty M, Jansen TL, Nuki G, Pascual E, Perez-Ruiz F, Punzi L, So AK, Bardin T (2012) Gout: why is this curable disease so seldom cured? Ann Rheum Dis 71(11):1765–1770
Yin R et al (2017) The rate of adherence to urate-lowering therapy and associated factors in Chinese gout patients: a cross-sectional study. Rheumatol Int 1187–1194
De Vera MA et al (2014) Medication adherence in gout: a systematic review. Arthritis Care Res 66(10):1551–1559
Cottrell E et al (2013) Improvement in the management of gout is vital and overdue: an audit from a UK primary care medical practice. BMC Fam Pract 14:170
Spaetgens B et al (2016) Knowledge, illness perceptions and stated clinical practice behaviour in management of gout: a mixed methods study in general practice. Clin Rheumatol 35(8):2053–2061
Latourte A, Bardin T, Clerson P, Ea HK, Flipo RM, Richette P (2018) Dyslipidemia, alcohol consumption, and obesity as main factors associated with poor control of urate levels in patients receiving urate-lowering therapy. Arthritis Care Res 70(6):918–924
Ridker PM, Torres J (2006) Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000-2005. J Am Med Assoc 295(19):2270–2274
Montaner JSG, O'Shaughnessy MV, Schechter MT (2001) Industry-sponsored clinical research: a double-edged sword. Lancet 358(9296):1893–1895
Acknowledgments
We thank Dr.Meng-tse Lee of Apply Medical Consulting in language editing.
Funding
This study is supported by the Taiwan National Ministry of Science and Technology Grants MOST 108-2314-B-038-073.
Author information
Authors and Affiliations
Contributions
Formal analysis, Y.-J.L., S.-Y.L, and S.-S.C.; Funding acquisition, S.-S.C.; Supervision, S.-S.C.; Writing – original draft, Y.-J.L. and S.-Y.L; Writing – review & editing, Y.-J.L., S.-Y.L; C.-H.L., S.-T.W., and S.-S.C.
Corresponding author
Ethics declarations
Disclaimer
No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 12230 kb)
Rights and permissions
About this article
Cite this article
Lin, YJ., Lin, SY., Lin, CH. et al. Evaluation of urate-lowering therapy in hyperuricemia patients: a systematic review and Bayesian network meta-analysis of randomized controlled trials. Clin Rheumatol 39, 1633–1648 (2020). https://doi.org/10.1007/s10067-019-04893-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04893-8