Abstract
Objectives
This work aimed to assess treatment adherence in rheumatoid arthritis patients with several tools and to identify factors associated with poor adherence.
Method
Between February and December 2015, 183 patients were included in this cross-sectional study. A homemade 23-item self-questionnaire was filled by patients during an outpatient consultation or a day hospitalization stay. Morisky Medication Adherence Scale (MMAS)-4, MMAS-8 and Girerd scores were extracted from this homemade questionnaire. Medication possession ratio (MPR) was then calculated. For identification of factors associated with nonadherence, patients were divided in two groups according to MMAS-8 results differentiating patients with good or bad adherence to treatments.
Results
Of the 183 patients, 59% received a combination of biologic and conventional synthetic disease-modifying drugs, 22% a biological treatment alone, and 19% a conventional DMARD alone. Respectively, 3%, 10%, and 7% were considered as low adherent according to MMAS-4, MMAS-8, and Girerd scores. MPR was calculated for 84/183 patients; 23% were low adherent. The need for a help in preparing the drugs (p = 0.05; OR = 6.12; 95% CI: 0.86 to 268.90) and concomitant diabetes (p < 0.001; OR = 0.045, 95% CI: 0.001 to 0.299) was higher in patients with good adherence. Presence of a patient’s relative reminding to take medications was associated with low adherence (p = 0.002; OR = 4.32, 95% CI: 1.41 to 13.11).
Conclusions
This study highlighted the difficulty of assessing treatment adherence in rheumatoid arthritis patients despite four different tools. Objective measures by MPR indicated a higher proportion of poor adherent patients than self-questionnaires.
Key Points • Proportion of patients considered as low adherent ranged from 3 to 27% according to the method of evaluation. • The use of a pillbox and/or the preparation of drugs by a patient’s relative was associated with good adherence. • The presence of a patient’s relative reminding to take medication was associated with low adherence. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adherence to treatment could be defined as the extent to which patients comply with recommendations made by the healthcare provider although this terminology seems to be more complex, including patient involvement and active participation in their therapeutic management [1, 2]. Adherence influences the success of treatments, and nonadherence is associated with increased morbidity, mortality, and healthcare costs [3]. In rheumatoid arthritis (RA), therapeutic strategies are based on targeted or conventional synthetic disease-modifying anti-rheumatoid drugs (csDMARDs) and/or biologic disease-modifying anti-rheumatoid drugs (bDMARDs) in order to control symptoms, induce remission, prevent structural damage, and avoid functional disability [4]. Medication adherence reported in RA is variable, ranging from 22% to more than 100% in cases of patients taking more medications than prescribed [5]. Non-optimal treatment adherence in RA is associated with less response to drugs and disease flares [6, 7]. Several methods exist to assess medication adherence. Self-questionnaires can be used, such as Morisky Medication Adherence Scale with 4 items (MMAS-4) or 8 items (MMAS-8) although they are currently not validated in RA [8, 9]. Moreover, calculation of the medication possession ratio (MPR) has been described as a more objective method of medication adherence assessment. Indeed MPR is a ratio of the number of doses dispensed relative to the dispensing period, often based upon pharmacy refill records [10, 11]. To date, no consensus has emerged on the best method of treatment adherence assessment in RA.
The main objective of this non-interventional, cross-sectional study was to assess treatment adherence in patients with RA treated with csDMARDs or bDMARDs using patient self-questionnaires and MPR calculation. A secondary objective was to identify factors associated with poor adherence.
Materials and methods
Patients
Between February and December 2015, patients fulfilling the following criteria were included in this non-interventional, cross-sectional study, conducted in the Department of Rheumatology in Rouen University Hospital: rheumatoid arthritis defined by the 2010 American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) [12], csDMARDs and/or bDMARDs for more than 6 months, a follow-up (day hospitalization and/or outpatient consultation) in Rouen University Hospital. Nonspeaking/nonreading French language, patient refusal to participate, and pregnancy were exclusion criteria. A meeting either with a nurse during patient routine rheumatologic medical follow-up or with a pharmacy student during planned day hospitalization stays was organized. This study received ethics board approval provided by the institutional Ethics Committee for Research of Rouen University Hospital with the number E2019-23. Information about purpose of the study and anonymity was given to patients. According to French Regulation, the patient written consent for publish was waived for this non-interventional study with no modification in the management of patients.
Demographic, clinical, and biological data
Sociodemographic data were prospectively obtained during patient meeting: marital status, ethnicity, educational level, working status, residence location, rheumatologic follow-up, and monthly income. Data about DMARDs were also collected. Health assessment questionnaire (HAQ) was proposed to patients during the meeting. Disease-related data were retrospectively collected in medical files: comorbidities, disease duration, DAS-28, radiologic structural damage, the presence of rheumatoid factor and/or anti-CCP antibodies, visual analogue scales (VAS) for pain, and global disease activity. The updated records about therapeutic education sessions planned in the rheumatology department were consulted.
Self-questionnaire
A 23-item self-questionnaire was proposed (online resource). At the end of patients’ visit, self-questionnaires were collected. This homemade 23-item questionnaire was elaborated in French, inspired from MMAS-4, MMAS-8, and Girerd questionnaire which is a 6-item French adaptation of MMAS scales [13]. Patients filled this questionnaire only for DMARDs and not for symptomatic treatments. This information was also written on the questionnaire. Items 4, 6, 9, and 11 were extracted from MMAS-4, items 4, 5, 6, 8, 10 ,12 , 14, and 18 from MMAS-8, and items 5, 6, 13, 16, 17, and 19 from Girerd questionnaire. Semantics from original MMAS-4, MMAS-8, and Girerd were adapted to RA patients by adding the terms “rheumatoid arthritis” when it seemed necessary. Item 5 was adapted from MMAS-8 and Girerd by modifying time interval. Indeed “last month” seemed more suitable for DMARDs than “yesterday” (MMAS-8) or “this morning” (Girerd). Other items were about treatment duration (item 1), treatment knowledge (item 2), medication taking omission (item 3), eventual patient relative reminding to take medication(s) (item 7), medication tolerance (items 10 and 20), routine influence (item 15), participation to therapeutic education sessions (item 21), pharmacy dispensing treatments (item 22), and medication satisfaction (item 23). Items 2–6, 8–18, and 20 were dichotomized for bDMARDs and csDMARDs.
Medication possession ratio calculation
When patients indicated a referral pharmacy in 23-item questionnaire, pharmacists were phone interviewed by a pharmacist student. The number of bDMARD and csDMARDs dispensed over a 6-month period before the day of questionnaire completion was asked. Medication possession ratio (MPR) was calculated by dividing the number of supplied medications by the number of prescribed medication, during the 6-month period before the day of questionnaire completion. Patients were considered as adherent when MPR was 0.8 or greater [14].
Risk factor identification
To identify factors associated with nonadherence, two groups were defined according to MMAS-8 results: patients with low adherence (0–5 points) and patients with medium (6–7 points) or high adherence (8 points). The entire clinical and biological characteristics were compared between these two groups of patients.
Statistical analysis
Qualitative data were expressed as number and percentage and were compared with chi-square test. Quantitative data were expressed as means with standard deviations and were compared by bilateral Wilcoxon Mann-Whitney test. P-values < 0.05 were considered statistically significant. Statistical analyses were performed using NCSS Software.
Results
Clinical and biological characteristics
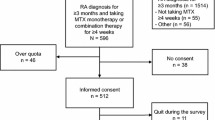
Among the 183 patients included in this study, 112 (61%) were enrolled during an outpatient consultation and 71 (39%) during a day hospitalization stay. Twelve patients were not included: 5 refused to fill the questionnaire, 2 because of an identification default, 2 for a rheumatologic pathology not yet labeled, and 3 patients did not speak French. Included patient characteristics are presented in Table 1.
Among the 183 patients, 59% (n = 108) of patients were treated with a bDMARD associated with a csDMARD, 22% (n = 40) with a bDMARD alone and 19% (n = 35) with a csDMARD alone (Table 2). The most prescribed csDMARD was methotrexate (n = 120, 84% of patients) administered orally (n = 75, 62.5%) or subcutaneously (n = 45, 37.5%). Of the 148 patients treated with bDMARD, the most prescribed drug was etanercept (n = 55, 37%). Overall, CsDMARDs and bDMARDs were administered either subcutaneously (43%), orally (34%), or intravenously (23%).
Assessment of treatment adherence with several tools
The answers to the 23-item self-questionnaire are presented in Table 3. Among the whole RA patients, 95% of them reported knowing their medication name (item 2), 65% were able to give details (name, dosage, and days of taking), and 60% detailed it correctly. Among the patients treated with csDMARD alone and the patients treated with DMARD, respectively, 80% and 87% reported a good tolerance to their medication (item 20). Reported adverse effects were digestive disorders and asthenia for csDMARDs and influenza-like syndrome after administration of bDMARDs. A total of 9.3% of patients reported having already stopped treatment without informing their doctor (item 10). A total of 12% of patients reported a participation in therapeutic education sessions. According to the records maintained in the department about this activity, 94 patients (51%) received at least 1 therapeutic education session. Most patients (92%) were satisfied with the therapeutic management of their RA (item 23). Three patients referred to the inefficacy of treatment as a cause of dissatisfaction, 1 patient referred to the mode of administration. The other patients did not declare any reason.
Using the results from the 23-item questionnaire, MMAS-4, MMAS-8, and Girerd scores were calculated (Table 4). A total of 3% of patients were considered poor adherent with MMAS-4, 10% with MMAS-8, and 7% with Girerd. The 3 scores agreed to define 3% of patients with poor adherence, 12% with medium adherence, and 21% with high adherence. Finally, for 84 patients (46%), it was also possible to calculate their MPR. The MPR indicated a rate of 27% of low-adherent patients.
Identification of factors associated with good or low adherence
MMAS-8 was used as reference questionnaire for the definition of 2 groups: patients with low adherence (n = 19) and with good adherence (n = 164). The comparison of the two group characteristics is presented in Table 5. The use of a pillbox and/or the preparation of drugs by a patient’s relative was slightly associated with good adherence (p = 0.05; OR = 6.12; 95% CI: 0.86 to 268.90). The presence of diabetes as comorbidity was significantly higher in the group of patients with good adhesion (p < 0.001; OR = 0.045, 95% CI: 0.001 to 0.299). The presence of a patient’s relative reminding him to take medications was higher in low adherence group (p = 0.002; OR = 4.32, 95% CI: 1.41 to 13.11).
Discussion
We conducted a cross-sectional study to assess medication adherence in patients with RA followed up in a University Hospital. First of all, we faced the absence of specifically validated RA questionnaires and the lack of consensus on this issue, which have recently been highlighted [15]. This is the reason why we did not choose a single questionnaire but decided to evaluate the adhesion using several questionnaires: MMAS-4, MMAS-8, and Girerd. These questionnaires are validated in several chronic pathologies but not yet in inflammatory rheumatisms such as RA. We chose to build a single synthetic questionnaire for the comfort of patients. Indeed, in our opinion, it would have been more a constraint for them to complete several questionnaires rather than a single one. We did not establish feasibility, reliability, and validity of our homemade questionnaire, which intended to be used punctually for this study and not in clinical routine. If it were to be used routinely, we should validate these psychometric characteristics. Psychometric characteristics of MMAS-4 and MMAS-8 have been evaluated in the case of hypertension. The sensitivity was 81% and 91%, and the specificity was 44% and 53% for MMAS-4 and MMAS-8, respectively. Cronbach’s alpha reliability was also better for MMAS-8 compared to MMAS-4 (0.83 vs 0.7) [16]. Girerd questionnaire is a 6-item French adaptation of Morisky’s scales also studied in patients with hypertension. But to our knowledge, its psychometric characteristics have not been yet established.
According the method used to assess the adherence, the proportion of patients with low adherence ranged from 3% to 27%. To our knowledge, this work is the first study with the objective to compare the results of four different methods to assess adherence in the same patients. Results obtained from these questionnaires are discordant. Indeed, adherence was evaluated at 74%, 40%, and 30%, respectively, with MMAS-4, MMAS-8, and Girerd. Only 20.8% of patients were defined as adherent with the 3 scores and 2.7% as low adherent in our study. The 3 scores agreed only for 65 patients (36%).
The “declarative” adherence rate reported with self-questionnaires was higher than a more “objective” rate, evaluated by calculation of the MPR. A first explanation is that declarative methods often tend to overestimate adherence [17]. Indeed a patient who fills a questionnaire knows that he is being observed and would tend to modify his behavior [3]. No method of adherence measurement is perfect, and they all display advantages and disadvantages [18]. The main advantage of MPR calculation as adherence assessment method is its objectivity compared to self-questionnaires. Then MPR calculation could be an accurate measure in case of the patients always go for their treatments in the same pharmacy. An underestimation of adherence could then be observed if the patient went to seek his treatment in another pharmacy than the usual one, even once. On the contrary, an overestimation of MPR could occur because the calculation is based on the number of dispensed treatments and not on the number of administered treatments. This trend is highlighted when MPRs greater than 100% are reported [5]. A lot of data were missing for MPR calculation, forcing us to interpret our results with caution. An explanation for these missing data is the design of our study; indeed, patients were included on the basis of the questionnaire and not on the availability of data from pharmacies. Then we faced several difficulties in obtaining data for MPR calculation. First 13 patients received medications in hospital pharmacy (intravenous bDMARD). Then among the 169 patients who declared to look for their medications in the same community pharmacy, 129 named it. Finally, data could not be obtained for 14 patients because the pharmacy could not be found according to information given by the patient, and for 18 patients, the pharmacist could not transmit the history of treatments dispensed.
This study confirms that each method of assessing adherence has its specificities and thus tends to detect different types of non-adherent behaviors. The combination of two or more methods improves the detection of “at risk” patients [19].
We observed that 59% of patients were treated with a combination of a csDMARD and a bDMARD. The influence of the number of DMARDs on adherence is unclear. On the one hand, adherence was shown to be better when a single anti-TNF was prescribed drug versus a combination of DMARDs [20]. In contrast, other studies suggest that adherence is better when a bDMARD is associated with methotrexate [21, 22]. The route of administration may also influence adherence. Intravenous administration induces little lack of adherence. In patients followed in our department, intravenous medications are often administered in combination with other drugs that are administered orally or subcutaneously. Thus, an effect of route of administration on adherence could be explained more by the follow-up of these patients. Indeed, these patients have regular and scheduled stays in day hospitalization. This close monitoring should allow to naturally eliminating some reasons for low adherence such as doubts about the effectiveness of treatment or poor tolerance.
We decided to use MMAS-8 questionnaire as reference to define patients with low adherence and good adherence. In fact, results of MMAS-4 (3% of patients with low adherence) were different compared to MMAS-8 (10%) and Girerd (7%). Then two questions from the Girerd questionnaire seemed less relevant to the drug mode of administration in RA: “Have you ever take your medication(s) late compared to the usual hour or day of taking?” (item 16) and “Do you think you take too much medication(s)” (item 19). In fact, almost half of the patients were treated with monotherapy (41%) and the other half with dual therapy (59%). Only one patient received simultaneously three DMARDs. On the other hand, the most commonly prescribed DMARDs were methotrexate and etanercept, which are mainly administered once a week and for whom the time of intake is of little importance, in contrast to treatments of arterial hypertension in which MMAS-4 has been developed [8]. Finally, we grouped patients with medium and high adherence according to the results of MMAS-8. Indeed the ranking between medium and high adherence was mainly determined by their weariness in following treatment (item 18).
A variety of associated/predictive factors has been studied in RA, including patient-related, treatment-related, and patient-healthcare provider relationship-related, with different results across studies making the interpretation difficult [15]. Some studies identified that older patients were more likely to be adherent [23]. We observed the same trend in this study even if it did not reach statistical significance, probably because of the small number of patients included in the analysis, especially in “low adherence” group.
Consistent associations between disease-related risk factors and adherence in RA were not identified [24]. Some studies suggest that low disease activity, the presence of rheumatoid factor, and shorter disease duration may be associated with better adherence [25,26,27]. We did not observe association with these disease-related factors, but these results should be interpreted with caution because of the small number of patients. Interestingly diabetes as comorbidity was significantly higher in the group of adherent patients. This result could be explained by the fact that these patients are more aware of the importance of being adherent because of the chronicity of their pathology and their close medical follow-up. The presence of comorbidities has already been associated with better adherence in RA [26].
In our study, the presence of a patient’s relative reminding to take medications as well as the presence of a help for the preparation of drugs (pill dispenser and/or patient’s relative) seem to be associated with low adherence. This result may reflect a behavior of poor adherence caused either by memory difficulties or by a disinvestment of the patient in his disease treatment. This observation is consistent with a recent literature review identifying increased professional or familial support as associated with better adherence as well as living alone with poorer adherence [28].
Therapeutic education seems to improve adherence [29]. We observed that participation in therapeutic education sessions seemed to be not associated with adherence. However, the proportion of patients who reported having participated in therapeutic education sessions was very low than reality since we compared this proportion with the current department records on this activity. Patients did not seem to have understood that the specialized interview they had at the initiation of a biotherapy or during their annual hospital follow-up was a therapeutic education session.
We did not focus on costs in this study whereas economic issues are of paramount where interesting on medication adherence. On the one hand, poor adherence seems to generate additional costs, and on the other hand, higher out-of-pocket costs have been shown to reduce medication adherence in a cohort of 2 285 RA patients [30]. In France, RA is classified as a “long-term condition” by the French healthcare system. As such, all care and medications are fully supported, and no out-of-pockets payments are needed.
Our study has a number of limitations. First questionnaires we used to assess adherence were not currently validated in the RA. Then MMAS-4, MMAS-8, and Girerd questionnaires display similar constructs. Other questionnaires with different structures could have been used, such as the Medication Adherence Self-Report Inventory (MASRI) which includes VAS [31]. Other questionnaires integrate the patient’s perceptions of medicines such as the “Beliefs about Medicines Questionnaire (BMQ)” [32]. This kind of questionnaire then focuses on intentional adherence, whereas it seems that Morisky’s scales study both dimensions: intentional and unintentional [16]. Finally a questionnaire was developed specifically in RA, but it measures satisfaction of patients with different forms of complex multidisciplinary care rather than adherence to medications [33].
We studied adherence but not persistence to DMARDs. Persistence could be defined as “the duration of time from initiation to discontinuation of therapy” [1]. Addressing both adherence and persistence would have provided a richer understanding of medication-taking behavior. In RA, persistence rates of 61%, 57.5%, and 63% with etanercept, adalimumab, or infliximab, respectively, were described using a 1-year time period. Persistence rates were even lower for abatacept, golimumab, rituximab, and certolizumab, and generally, persistence was better when patients received a combination of a bDMARD and csDMARDs than in patients receiving a combination of csDMARDs [34].
Finally, in our study, the number of patients did not allow us to make separate analysis depending on the drug. Adherence may indeed be different between two drugs even if they have the same route and the same rate of administration [11, 35].
This study highlighted the difficulty of assessing adherence in patients with RA. A gap appears between the patient perception and what could be objectified. Methods of adherence assessment explore only certain facets of a set that is patient’s involvement in his therapeutic management. However, it seems obvious that therapeutic adherence is not optimal in RA and that solutions to improve remain to be found.
References
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK (2008) Medication compliance and persistence: terminology and definitions. Value Health 11:44–47
Martin LR, Williams SL, Haskard KB, Dimatteo MR (2005) The challenge of patient adherence. Ther Clin Risk Manag 1:189–199
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353:487–497
Scott DL, Wolfe F, Huizinga TWJ (2010) Rheumatoid arthritis. Lancet 376:1094–1108
de Achaval S, Suarez-Almazor ME (2010) Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumatol 5:313–326
Contreras-Yáñez I, Ponce De León S, Cabiedes J et al (2010) Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease-modifying antirheumatic drugs. Am J Med Sci 340:282–290
Bluett J, Morgan C, Thurston L et al (2015) Impact of inadequate adherence on response to subcutaneously administered anti-tumour necrosis factor drugs: results from the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate cohort. Rheumatology 54:494–499
Morisky DE, Green LW, Levine DM (1986) Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 24:67–74
Morisky DE, Ang A, Krousel-Wood M, Ward HJ (2008) Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens 10:348–354
Blum MA, Koo D, Doshi JA (2011) Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther 33:901–913
Tkacz J, Ellis L, Bolge SC, Meyer R, Brady BL, Ruetsch C (2014) Utilization and adherence patterns of subcutaneously administered anti-tumor necrosis factor treatment among rheumatoid arthritis patients. Clin Ther 36:737–747
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581
Girerd X, Hanon O, Anagnostopoulos K et al (2001) Assessment of antihypertensive compliance using a self-administered questionnaire: development and use in a hypertension clinic. Presse Med 30:1044–1048
Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC (2009) Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin 25:2303–2310
Gossec L, Molto A, Romand X et al (2019) Recommendations for the assessment and optimization of adherence to disease-modifying drugs in chronic inflammatory rheumatic diseases: a process based on literature reviews and expert consensus. Joint Bone Spine 86:13–19
Tan X, Patel I, Chang J (2014) Review of the four item Morisky Medication Adherence Scale (MMAS-4) and eight item Morisky Medication Adherence Scale (MMAS-8). Innov Pharm. https://doi.org/10.24926/iip.v5i3.347
Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD (2009) Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother 43:413–422
van den Bemt BJF, Zwikker HE, van den Ende CHM (2012) Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol 8:337–351
Ho PM, Bryson CL, Rumsfeld JS (2009) Medication adherence: its importance in cardiovascular outcomes. Circulation 119:3028–3035
Harley CR, Frytak JR, Tandon N (2003) Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. Am J Manag Care 9:S136–S143
Kristensen LE, Saxne T, Nilsson J-A, Geborek P (2006) Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res Ther 8:R174
Voulgari PV, Alamanos Y, Nikas SN, Bougias DV, Temekonidis TI, Drosos AA (2005) Infliximab therapy in established rheumatoid arthritis: an observational study. Am J Med 118:515–520
Morgan C, McBeth J, Cordingley L, Watson K, Hyrich KL, Symmons DP, Bruce IN (2015) The influence of behavioural and psychological factors on medication adherence over time in rheumatoid arthritis patients: a study in the biologics era. Rheumatology 54:1780–1791
Vangeli E, Bakhshi S, Baker A, Fisher A, Bucknor D, Mrowietz U, Östör AJ, Peyrin-Biroulet L, Lacerda AP, Weinman J (2015) A systematic review of factors associated with non-adherence to treatment for immune-mediated inflammatory diseases. Adv Ther 32:983–1028
Zink A, Listing J, Kary S, Ramlau P, Stoyanova-Scholz M, Babinsky K, von Hinueber U, Gromnica-Ihle E, Wassenberg S, Antoni C, Herzer P, Kekow J, Schneider M, Rau R (2005) Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann Rheum Dis 64:1274–1279
De Cuyper E, De Gucht V, Maes S et al (2016) Determinants of methotrexate adherence in rheumatoid arthritis patients. Clin Rheumatol 35:1335–1339
Gadallah MA, Boulos DNK, Gebrel A et al (2015) Assessment of rheumatoid arthritis patients’ adherence to treatment. Am J Med Sci 349:151–156
Anghel L-A, Farcaş AM, Oprean RN (2018) Medication adherence and persistence in patients with autoimmune rheumatic diseases: a narrative review. Patient Prefer Adherence 12:1151–1166
Hill J, Bird H, Johnson S (2001) Effect of patient education on adherence to drug treatment for rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis 60:869–875
Curkendall S, Patel V, Gleeson M, Campbell RS, Zagari M, Dubois R (2008) Compliance with biologic therapies for rheumatoid arthritis: do patient out-of-pocket payments matter? Arthritis Rheum 59:1519–1526
Walsh JC, Mandalia S, Gazzard BG (2002) Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS 16:269–277
Porteous T, Francis J, Bond C, Hannaford P (2010) Temporal stability of beliefs about medicines: implications for optimising adherence. Patient Educ Couns 79:225–230
Tijhuis GJ, Kooiman KG, Zwinderman AH, Hazes JM, Breedveld FC, Vliet Vlieland TP (2003) Validation of a novel satisfaction questionnaire for patients with rheumatoid arthritis receiving outpatient clinical nurse specialist care, inpatient care, or day patient team care. Arthritis Rheum 49:193–199
Murage MJ, Tongbram V, Feldman SR et al (2018) Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence 12:1483–1503
Carter CT, Changolkar AK, Scott McKenzie R (2012) Adalimumab, etanercept, and infliximab utilization patterns and drug costs among rheumatoid arthritis patients. J Med Econ 15:332–339
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T.L. has received research grants and/or honoraria from AbbVie, Amgen, BMS, Lilly, MSD, Novartis, Pfizer, Sanofi Aventis, and UCB. Other authors do not declare conflicts of interest.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 168 kb)
Rights and permissions
About this article
Cite this article
Monchablon, C., Gondé, H., Pouplin, S. et al. Assessment of adherence to disease-modifying anti-rheumatic drugs in rheumatoid arthritis. Clin Rheumatol 39, 207–216 (2020). https://doi.org/10.1007/s10067-019-04837-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04837-2