Abstract
Rituximab (RTX) is an approved treatment for rheumatoid arthritis (RA) patients that do not respond adequately to disease-modifying antirheumatic drugs. However, different new concerns, such as efficacy, optimum dose, safety issues, prediction of response to RTX, and pregnancy outcomes have attracted a lot of attention. The PubMed database was systematically reviewed for the last published articles, new findings, and controversial issues regarding RTX therapy in RA using “Rheumatoid arthritis” AND “rituximab” keywords, last updated on June 18, 2019. From 1812 initial recorders, 162 studies met the criteria. Regarding the optimum dose, low-dose RTX therapy (2 × 500 mg) seems as effective as standard dose (2 × 1000 mg), safer, and more cost-effective. The most common reported safety challenges included de novo infections, false negative serologic tests of viral infections, reactivation of chronic infections, interfering with vaccination outcome, and development of de novo psoriasis. Other less reported side effects are infusion reactions, nervous system disorders, and gastrointestinal disorders. Lower exposure to other biologics, presence of some serological markers (e.g., anti-RF, anti-CCP, IL-33, ESR), specific variations in FCGR3A, FCGR2A, TGFβ1, IL6, IRF5, BAFF genes, and also EBV-positivity could be used to predict response to RTX. Although there is no evidence of the teratogenic effect of RTX, it is recommended that women do not expose themselves to RTX at least 6 months before the conception. Only a reversible reduction of B cell-count in the offspring may be the pregnancy-related outcome. Although RTX is an effective therapeutic option for RA, more studies on optimum doses, prevention of RTX-related side effects, prediction of RTX response, and safety during the pregnancy are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease and also one of the most disabling types of arthritis, which is frequently observed among adults. Erosive joint damage, symmetric polyarticular inflammation, and functional impairments as the results of persistent inflammation are the most common complaints of RA patients. Disease Activity Score (DAS) is widely used as a measure of inflammatory disease activity in people with RA and is used to objectively evaluate a patient’s response to treatment. The DAS28 is based on a count of 28 swollen and tender joints, with a score ranging from 0 to 9.4. A value of ≤ 3.2 was defined as the threshold for a low disease activity state and < 2.6 as the threshold for the remission [1].
Different types of autoantibodies, such as rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP), seem to be involved in the pathogenesis of RA. They are not only considered useful biological markers for the diagnosis of RA but also have been suggested as predictors of drug responses in RA [2, 3]. A large number of risk factors have been suggested related to RA, such as sex, genetics, and epigenetic factors, as well as various environmental factors (e.g., smoking) which some of them were found to be associated with response to biological agents [4].
Considering the fact that RA is a progressive disease, accumulation of joint damage can cause irreversible disability, if left untreated or even improperly treated. Numerous RA patients all over the world are treated with conventional agents, such as glucocorticoids and methotrexate (MTX) [4]. Nonetheless, some of the patients may not tolerate them or even may not achieve disease remission. Although etiology and the pathogenesis of RA are complex, different newly employed biologics have revolutionized therapeutic approaches. They usually suppress the immune system by targeting particular signaling pathways and act in a more specific manner. It was suggested that biologic agents, such as tumor necrosis factor-alpha (TNFα) inhibitors and rituximab (RTX) could significantly reduce mortality risk in RA patients, as compared to the disease-modifying antirheumatic drugs (DMARDs) [5]. RTX, a chimeric anti-CD20 monoclonal antibody, depletes B cells through different mechanisms, including apoptosis of B cells, complement-dependent cytotoxicity, and mediation of antibody-dependent cellular cytotoxicity [6]. To date, the role of different arms of the immune system during the RA have remained largely unknown. However, achieving clinical remission in patients following B cell depletion confirmed the previous findings related to the critical roles of the humoral immune system in the pathogenesis of RA [7, 8].
During the last two decades, an increasing amount of evidence for the efficacy of RTX in RA patients has made this drug an attractive topic for several researchers and clinicians. Promising results of using RTX in RA patients had led to approving RTX by the Food And Drug Administration (FDA) with concomitant MTX for the treatment of moderate-to-severe RA on March 1, 2006 [9]. From that date, several studies had reported the significant reduction in DAS28 score and improvement of Health Assessment Questionnaire (HAQ), with no serious adverse events. This is consistent with the high efficacy and safety profile of RTX in RA patients [10,11,12,13,14,15]. Overall, among the different biologic drugs to target B cells, such as ocrelizumab, ofatumumab, belimumab, and atacicept, RTX is the only one with promising results and acceptable safety profile for the treatment of RA patients. Despite the approval of RTX for RA patients, promising results, and less adverse effects as compared to the conventional treatments, there are growing concerns over the safety of this drug. Additionally, there is no consensus regarding optimum dosage, biomarkers for RTX response, and treatment of pregnant women with RTX, which need to be addressed. Having a comprehensive insight into these matters could open an avenue for developing more effective and safer treatments for RA patients.
Search strategy and selection criteria
The PubMed database was searched for any study associated with the RTX in RA. Accordingly, two terms of “Rheumatoid arthritis” AND “Rituximab” were used to find relative studies. The search result was updated on June 18, 2019. The references for the selected articles were also checked for any missed articles.
Characteristics of included studies are listed below
-
The original study in the English language.
-
Association with using RTX in RA.
-
Presentation or confirmation of a unique/novel clinical outcome(s), a biomarker for RTX response, specific recommendation, and any protocol of treatment. Critical meta-analyses and systematic reviews, which help to reach a clear message regarding uncertain issues as well as case reports with novel findings had been included.
After identification of studies, eligible studies were carefully read to extract any novel data regarding efficacy, safety, treatment protocol, RTX response’s biomarker, and use of RTX in RA patients during the pregnancy. In the case of very repetitive results, which were confirmed by several studies, only those with higher number of patients, more clear message, and a higher quality of study design had been chosen and discussed.
Results
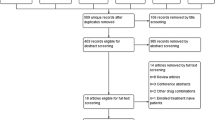
Among the 1805 found records in database searching, in addition to seven identified articles through searching in the reference list of full-text studied articles, 1369 records were selected for initial screening. Subsequently, 1161 records excluded due to presenting not original findings (n = 629), non-English studies (n = 188), and irrelevant data to using RTX in RA (n = 344). Reading the full-text of 208 studies had led to including 162 studies, which met the inclusion criteria. The reasons for exclusion of 46 ignored studies were the lack of association with RTX or RA (n = 17), not informative/novel data (n = 26), and no clear message (n = 3). The most important findings of such studies have been categorized into some major groups, including efficacy, optimum RTX doses, safety concerns, prediction of RTX response, and outcome of exposing pregnant RA patients to the RTX; and have been discussed in details. The study selection process and reasons for exclusions are presented in Fig. 1.
Efficacy
Some years after the introduction of RTX, different studies showed its efficacy in refractory and active RA patients [16,17,18]. On those years, the high efficacy of RTX was reported in various studies. For example, Moore et al. [19] demonstrated a successful induction of clinical remission in a small number of patients. Employment of RTX plus MTX in patients with active disease and a history of inadequate response to at least one anti-TNF agent was another innovative attempt [15]. As the results, it was shown that a single course of RTX (2 × 1000 mg) with concomitant MTX therapy could significantly improve the clinical feature of RA patients within 24 weeks. Subsequently, using RTX as an alternative treatment in anti-TNF therapy-resistant RA patients was encouraged in several other studies [20,21,22]. However, Porter et al. [23] conducted a multi-center open-label randomized non-inferiority trial and demonstrated that initial treatment with RTX is non-inferior to initial treatment with anti-TNF agents in RA patients. In addition to the significant reduction of DAS28 [15], RTX was shown to be effective in reducing radiographic damage [24, 25]. It also caused a significantly improvment in the HAQ disability index [14]. Additionally, refractory RA patients experienced significant improvement in patient-reported outcomes, such as patient global, pain, and fatigue, 1 year after initiating RTX [26]. It was also shown that RTX is significantly more effective than abatacept in a 2-year follow-up of outcomes [27].
Recently, it was found that RTX could be even more effective in some cases than we thought. For example, following three consecutive RTX courses, 9 years of sustained biologic-free clinical remission as the results of persistent peripheral B cell depletion was reported [28]. However, in spite of frequently reporting of complete B cell depletion followed by clinical improvement of RTX-exposed patients [18], some evidence implying to the inability of RTX incomplete depletion of synovial or bone marrow CD19+ B cells number in few patients, which is probably followed by non-favorable clinical responses [29,30,31].
After general acceptance of the efficacy of RTX in RA patients, the influence of response to previous treatments, especially anti-TNF agents has become another hot topic among the researchers. It was suggested that naïve patients for biological drugs might be better RTX responders [32]. Moreover, a higher failure of the previous anti-TNF agents or even previously exposure to them seem to be associated with a less favorable response [32,33,34,35]. In this regard, it was observed that those with a lower number of previously failed TNF blockers usually experience a more favorable clinical response following RTX therapy [36]. However, the association between the numbers of previously failed attempts with anti-TNF agents was not confirmed in the later study with a higher number of RA patients [37]. Additionally, Soliman et al. [22] suggested that non-responders to at least one anti-TNF agents might benefit more from switching to RTX than switching to alternative anti-TNF therapy. Concomitant MTX was also suggested as a predictor of low disease activity/remission at 24 months [38].
In 2011, it was speculated that a history of treatment with statins might be related to less efficacy of RTX in RA patients [39]. However, this belief did not last long and was revised by later studies. Considering the fact that age is usually higher in patients receiving a statin, Das et al. [40], suggested the probable involvement of ages of patients. Subsequently, other authors with a long-term follow-up did not find any association between RTX clinical efficiency and concomitant use of statin [41, 42]. The key points related to RTX efficacy are summarized in Table 1.
Initial and maintenance doses of rituximab in rheumatoid arthritis
Before approval of RTX for RA patients, different researchers have designed studies to show its high efficacy in refractory and seropositive active RA patients, while there was no consensus on the optimal dosage and schedule [16,17,18]. After evaluation and designing several studies, the licensed dose of RTX in RA was introduced as the two intravenous infusions of 1000 mg, given 2 weeks apart (days 1 and 15). However, different other RTX doses were tested to assess efficacy, safety, and cost analysis. One of the most attractive strategies was comparing the effectiveness and safety of standard dose (2 × 1000 mg) with low-dose of RTX (2 × 500 mg). In comparing RTX therapy with a low-dose regimen and standard dose, no significant difference in either clinical or safety outcomes was detected [43, 44]. Moreover, in Phase III randomized study, efficacy, and safety could not be clearly differentiated between the RTX at low-dose and standard dose [45]. These findings were also confirmed in a meta-analysis, containing eight studies. As the results, the low-dose regimen of RTX regimen not only a resulted in similar effectiveness but also it was associated with a lower cost [46]. The same findings have also been reported in the next updated meta-analysis [47]. As another attempt, treating RA patients with a standard dose and reduced doses (a total dose lower than 2000 mg) have revealed no significant difference between the strategies when maintenance of RTX at 5 years was evaluated [48]. Additionally, the total cumulative doses of RTX, as well as the rate of serious infections were found to be significantly lower in the reduced-dose group [48]. In accordance with some of the studies, it seems that cumulative doses of the second course of RTX therapy could be lower than the standard dose, with no impairment in drug efficacy. It probably leads to more cost-saving and is associated with a lower rate of severe adverse events [49, 50].
In addition to trying to find optimum doses, the difference between the efficacy of fixed-interval RTX retreatment and on-flare retreatment as well as the influence of a number of RTX courses is another critical issue. Regarding the former topic, there is some controversy. In fact, in a cohort study containing a large number of patients, evaluation of DAS28 showed that a fixed-interval retreatment group yielded significantly better results than the on-flare group [51]. However, in a recently published study with a lower number of patients, it was reported fixed-interval strategy does not lead to better disease control or more drug use compared to on-flare retreatment strategy [52]. Meanwhile, more studies had been conducted to address the latter issue. Vital et al. [53] proposed that an extra 1000 mg infusion of RTX at 4 weeks improves the levels of B cells depletion in those who show incomplete B cell depletion. As it was expected, complete depletion led to better clinical response without any additional safety concern. In a trial containing more than 1000 RA patients exposed to RA, it was suggested that repeated courses of RTX could be associated with sustained clinical responses with no new adverse events [54]. Additionally, non-responders to the first RTX cycle may benefit from early retreatment with RTX [55]. It is worthy to note that the administration rate of RTX could even be faster at the second and subsequent infusions with no concern related to increasing the rate or severity of infusion-related reactions [56]. The key points related to initial RTX doses and retreatment are briefly brought in Table 2.
Safety concerns
Generally, RTX administration in RA patients has been accepted as a safe procedure, and prolonged exposure to RTX does not seem to be related to additional adverse events [10,11,12,13, 20]. However, there are some increasingly concerns related to the safety profiles of RTX in RA patients, such as development/reactivation of infections, failure of immunization, and paradoxical reactions. Table 3 summarizes the most critical points related to the RTX safety profiles.
Infections
Development of de novo infections has been recognized as the most challenging RTX-related side effects. Recently, infection rates (both serious and non-serious) were reported 0.30 to 0.41 per patient-year [79]; and between 0.058 and 0.089 per patient-year reported in different studies for significant infections [57, 58]. In a study with 989 RA patients and a mean of 3.9 years follow-up, the rate of infection was reported 20%, which 8% of patients had ≥ 2 significant infections, and 1.6% of them expired due to infection [57]. Interestingly, in a study with a large number of patients by the French Society of Rheumatology, around half of the most common RTX-related severe infections in RA patients occurred within the first 3 months [59]. In another study, containing 3363 courses of RTX, the risks of serious infections were highest within the first 6 months after RTX start [58].
It is worth to note that observations of Silva-Fernandez et al. [80] suggest that serious infections related to anti-TNF agents are comparable to RTX in non-responders to TNF blockers within the first year of follow-up. In a recently conducted systematic review, it was concluded that RTX treatment has no additional risks for infections over non-RTX treatment in RA patients [81].
Less frequently reported safety concerns following RTX therapy in RA patients across the literature includes Campylobacter fetus infection [82], tuberculosis arthritis [83], Pasteurella multocida infection [84], bronchiectasis [85], and progressive multifocal leukoencephalopathy (PML) [86]. In another study, which reported seronegative West Nile virus in RA patients under treatment with RTX, it was warned that RTX not only predisposes patients to the infections but may also lead to a false negative serologic test of viral infections, which is expected to follow by delayed diagnosis [60]. RTX in RA may also cause a delay in clearance of some infections, such as Babesia microti infection [61]. Additionally, late-onset neutropenia might occur after RTX administration and might lead to serious infections [87].
Regarding prediction of severe infection risk, higher age, higher body mass index (BMI), diabetes condition, and presence of infection during the last years before RTX administration, RA-related extra-articular involvement, lung and cardiac comorbidities as well as low baseline IgG level (< 6 g/l) has been suggested [57, 59]. However, increasing in cumulative dose of RTX did not cause a higher incidence of significant infections [57].
In addition to the development of new infections, reactivation of chronic infections (e.g., hepatitis B virus [HBV]) is a problematic issue. Reactivation of chronic HBV (CHB) after exposure to RTX is one of the most critical concerns [88]. In addition to HBsAg positive patients, those with resolved or occults HBV (HBsAg−/anti-HBc+) are also at the risk of HBV reactivation [62,63,64]. This phenomenon was reported with the incidence of 9.1% among the HBsAg−/anti-HBc+ patients within the mean duration of 25.4 ± 4.6 months from the first RTX infusion [65]. In another study, the incidence of HBV reactivation was reported by 8.7%, during the mean 61 months from the first RTX infusion in HBsAg-negative patients [66]. In contrast, in a retrospective multicenter Italian study, a very low risk of HBV reactivation was noted as the result of RTX administration [89]. Similar to the CHB infected patients, there is also some evidence of the capability of RTX in the induction of hepatitis C virus (HCV) reactivation [67, 68]. There are also pieces of evidence that during the RTX treatment in rheumatic diseases, including RA, clinicians should be aware of a potential, albeit modest, risk of developing progressive multifocal leukoencephalopathy (PML) [86]. It is a demyelinating disease of the central nervous system caused by the John Cunningham (JC) virus. RTX was found to be associated with the highest risk of PML development among the biologics used for RA treatment [90].
Surprisingly, there are some reports of successful administration of RTX in patients under the risk of reactivation of certain types of infections or even those who were suffering from a reactivated infection following other treatments. A decade ago, the first report of efficiently and safely using RTX in an RA patient who had developed tuberculosis (TB) as the results of the anti-TNF agents was reported [91]. The safety of RTX in treating RA with concomitant pulmonary TB was also reported in another study [92]. Moreover, two cases with active TB have been reported, who reached not only RA remission but also experienced inactivation of TB following the RTX treatment [93]. As another surprising finding, it was reported that RTX eradicates all traces of Epstein-Barr virus (EBV) [94].
Immune response to vaccine antigens
In addition to the risk of development or reactivation of infections following the weakening of immune responses, successful immunization is another challenging issue related to RTX therapy. It was demonstrated that few RA patients exposed to the RTX may not be sufficiently protected against influenza infection, while it could be effective in the majority of patients [69, 70, 95]. The total absence of influenza-specific IgG production was also reported in around half of the patients, exposed to RTX 6 months before vaccination [69]. However, the impaired humoral immune responses against influenza seem to modestly restore within 6–10 months after the last infusion [70]. Regarding hepatitis B vaccination, although the hepatitis B vaccination did not lead to increasing the rate of RA flare, and RA patients well-tolerated, RTX was associated with impaired response to hepatitis B vaccination [71].
Paradoxical reactions and rare side effects
One of the most frequently reported paradoxical effect related to RTX therapy in RA patients is related to the development of psoriasis, a T cell-mediated autoimmune skin disease. In the first year after the approval of RTX by FDA, an unexpected and serious paradoxical reaction was reported. Dass et al. [72] have reported three patients with no known risk factor for psoriasis, who developed psoriasis as the result of RTX therapy. Interestingly, the underlying disease well-responded to RTX in all the patients. After that, the development of psoriasis was reported in more patients by other authors [73,74,75]. Plantar pustulosis development following successful treatment with RTX in RA patients was also reported [76]. In contrast to these results, evaluation of a large number of patients supports a causative role of RTX in neither new-onset nor flare of preexisting psoriasis in RA patients [96]. It is worthy to note that RTX-specific psoriasis may also occur with anti-TNF therapy. In fact, despite the indicated for the treatment of psoriasis, anti-TNF agents may paradoxically trigger a psoriasiform condition [97]. Other less frequent reports of paradoxical effects include de novo ulcerative colitis developing probably due to RTX administration in RA patients [77], progressive reduction of lung function parameters, and some lung-related side effects, such as interstitial lung diseases have been reported as paradoxical or rare side effects of RTX therapy [98, 99].
Other side effects
In addition to the infections and infestations, which have been frequently reported in RA patients exposed to RTX, and rare reports implying the paradoxical reactions, different other adverse reactions also seem probable. Infusion reactions (e.g., headache, skin itchiness, throat irritation) which usually are mild to moderate in severity, could appear in approximately a quarter of patients [9, 78]. It has been suggested that cytokine-release plays a crucial role, and CD20 cells, as well as CD16 positive natural killer (NK) cells, are involved in infusion reaction [100]. Recently, it was proposed that anti-CCP positivity and absence of concomitant treatment with a synthetic DMARDs increase the risk of serious infusion-related reactions [101].
In a study containing 144 RTX-exposed RA patients, the frequently observed adverse reactions (> 20%) include nervous system disorders, gastrointestinal disorders, and general disorders and administration site conditions [23]. Additionally, a significant decrease in bone mineral density (BMD) at the femoral neck and total femur, but not in bone density at the lumbar spine or ultra-distal forearm was reported following 12 months follow-up in RTX-exposed refractory RA patients [102]. Increased risk of hypogammaglobulinemia as the result of RTX administration in RA patients is another challenging issue [103]. The main risk factors for hypogammaglobulinemia after B cell-targeted therapy in the autoimmune rheumatic disease was found a low baseline serum IgG levels [104]. Thus, the use of prophylactic intravenous immunoglobulins (IVIg) and IgG monitoring may be useful for those receiving RTX. Furthermore, recently, a severe anaphylactic reaction to RTX in two patients was also reported [101]. These side effects, such as sustained secondary antibody deficiency should be considered for evaluation of risk to benefit of RTX in RA patients [104].
Probable allowable conditions for rituximab therapy
Although RTX is related to different safety concerns, there is some evidence of safe administration of RTX in specific groups of RA patients. For example, low-dose RTX (2 × 500 mg) was safely used in a 76 years old man with heart failure (HF), which did not lead to aggravation of HF status [105]. Moreover, RTX was effective and safe in the treatment of subcutaneous nodulosis in an RA patient [106]. Moreover, no relation between surgical complications and RTX administration in RA patients was reported [107].
Some of the potential long-term side effects of RTX had been checked in some studies, which suggest RTX not a risk for increased risk of future incident malignancy in RA patients [78, 108, 109]. Moreover, evaluation of chromosomal changes in patients exposed to the RTX was associated with neither clastogenic nor aneugenic effects [110]. Interestingly, the beneficial role of RTX in not only controlling RA activity but also resolving drug-induced lupus as the result of treatment with anti-TNF agents was observed [111].
Predition of response to rituximab
Despite the several reports implying high efficacy of RTX therapy in a large number of patients, many of them do not respond, and different patients may experience related adverse events. For example, in the recent study with 1629 RA patients who continued RTX for more than 4 years, 240 (16%) discontinued RTX treatment because of ineffectiveness and 95 (5.8%) for adverse events [112]. Over the last decade, we have witnessed rapid progress in the prediction of response to the particular therapeutic options in autoimmune diseases, such as RA [113, 114]. Although several pharmacogenetics studies have been conducted [115], we still could not consider it very practical. However, to date, different markers, including serological, cells numbers, genes expression, and genetic variations have been suggested, which discussed below. Despite the suggestion of several markers for prediction of RTX response in RA patients, only a few of them are routinely used by the majority of rheumatologists. Table 4 summarizes some of the most important predictors of a better response to RTX.
Cell counts
Considering the RTX mechanism of action, it is expected that patients with complete and rapid depletion of B cells benefit more from the RTX; however, there are conflicting results. As one of the first studies related to the prediction of response to RTX, Dass et al. [143] measured peripheral B cell numbers using minimal residual disease (MRD) flow cytometry before and after each infusion. They have concluded that a lack of complete depletion of CD20+ cells following the first infusion is associated with a poorer outcome. However, other groups did not find any relationship between B cell depletion and clinical response in RA patients exposed to RTX after either tissue examination, using flow cytometry, immunohistochemistry, and quantitative PCR or blood examination by fluorescence-activated cell sorting [144, 145]. Additionally, baseline or post-treatment frequencies of activated T cells do not seem to be a predictor of treatment response [145].
A higher number of circulating pre-plasma cells (cells that usually do not express CD20 and cause resistance to RTX) at the baseline were also related to not responding to RTX as well as incomplete depletion of B cells [31]. A higher elimination rate constant of RTX (the rate at which RTX is removed) was significantly associated with the CD19+ count as well as IgG concentration [146]. However, Sellam et al. [147] reported comparable levels of CD19+ B cells among the responders and non-responders to RTX. Association of low baseline CD27+ memory B cell frequency, normal levels of RF+CD19+ and increased levels of CD19+CD27−IgD− B cells are other reported biomarkers to predict response to RTX in RA patients [147, 148]. Baseline CD95+ pre-switch B cells frequency were also observed as a negative predictor of response to RTX [149].
Not only B cells but also T lymphocytes and other immune cells seem to be involved in RTX response. For example, total lymphocyte counts and CD4+ T cells at baseline were found as the independent predictors of EULAR response [116]. In addition to higher baseline levels of the total lymphocytes, higher plasmablast frequency (> 2.85%) at the baseline was also able to sensitively identifies RA patients who will not benefit from RTX. Because depletion of substantial T cell, particularly CD4+ cells is one of the outcomes of RTX infusion and probably is associated with the clinical response, monitoring of T cells could be considered one of the possible approaches to early detection of likely non-responders [8, 117]. Indeed, a significant reduction in the circulating CD4+ T cell may increase the chance of responding to RTX. Innate immune system’s components are also involved, and a lower count of NK cells and also a higher frequency of iNKT cells could predict a better response to RTX in RA patients [118,119,120].
Serological markers
During recent years, three markers of total IgG serum concentrations, RF, and anti-CCP in RA patients with the aim of predicting response to RTX have been widely studied. Regarding the association between the elevated baseline IgG serum concentrations and a better response to RTX, there are conflicting data. Some studies have shown it as a predictor of RTX response [121, 122], while some others do not agree [123, 147]. A higher titer of all autoantibodies at the baseline was found to be related to a better response to RTX in RA patients [150].
Majority of studies implying a higher efficacy of RTX therapy in patients with both positive RF and anti-CCP as compared to seronegative patients [32,33,34, 121,122,123,124,125,126]. However, there are some other pieces of evidence, which only suggest one of the RF or anti-CCP as the marker of clinical response [36, 123, 127, 147]. Recently, RTX discontinuation (mainly as the result of ineffectiveness, 46%; and death, 24%) was associated with RF negativity [112]. The lower baseline value of erythrocyte sedimentation rate (ESR) is another suggested factor prediction be a better response to RTX [127]. Multi-bio-marker disease activity (MBDA) score, which is calculated by measuring 12 serum proteins and clinically validated as a measure of disease activity in patients with RA was found to be valid for tracking disease activity in RA patients treated with RTX [151]. Moreover, change in MBDA score reflected the degree of treatment response [151]. In contrast, no correlation was found between the response to RTX and neither baseline B cell-activating factor (BAFF) level and BAFF-R expression nor 25(OH)D levels in RA patients [152, 153]. Detectable serum level of IL-33 was found to be associated with EULAR responders to RTX [122]. However, no association between IL-33 detection and response to RTX was found in another study [154].
Genetic markers
One of the most attractive markers in pharmacogenomics of autoimmune diseases is genetic polymorphisms [115]. Genetic polymorphisms in different genes have been found to be associated with treatment response and might be considered a predictor for both efficacy and safety profile of RTX therapy. Since cytokines orchestrate the fate of autoreactive lymphocytes and autoimmune reactivity, their polymorphisms have attracted the attention of many researchers owing to their potential applications in the prediction of treatment response to RTX. It was demonstrated that patients with GC/GG genotypes in interleukin (IL)-6 promoter at position -174 (rs1800795) were more susceptible to a better response at month + 6 as compared to those with CC genotypes [128]. SNPs at TGFβ1 codon 10 (rs1800470) and TGFβ1 codon 25 (rs1800471) are other associated polymorphisms with clinical response to RTX [129]. Indeed, it was observed that CT genotype for rs1800470 and GC genotype for rs1800471 could be the criterion of response to RTX therapy in RA patients [129]. BAFF is a cytokine essential for B cell maturation, which significantly contributes to the pathogenesis of different autoimmune diseases [155]. Although there was no relation between the BAFF−871 promoter polymorphisms (rs9514828; C>T) and BAFF serum level in RA patients, homozygous carriers of the BAFF rs9514828 C were found as better responders to RTX as compared to the homozygotes for BAFF rs9514828 T (response rate: 92% for C/C vs. 64% for T/T) [130].
Polymorphisms in FCGR3A gene is another interesting research topic. In fact, the variations in this gene influencing the outcome of B cell-depleting therapy with RTX in malignancies, probably through the induction of differential affinity of the receptors [156]. Regarding RA, FCGR3A F158V (rs396991; T>G) polymorphism seems to contribute to having a higher response rate to RTX. In fact, those with V allele (code Valine) and/or G allele were found to be better responders in different studies conducted in North America [132], France [133], Italia [134], Hungary [135], and Spain [127]. The presence of FCGR2A rs1801274-TT genotype is another novel probably involved factor in RTX response in RA patients [127]. Additionally, IRF5 rs2004640 and TNFSF13B rs9514828 influenced response to RTX at week 24 [131]. Association between the RTX response and the allele*2 of the HS1,2A enhancer in seropositive RA patients was also reported [157].
The role of genetics in the prediction of response to RTX is not limited to the genetic polymorphisms. Different studies have evaluated genes’ expression in responders and non-responders. Low or absent baseline IFN type-I response gene (IRG) expression levels (consist of LY6E, HERC5, IFI44L, ISG15, MxA, MxB, EPSTI1, and RSAD2) means better response to RTX in RA patients [136, 137]. In a small cohort study containing nine RA patients, it was found that lower expression of ARG1 and a higher expression of TRAF1 in whole blood and TLR4 in CD4+ T cells were associated with better response to RTX [138]. As the results of another study, the response to RTX was associated with a decrease in the expression of mTOR, p21 (CDKN1A), caspase 3 (CASP3), ULK1, TNFα, IL-1β, and cathepsin K (CTSK) [140]. Moreover, increased CD46 expression, but not CD35, seems to be able to predict time to the repopulation of B cells in RA patient treated with RTX [158]. In contrast to leukemia B cells [159], the alternative CD20 transcript could not predict resistance to RTX in RA patients [160]. High serum levels of miR-125b, which are overexpressed in RA patients, increase the success of the response to RTX [139].
Other biomarkers
As it was discussed, being naïve to biologics [32] or a smaller number of previous biological therapies [127] are associated with better response to RTX. Some studies have highlighted the positive role of the presence of EBV genome in responding to RTX. Indeed, RA patients who are also EBV carriers not only respond better to RTX therapy but also need an additional course of treatment within a longer time [94, 141]. However, this did not confirm in a later conducted study [123]. Smoking is another suggested factor that influences RTX response. Active smokers, especially those with negative auto-antibody status were found to be more susceptible to not respond to RTX [125], but this was not found in another study [142]. Additionally, lower baseline DAS28 was found as an independent predictor of good EULAR response at 6 months [142] and 24 months [38].
Pregnancy and fetal risk
Despite the several conducted studies on the efficacy and safety of RTX in RA patients, there are few data concerning risk assessment of RTX therapy in pregnant women. A study on pregnant animal models (macaque species) exposed to RTX had shown no evidence of teratogenic effects, but only a reversible reduction of B-cell count in the offspring [161]. Since RTX is an IgG-based antibody, it could cross the placental barrier, which increases as pregnancy progresses and may lead to increased susceptibility to infections through impairing fetal and neonatal B cell development [162]. Hence, different studies have recommended stopping RTX therapy at least 12 months before starting a pregnancy [163]. However, it seems that the RTX administration before conception is safer than we thought [164]. According to the EULAR recommendation, RTX probably does not increase the rate of congenital malformations; even in exceptional cases, RTX usage was allowed early in gestation [165]. However, in later stages of pregnancy, the risk of B cell depletion and other cytopenias in the neonate had been warned. However, according to the British Society for Rheumatology and British Health Professionals in Rheumatology (BSR-BHPR) guideline, RA patients should not be exposed to RTX, at least 6 months before conception [166]. However, inadvertent pregnancy may occur during or after RTX treatment. Thus, it is recommended that before starting RTX infusion, all women of childbearing age must take a pregnancy test before RTX administration; those with a positive result must avoid RTX therapy. Additionally, women of childbearing age are advised to use effective contraception for 6 months after the last infusion of RTX [167].
Recently reported records from the British Society for Rheumatology Biologics Register on pregnancy outcomes in RTX-exposed RA patients (BSRBR-RA) are registering 66% (n = 6) live births, 22% (n = 2) miscarriages/stillbirths, and 11% (n = 1) termination among expecting mothers, who had been exposed to RTX within 6 months of conception [168]. These records change to 86% (n = 6), 14% (n = 1) and 0% of live births, miscarriages/stillbirths, and termination, respectively, for those who had been exposed to RTX between 6 and 12 months before conception. Surprisingly, the safety of RTX administration within the first trimester of pregnancy in two RA patients was also reported [169]. This unexpected outcome was explained by very low transplacental maternofetal transfer of this monoclonal antibody during the first trimester of pregnancy. In eight pregnancies that occurred during a study for evaluation of the safety of RTX, except two spontaneous abortions, one elective termination, and one preterm birth, others were successful and no congenital abnormality was found [57]. As EULAR had recommended, due to the lack of evidence regarding RTX in breast milk, patients must avoid exposure to the RTX in breastfeeding [165].
Rituximab biosimilars
During recent years, different RTX biosimilars have been introduced, and many clinical trials are being conducted to evaluate their efficacy and safety, and compare to originator [170]. These alternative drugs, such as Truxima (CT-P10) and Rixathon (GP2013) are increasingly being used due to availability and lower cost. It was shown that switching from reference RTX to GP2013 is not associated with any additional safety and immunogenicity problems [171]. Regarding CT-P10, there was no significant difference between the efficacy, safety, and immunogenicity of reference RTX and biosimilar RTX in 24, 48, and 72 weeks follow-up [172,173,174]. The similar findings in term of efficacy, safety, and immunogenicity were reported for PF-05280586, another RTX biosimilar [175].
Conclusion and recommendations
According to the literature review, it could be concluded that RTX is an effective treatment with long-lasting effects on majority of RA patients. Although the superiority of RTX to anti-TNF agents is not very clear, non-responders to TNF blockers have a great chance to achieve remission by RTX therapy. Regarding the optimum doses of RTX, there is no consensus. However, low-dose RTX might be an alternative for standard dose for patients who cannot tolerate or are at the risk of infection development/reactivation. RTX seems a safe treatment for RA patients, while particular attention to those with a chronic infection, such as CHB, is required. Additionally, because of some reported paradoxical reaction, close monitoring of patients is recommended. Essential vaccination also should be administrated before RTX initiation. Prediction of response to RTX therapy is still in its juvenile stages. To date, the different genetic factors, as well as the presence of specific cells or proteins, have been identified as the probable predictor of RTX response. Evaluation of sensitivity and specificity of them before adopting them for use in clinical practice is crucial. In this regard, it could be recommended that the determination of the efficient and safe dose might be related to such predictors or markers, which needs further studies. The lack of studies on safety of RTX exposure before/during the pregnancy has led to concerns regarding RTX safety during conception. Until reaching a consensus, following the last released guidelines are recommended. Figure 2 summarizes these findings and recommendations.
References
van Gestel AM, Haagsma CJ, van Riel PL (1998) Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 41(10):1845–1850. https://doi.org/10.1002/1529-0131(199810)41:10<1845::aid-art17>3.0.co;2-k
Derksen VFAM, Huizinga TWJ, van der Woude D (2017) The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin Immunopathol 39(4):437–446. https://doi.org/10.1007/s00281-017-0627-z
Wijbrandts CA, Tak PP (2017) Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc 92(7):1129–1143. https://doi.org/10.1016/j.mayocp.2017.05.009
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K (2018) Rheumatoid arthritis. Nat Rev Dis Primers 4:18001. https://doi.org/10.1038/nrdp.2018.1
Listing J, Kekow J, Manger B, Burmester GR, Pattloch D, Zink A, Strangfeld A (2015) Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFalpha inhibitors and rituximab. Ann Rheum Dis 74(2):415–421. https://doi.org/10.1136/annrheumdis-2013-204021
Clark E, Ledbetter J (2005) How does B cell depletion therapy work, and how can it be improved? Ann Rheum Dis 64(Suppl 4):iv77–iv80. https://doi.org/10.1136/ard.2005.042507
Cambridge G, Stohl W, Leandro MJ, Migone TS, Hilbert DM, Edwards JC (2006) Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum 54(3):723–732. https://doi.org/10.1002/art.21650
Melet J, Mulleman D, Goupille P, Ribourtout B, Watier H, Thibault G (2013) Rituximab-induced T cell depletion in patients with rheumatoid arthritis: association with clinical response. Arthritis Rheum 65(11):2783–2790. https://doi.org/10.1002/art.38107
Buch MH, Smolen JS, Betteridge N, Breedveld FC, Burmester G, Dorner T, Ferraccioli G, Gottenberg JE, Isaacs J, Kvien TK, Mariette X, Martin-Mola E, Pavelka K, Tak PP, van der Heijde D, van Vollenhoven RF, Emery P (2011) Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 70(6):909–920. https://doi.org/10.1136/ard.2010.144998
van Vollenhoven RF, Emery P, Bingham CO 3rd, Keystone EC, Fleischmann RM, Furst DE, Tyson N, Collinson N, Lehane PB (2013) Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis 72(9):1496–1502. https://doi.org/10.1136/annrheumdis-2012-201956
Nicholls D, Zochling J, Boers A, Champion G, Mathers D, Riordan J, Youssef P, Scott J, Griffiths H (2014) A retrospective chart review of the use of rituximab for the treatment of rheumatoid arthritis in Australian rheumatology practice. Int J Rheum Dis 17(7):755–761. https://doi.org/10.1111/1756-185x.12164
Aaltonen KJ, Joensuu JT, Virkki L, Sokka T, Aronen P, Relas H, Valleala H, Rantalaiho V, Pirila L, Puolakka K, Uusitalo T, Blom M, Konttinen YT, Nordstrom D (2015) Rates of serious infections and malignancies among patients with rheumatoid arthritis receiving either tumor necrosis factor inhibitor or rituximab therapy. J Rheumatol 42(3):372–378. https://doi.org/10.3899/jrheum.140853
van Vollenhoven RF, Fleischmann RM, Furst DE, Lacey S, Lehane PB (2015) Longterm safety of rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol 42(10):1761–1766. https://doi.org/10.3899/jrheum.150051
Rigby W, Ferraccioli G, Greenwald M, Zazueta-Montiel B, Fleischmann R, Wassenberg S, Ogale S, Armstrong G, Jahreis A, Burke L, Mela C, Chen A (2011) Effect of rituximab on physical function and quality of life in patients with rheumatoid arthritis previously untreated with methotrexate. Arthritis Care Res (Hoboken) 63(5):711–720. https://doi.org/10.1002/acr.20419
Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, Hessey EW, Shaw T, Totoritis MC (2006) Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 54(9):2793–2806. https://doi.org/10.1002/art.22025
Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, Hessey EW, Shaw TM (2006) The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 54(5):1390–1400. https://doi.org/10.1002/art.21778
Higashida J, Wun T, Schmidt S, Naguwa SM, Tuscano JM (2005) Safety and efficacy of rituximab in patients with rheumatoid arthritis refractory to disease modifying antirheumatic drugs and anti-tumor necrosis factor-alpha treatment. J Rheumatol 32(11):2109–2115
Ho LY, Mok CC, To CH, Anselm M, Cheung MY, Yu KL (2007) Rituximab for refractory rheumatoid arthritis: a 24-week open-label prospective study. Open Rheumatol J 1:1–4. https://doi.org/10.2174/1874312900701010001
Moore J, Ma D, Will R, Cannell P, Handel M, Milliken S (2004) A phase II study of rituximab in rheumatoid arthritis patients with recurrent disease following haematopoietic stem cell transplantation. Bone Marrow Transplant 34(3):241–247. https://doi.org/10.1038/sj.bmt.1704570
Jois RN, Masding A, Somerville M, Gaffney K, Scott DG (2007) Rituximab therapy in patients with resistant rheumatoid arthritis: real-life experience. Rheumatology (Oxford) 46(6):980–982. https://doi.org/10.1093/rheumatology/kel453
Keystone E, Burmester GR, Furie R, Loveless JE, Emery P, Kremer J, Tak PP, Broder MS, Yu E, Cravets M, Magrini F, Jost F (2008) Improvement in patient-reported outcomes in a rituximab trial in patients with severe rheumatoid arthritis refractory to anti-tumor necrosis factor therapy. Arthritis Rheum 59(6):785–793. https://doi.org/10.1002/art.23715
Soliman MM, Hyrich KL, Lunt M, Watson KD, Symmons DP, Ashcroft DM (2012) Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology biologics register. Arthritis Care Res (Hoboken) 64(8):1108–1115. https://doi.org/10.1002/acr.21663
Porter D, van Melckebeke J, Dale J, Messow CM, McConnachie A, Walker A, Munro R, McLaren J, McRorie E, Packham J, Buckley CD, Harvie J, Taylor P, Choy E, Pitzalis C, McInnes IB (2016) Tumour necrosis factor inhibition versus rituximab for patients with rheumatoid arthritis who require biological treatment (ORBIT): an open-label, randomised controlled, non-inferiority, trial. Lancet 388(10041):239–247. https://doi.org/10.1016/s0140-6736(16)00380-9
Tak PP, Rigby WF, Rubbert-Roth A, Peterfy CG, van Vollenhoven RF, Stohl W, Hessey E, Chen A, Tyrrell H, Shaw TM (2011) Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis 70(1):39–46. https://doi.org/10.1136/ard.2010.137703
Tak PP, Rigby W, Rubbert-Roth A, Peterfy C, van Vollenhoven RF, Stohl W, Healy E, Hessey E, Reynard M, Shaw T (2012) Sustained inhibition of progressive joint damage with rituximab plus methotrexate in early active rheumatoid arthritis: 2-year results from the randomised controlled trial IMAGE. Ann Rheum Dis 71(3):351–357. https://doi.org/10.1136/annrheumdis-2011-200170
Harrold LR, John A, Best J, Zlotnick S, Karki C, Li Y, Greenberg JD, Kremer JM (2017) Impact of rituximab on patient-reported outcomes in patients with rheumatoid arthritis from the US Corrona Registry. Clin Rheumatol 36(9):2135–2140. https://doi.org/10.1007/s10067-017-3742-2
Gottenberg JE, Morel J, Perrodeau E, Bardin T, Combe B, Dougados M, Flipo RM, Saraux A, Schaeverbeke T, Sibilia J, Soubrier M, Vittecoq O, Baron G, Constantin A, Ravaud P, Mariette X (2019) Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. Bmj 364:l67. https://doi.org/10.1136/bmj.l67
Coutant F, Rouzaire P, Soubrier M (2017) An unexpected response to rituximab in a patient with rheumatoid arthritis. Rheumatology (Oxford) 57:580–582. https://doi.org/10.1093/rheumatology/kex418
Vos K, Thurlings RM, Wijbrandts CA, van Schaardenburg D, Gerlag DM, Tak PP (2007) Early effects of rituximab on the synovial cell infiltrate in patients with rheumatoid arthritis. Arthritis Rheum 56(3):772–778. https://doi.org/10.1002/art.22400
Nakou M, Katsikas G, Sidiropoulos P, Bertsias G, Papadimitraki E, Raptopoulou A, Koutala H, Papadaki HA, Kritikos H, Boumpas DT (2009) Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response. Arthritis Res Ther 11(4):R131. https://doi.org/10.1186/ar2798
Vital EM, Dass S, Rawstron AC, Buch MH, Goeb V, Henshaw K, Ponchel F, Emery P (2010) Management of nonresponse to rituximab in rheumatoid arthritis: predictors and outcome of re-treatment. Arthritis Rheum 62(5):1273–1279. https://doi.org/10.1002/art.27359
Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, Gabay C, van Riel PL, Nordstrom DC, Gomez-Reino J, Pavelka K, Tomsic M, Kvien TK, van Vollenhoven RF (2011) Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann Rheum Dis 70(9):1575–1580. https://doi.org/10.1136/ard.2010.148759
Narvaez J, Diaz-Torne C, Ruiz JM, Hernandez MV, Torrente-Segarra V, Ros S, Rodriguez de la Serna A, Diaz-Lopez C, Sanmarti R, Nolla JM (2011) Predictors of response to rituximab in patients with active rheumatoid arthritis and inadequate response to anti-TNF agents or traditional DMARDs. Clin Exp Rheumatol 29(6):991–997
De Keyser F, Hoffman I, Durez P, Kaiser MJ, Westhovens R (2014) Longterm followup of rituximab therapy in patients with rheumatoid arthritis: results from the Belgian MabThera in Rheumatoid Arthritis registry. J Rheumatol 41(9):1761–1765. https://doi.org/10.3899/jrheum.131279
Harrold LR, Reed GW, Shewade A, Magner R, Saunders KC, John A, Kremer JM, Greenberg JD (2015) Effectiveness of rituximab for the treatment of rheumatoid arthritis in patients with prior exposure to anti-TNF: results from the CORRONA registry. J Rheumatol 42(7):1090–1098. https://doi.org/10.3899/jrheum.141043
Quartuccio L, Fabris M, Salvin S, Atzeni F, Saracco M, Benucci M, Cimmino M, Morassi P, Masolini P, Pellerito R, Cutolo M, Puttini PS, De Vita S (2009) Rheumatoid factor positivity rather than anti-CCP positivity, a lower disability and a lower number of anti-TNF agents failed are associated with response to rituximab in rheumatoid arthritis. Rheumatology (Oxford) 48(12):1557–1559. https://doi.org/10.1093/rheumatology/kep314
Valleala H, Korpela M, Hienonen-Kempas T, Immonen K, Lahteenmaki J, Uusitalo T, Komulainen R, Mottonen T, Hannonen P (2015) Long-term real-life experience with rituximab in adult Finnish patients with rheumatoid arthritis refractory or with contraindication to anti-tumor necrosis factor drugs. J Clin Rheumatol 21(1):24–30. https://doi.org/10.1097/rhu.0000000000000166
Wang KC, Liao HT, Chen WS, Lai CC, Chou CT, Chen MH, Tsai CY (2019) Real-world effectiveness and safety of rituximab in the treatment of rheumatoid arthritis: a single-center experience in Taiwan. Int J Rheum Dis 22(5):860–868. https://doi.org/10.1111/1756-185x.13511
Arts EE, Jansen TL, Den Broeder A, Vonkeman HE, Dutmer E, Van de Laar MA, Van Riel PL, Fransen J (2011) Statins inhibit the antirheumatic effects of rituximab in rheumatoid arthritis: results from the Dutch rheumatoid arthritis monitoring (DREAM) registry. Ann Rheum Dis 70(5):877–878. https://doi.org/10.1136/ard.2010.136093
Das S, Fernandez Matilla M, Dass S, Buch MH, Rawstron AC, Vital EM, Emery P (2013) Statins do not influence clinical response and B cell depletion after rituximab treatment in rheumatoid arthritis. Ann Rheum Dis 72(3):463–464. https://doi.org/10.1136/annrheumdis-2012-202454
Mazilu D, Gudu T, Ionescu R, Opris D (2014) Statins do not influence long-term rituximab clinical efficiency in rheumatoid arthritis patients. Biomed Res Int 2014:689426–689424. https://doi.org/10.1155/2014/689426
Lehane PB, Lacey S, Hessey EW, Jahreis A (2014) Effect of concomitant statins on rituximab efficacy in patients with rheumatoid arthritis. Ann Rheum Dis 73(10):1906–1908. https://doi.org/10.1136/annrheumdis-2014-205474
Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, Latinis K, Abud-Mendoza C, Szczepanski LJ, Roschmann RA, Chen A, Armstrong GK, Douglass W, Tyrrell H (2010) Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab's Efficacy in MTX iNadequate rEsponders (SERENE)). Ann Rheum Dis 69(9):1629–1635. https://doi.org/10.1136/ard.2009.119933
Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, Ancuta I, Pavelka K, Nordstrom DC, Gabay C, Canhao H, Tomsic M, van Riel PL, Gomez-Reino J, Kvien TK, van Vollenhoven RF (2016) Effectiveness of two different doses of rituximab for the treatment of rheumatoid arthritis in an international cohort: data from the CERERRA collaboration. Arthritis Res Ther 18:50. https://doi.org/10.1186/s13075-016-0951-z
Rubbert-Roth A, Tak PP, Zerbini C, Tremblay JL, Carreno L, Armstrong G, Collinson N, Shaw TM (2010) Efficacy and safety of various repeat treatment dosing regimens of rituximab in patients with active rheumatoid arthritis: results of a phase III randomized study (MIRROR). Rheumatology (Oxford) 49(9):1683–1693. https://doi.org/10.1093/rheumatology/keq116
Bredemeier M, de Oliveira FK, Rocha CM (2014) Low- versus high-dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 66(2):228–235. https://doi.org/10.1002/acr.22116
Bredemeier M, Campos GG, de Oliveira FK (2015) Updated systematic review and meta-analysis of randomized controlled trials comparing low- versus high-dose rituximab for rheumatoid arthritis. Clin Rheumatol 34(10):1801–1805. https://doi.org/10.1007/s10067-015-2977-z
Henry J, Gottenberg JE, Rouanet S, Pavy S, Sellam J, Tubach F, Belkhir R, Mariette X, Seror R (2017) Doses of rituximab for retreatment in rheumatoid arthritis: influence on maintenance and risk of serious infection. Rheumatology (Oxford) 57:538–547. https://doi.org/10.1093/rheumatology/kex446
Mena-Vazquez N, Manrique-Arija S, Urena-Garnica I, Romero-Barco CM, Jimenez-Nunez FG, Coret V, Irigoyen-Oyarzabal MV, Fernandez-Nebro A (2016) Eficiency of different doses of rituximab in rheumatoid arthritis. Reumatol Clin 12(3):139–145. https://doi.org/10.1016/j.reuma.2015.07.003
Mariette X, Rouanet S, Sibilia J, Combe B, Le Loet X, Tebib J, Jourdan R, Dougados M (2014) Evaluation of low-dose rituximab for the retreatment of patients with active rheumatoid arthritis: a non-inferiority randomised controlled trial. Ann Rheum Dis 73(8):1508–1514. https://doi.org/10.1136/annrheumdis-2013-203480
Chatzidionysiou K, Lie E, Lukina G, Hetland ML, Hauge EM, Pavelka K, Gabay C, Scherer A, Nordstrom D, Canhao H, Santos MJ, Tomsic M, Rotar Z, Hernandez MV, Gomez-Reino J, Ancuta I, Kvien TK, van Vollenhoven R (2017) Rituximab retreatment in rheumatoid arthritis in a real-life cohort: data from the CERERRA collaboration. J Rheumatol 44(2):162–169. https://doi.org/10.3899/jrheum.160460
Schapink L, Nd B, Ad B, Verhoef L (2019) AB0408 fixed-interval versus on-demand retreatment strategy with rituximab in rheumatoid arthritis: a retrospective cohort study. Ann Rheum Dis 78(Suppl 2):1664–1665. https://doi.org/10.1136/annrheumdis-2019-eular.6215
Vital EM, Dass S, Buch MH, Rawstron AC, Emery P (2015) An extra dose of rituximab improves clinical response in rheumatoid arthritis patients with initial incomplete B cell depletion: a randomised controlled trial. Ann Rheum Dis 74(6):1195–1201. https://doi.org/10.1136/annrheumdis-2013-204544
Keystone E, Fleischmann R, Emery P, Furst DE, van Vollenhoven R, Bathon J, Dougados M, Baldassare A, Ferraccioli G, Chubick A, Udell J, Cravets MW, Agarwal S, Cooper S, Magrini F (2007) Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum 56(12):3896–3908. https://doi.org/10.1002/art.23059
Bastian H, Zinke S, Egerer K, Breuer S, Safari F, Burmester GR, Feist E (2010) Effects of early rituximab retreatment in rheumatoid arthritis patients with an inadequate response after the first cycle: retrospective arthritis cohort study. J Rheumatol 37(5):1069–1071. https://doi.org/10.3899/jrheum.091127
Pritchard CH, Greenwald MW, Kremer JM, Gaylis NB, Rigby W, Zlotnick S, Chung C, Jaber B, Reiss W (2014) Safety of infusing rituximab at a more rapid rate in patients with rheumatoid arthritis: results from the RATE-RA study. BMC Musculoskelet Disord 15:177. https://doi.org/10.1186/1471-2474-15-177
Winthrop KL, Saag K, Cascino MD, Pei J, John A, Jahreis A, Haselkorn T, Furst DE (2018) Long-term safety of rituximab in rheumatoid arthritis: analysis from the SUNSTONE registry. Arthritis Care Res (Hoboken). https://doi.org/10.1002/acr.23781
Gron KL, Arkema EV, Glintborg B, Mehnert F, Ostergaard M, Dreyer L, Norgaard M, Krogh NS, Askling J, Hetland ML (2019) Risk of serious infections in patients with rheumatoid arthritis treated in routine care with abatacept, rituximab and tocilizumab in Denmark and Sweden. Ann Rheum Dis 78(3):320–327. https://doi.org/10.1136/annrheumdis-2018-214326
Gottenberg JE, Ravaud P, Bardin T, Cacoub P, Cantagrel A, Combe B, Dougados M, Flipo RM, Godeau B, Guillevin L, Le Loet X, Hachulla E, Schaeverbeke T, Sibilia J, Baron G, Mariette X (2010) Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum 62(9):2625–2632. https://doi.org/10.1002/art.27555
Goates C, Tsuha S, Working S, Carey J, Spivak ES (2017) Seronegative West Nile virus infection in a patient treated with rituximab for rheumatoid arthritis. Am J Med 130(6):e257–e258. https://doi.org/10.1016/j.amjmed.2017.01.014
Raffalli J, Wormser GP (2016) Persistence of babesiosis for >2 years in a patient on rituximab for rheumatoid arthritis. Diagn Microbiol Infect Dis 85(2):231–232. https://doi.org/10.1016/j.diagmicrobio.2016.02.016
Ghrenassia E, Mekinian A, Rouaghe S, Ganne N, Fain O (2012) Reactivation of resolved hepatitis B during rituximab therapy for rheumatoid arthritis. Joint Bone Spine 79(1):100–101. https://doi.org/10.1016/j.jbspin.2011.07.003
Gigi E, Georgiou T, Mougiou D, Boura P, Raptopoulou-Gigi M (2013) Hepatitis B reactivation in a patient with rheumatoid arthritis with antibodies to hepatitis B surface antigen treated with rituximab. Hippokratia 17(1):91–93
Salman-Monte TC, Lisbona MP, Garcia-Retortillo M, Maymo J (2014) Reactivation of hepatitis virus B infection in a patient with rheumatoid arthritis after treatment with rituximab. Reumatol Clin 10(3):196–197. https://doi.org/10.1016/j.reuma.2013.05.011
Tien YC, Yen HH, Chiu YM (2017) Incidence and clinical characteristics of hepatitis B virus reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for rheumatoid arthritis. Clin Exp Rheumatol 35(5):831–836
Chen YM, Chen HH, Huang WN, Chen YH, Hsieh TY, Yang SS, Lan JL, Chen DY (2019) Reactivation of hepatitis B virus infection following rituximab treatment in HBsAg-negative, HBcAb-positive rheumatoid arthritis patients: a long-term, real-world observation. Int J Rheum Dis. https://doi.org/10.1111/1756-185x.13582
Lin KM, Lin JC, Tseng WY, Cheng TT (2013) Rituximab-induced hepatitis C virus reactivation in rheumatoid arthritis. J Microbiol Immunol Infect 46(1):65–67. https://doi.org/10.1016/j.jmii.2011.12.020
Chen YM, Chen HH, Chen YH, Hsieh TY, Hsieh CW, Hung WT, Lan JL, Chen DY (2015) A comparison of safety profiles of tumour necrosis factor alpha inhibitors and rituximab therapy in patients with rheumatoid arthritis and chronic hepatitis C. Ann Rheum Dis 74(3):626–627. https://doi.org/10.1136/annrheumdis-2014-206711
Rehnberg M, Brisslert M, Amu S, Zendjanchi K, Hawi G, Bokarewa MI (2010) Vaccination response to protein and carbohydrate antigens in patients with rheumatoid arthritis after rituximab treatment. Arthritis Res Ther 12(3):R111. https://doi.org/10.1186/ar3047
van Assen S, Holvast A, Benne CA, Posthumus MD, van Leeuwen MA, Voskuyl AE, Blom M, Risselada AP, de Haan A, Westra J, Kallenberg CG, Bijl M (2010) Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 62(1):75–81. https://doi.org/10.1002/art.25033
Intongkam S, Samakarnthai P, Pakchotanon R, Narongroeknawin P, Assavatanabodee P, Chaiamnuay S (2018) Efficacy and safety of hepatitis B vaccination in rheumatoid arthritis patients receiving disease-modifying Antirheumatic drugs and/or biologics therapy. J Clin Rheumatol:1. https://doi.org/10.1097/rhu.0000000000000877
Dass S, Vital EM, Emery P (2007) Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum 56(8):2715–2718. https://doi.org/10.1002/art.22811
Hardcastle SA, Gibbs S, Williamson L (2012) Atypical psoriasis following rituximab for rheumatoid arthritis. J Rheumatol 39(6):1303–1304. https://doi.org/10.3899/jrheum.111256
Guidelli GM, Fioravanti A, Rubegni P, Feci L (2013) Induced psoriasis after rituximab therapy for rheumatoid arthritis: a case report and review of the literature. Rheumatol Int 33(11):2927–2930. https://doi.org/10.1007/s00296-012-2581-3
Ozen G, Ergun T, Oner SY, Demirkesen C, Inanc N (2013) Widespread psoriasis induced by rituximab in a patient with rheumatoid arthritis: an unexpected adverse reaction. Joint Bone Spine 80(5):545–547. https://doi.org/10.1016/j.jbspin.2013.02.001
Brunasso AM, Massone C (2012) Plantar pustulosis during rituximab therapy for rheumatoid arthritis. J Am Acad Dermatol 67(4):e148–e150. https://doi.org/10.1016/j.jaad.2011.12.010
Bhalme M, Hayes S, Norton A, Lal S, Chinoy H, Paine P (2013) Rituximab-associated colitis. Inflamm Bowel Dis 19(3):E41–E43. https://doi.org/10.1002/ibd.22963
van Vollenhoven RF, Emery P, Bingham CO 3rd, Keystone EC, Fleischmann R, Furst DE, Macey K, Sweetser M, Kelman A, Rao R (2010) Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol 37(3):558–567. https://doi.org/10.3899/jrheum.090856
Harrold LR, Reed GW, Karki C, Magner R, Shewade A, John A, Kremer JM, Greenberg JD (2016) Risk of infection associated with subsequent biologic agent use after rituximab: results from a National Rheumatoid Arthritis Patient registry. Arthritis Care Res (Hoboken) 68(12):1888–1893. https://doi.org/10.1002/acr.22912
Silva-Fernandez L, De Cock D, Lunt M, Low AS, Watson KD, Symmons DPM, Hyrich KL (2017) Serious infection risk after 1 year between patients with rheumatoid arthritis treated with rituximab or with a second TNFi after initial TNFi failure: results from the British Society for Rheumatology Biologics Register for rheumatoid arthritis. Rheumatology (Oxford) 57:1533–1540. https://doi.org/10.1093/rheumatology/kex304
Shi Y, Wu Y, Ren Y, Jiang Y, Chen Y (2019) Infection risks of rituximab versus non-rituximab treatment for rheumatoid arthritis: a systematic review and meta-analysis. Int J Rheum Dis. https://doi.org/10.1111/1756-185x.13596
Meyer A, Theulin A, Chatelus E, Argemi X, Sordet C, Javier RM, Hansmann Y, Sibilia J, Gottenberg JE (2012) Campylobacter fetus infection in three rheumatoid arthritis patients treated with rituximab. Ann Rheum Dis 71(6):1094–1095. https://doi.org/10.1136/annrheumdis-2011-200719
Ottaviani S, Tiendrebeogo J, Choudat L, Gill G, Palazzo E, Meyer O, Dieude P (2013) Knee tuberculosis under rituximab therapy for rheumatoid arthritis. Joint Bone Spine 80(4):435–436. https://doi.org/10.1016/j.jbspin.2012.10.018
Rouil A, Pollet S, Martin A, Tattevin P, Perdriger A (2013) Severe Pasteurella multocida infection in a patient on rituximab therapy for rheumatoid arthritis. Joint Bone Spine 80(2):224–225. https://doi.org/10.1016/j.jbspin.2012.07.011
Santos VA, Tobon GJ, Canas CA (2018) Development of bronchiectasis during long-term rituximab treatment for rheumatoid arthritis. Adv Respir Med. https://doi.org/10.5603/ARM.a2018.0050
Clifford DB, Ances B, Costello C, Rosen-Schmidt S, Andersson M, Parks D, Perry A, Yerra R, Schmidt R, Alvarez E, Tyler KL (2011) Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol 68(9):1156–1164. https://doi.org/10.1001/archneurol.2011.103
Abdulkader R, Dharmapalaiah C, Rose G, Shand LM, Clunie GP, Watts RA (2014) Late-onset neutropenia in patients with rheumatoid arthritis after treatment with rituximab. J Rheumatol 41(5):858–861. https://doi.org/10.3899/jrheum.130526
Pyrpasopoulou A, Douma S, Vassiliadis T, Chatzimichailidou S, Triantafyllou A, Aslanidis S (2011) Reactivation of chronic hepatitis B virus infection following rituximab administration for rheumatoid arthritis. Rheumatol Int 31(3):403–404. https://doi.org/10.1007/s00296-009-1202-2
Varisco V, Vigano M, Batticciotto A, Lampertico P, Marchesoni A, Gibertini P, Pellerito R, Rovera G, Caporali R, Todoerti M, Covelli M, Notarnicola A, Atzeni F, Sarzi-Puttini P (2016) Low risk of hepatitis B virus reactivation in HBsAg-negative/anti-HBc-positive carriers receiving rituximab for rheumatoid arthritis: a retrospective multicenter Italian study. J Rheumatol 43(5):869–874. https://doi.org/10.3899/jrheum.151105
Clavel G, Moulignier A, Semerano L (2017) Progressive multifocal leukoencephalopathy and rheumatoid arthritis treatments. Joint Bone Spine 84(6):671–675. https://doi.org/10.1016/j.jbspin.2017.03.002
Burr ML, Malaviya AP, Gaston JH, Carmichael AJ, Ostor AJ (2008) Rituximab in rheumatoid arthritis following anti-TNF-associated tuberculosis. Rheumatology (Oxford) 47(5):738–739. https://doi.org/10.1093/rheumatology/ken113
Jung N, Owczarczyk K, Hellmann M, Lehmann C, Fatkenheuer G, Hallek M, Rubbert A (2008) Efficacy and safety of rituximab in a patient with active rheumatoid arthritis and chronic disseminated pulmonary aspergillosis and history of tuberculosis. Rheumatology (Oxford) 47(6):932–933. https://doi.org/10.1093/rheumatology/ken143
Pehlivan Y, Kisacik B, Bosnak VK, Onat AM (2013) Rituximab seems to be a safer alternative in patients with active rheumatoid arthritis with tuberculosis. BMJ Case Rep 2013:bcr2012006585. https://doi.org/10.1136/bcr-2012-006585
Magnusson M, Brisslert M, Zendjanchi K, Lindh M, Bokarewa MI (2010) Epstein-Barr virus in bone marrow of rheumatoid arthritis patients predicts response to rituximab treatment. Rheumatology (Oxford) 49(10):1911–1919. https://doi.org/10.1093/rheumatology/keq159
Gelinck LB, Teng YK, Rimmelzwaan GF, van den Bemt BJ, Kroon FP, van Laar JM (2007) Poor serological responses upon influenza vaccination in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis 66(10):1402–1403. https://doi.org/10.1136/ard.2007.071878
Thomas L, Canoui-Poitrine F, Gottenberg JE, Economu-Dubosc A, Medkour F, Chevalier X, Bastuji-Garin S, Le Louet H, Farrenq V, Claudepierre P (2012) Incidence of new-onset and flare of preexisting psoriasis during rituximab therapy for rheumatoid arthritis: data from the French AIR registry. J Rheumatol 39(5):893–898. https://doi.org/10.3899/jrheum.111347
Vasconcellos JB, Pereira DD, Vargas TJ, Levy RA, Pinheiro GD, Cursi IB (2016) Paradoxical psoriasis after the use of anti-TNF in a patient with rheumatoid arthritis. An Bras Dermatol 91(5 suppl 1):137–139. https://doi.org/10.1590/abd1806-4841.20164456
Fui A, Bergantini L, Selvi E, Mazzei MA, Bennett D, Pieroni MG, Rottoli P, Bargagli E (2019) Rituximab therapy in interstitial lung disease associated with rheumatoid arthritis. Intern Med J. https://doi.org/10.1111/imj.14306
Md Yusof MY, Kabia A, Darby M, Lettieri G, Beirne P, Vital EM, Dass S, Emery P (2017) Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single Centre. Rheumatology (Oxford) 56(8):1348–1357. https://doi.org/10.1093/rheumatology/kex072
Paul F, Cartron G (2019) Infusion-related reactions to rituximab: frequency, mechanisms and predictors. Expert Rev Clin Immunol 15(4):383–389. https://doi.org/10.1080/1744666x.2019.1562905
Salmon JH, Perotin JM, Morel J, Drame M, Cantagrel A, Ziegler LE, Ravaud P, Sibilia J, Pane I, Mariette X, Gottenberg JE (2018) Serious infusion-related reaction after rituximab, abatacept and tocilizumab in rheumatoid arthritis: prospective registry data. Rheumatology (Oxford) 57(1):134–139. https://doi.org/10.1093/rheumatology/kex403
Wheater G, Elshahaly M, Naraghi K, Tuck SP, Datta HK, van Laar JM (2018) Changes in bone density and bone turnover in patients with rheumatoid arthritis treated with rituximab, results from an exploratory, prospective study. PLoS One 13(8):e0201527. https://doi.org/10.1371/journal.pone.0201527
Roberts DM, Jones RB, Smith RM, Alberici F, Kumaratne DS, Burns S, Jayne DR (2015) Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun 57:60–65. https://doi.org/10.1016/j.jaut.2014.11.009
Wijetilleka S, Mukhtyar C, Jayne D, Ala A, Bright P, Chinoy H, Harper L, Kazmi M, Kiani-Alikhan S, Li C, Misbah S, Oni L, Price-Kuehne F, Salama A, Workman S, Wrench D, Karim MY (2019) Immunoglobulin replacement for secondary immunodeficiency after B-cell targeted therapies in autoimmune rheumatic disease: systematic literature review. Autoimmun Rev 18(5):535–541. https://doi.org/10.1016/j.autrev.2019.03.010
Lee S, Kim W, Seo W (2017) Safe use of rituximab in an elderly patient with rheumatoid arthritis and severe heart failure: a case report. J Clin Rheumatol:1. https://doi.org/10.1097/rhu.0000000000000648
Sautner J, Rintelen B, Leeb BF (2013) Rituximab as effective treatment in a case of severe subcutaneous nodulosis in rheumatoid arthritis. Rheumatology (Oxford) 52(8):1535–1537. https://doi.org/10.1093/rheumatology/kes406
Godot S, Gottenberg JE, Paternotte S, Pane I, Combe B, Sibilia J, Flipo RM, Schaeverbeke T, Ravaud P, Toussirot E, Berenbaum F, Mariette X, Wendling D, Sellam J (2013) Safety of surgery after rituximab therapy in 133 patients with rheumatoid arthritis: data from the autoimmunity and rituximab registry. Arthritis Care Res (Hoboken) 65(11):1874–1879. https://doi.org/10.1002/acr.22056
Slimani S, Lukas C, Combe B, Morel J (2011) Rituximab in rheumatoid arthritis and the risk of malignancies: report from a French cohort. Joint Bone Spine 78(5):484–487. https://doi.org/10.1016/j.jbspin.2010.11.012
Silva-Fernandez L, Lunt M, Kearsley-Fleet L, Watson KD, Dixon WG, Symmons DP, Hyrich KL (2016) The incidence of cancer in patients with rheumatoid arthritis and a prior malignancy who receive TNF inhibitors or rituximab: results from the British Society for Rheumatology Biologics Register-rheumatoid arthritis. Rheumatology (Oxford) 55(11):2033–2039. https://doi.org/10.1093/rheumatology/kew314
Sokolovic S, Kasumagic S, Mackic-Durovic M, Aganovic-Musinovic I (2010) The impact of rituximab therapy on the chromosomes of patients with rheumatoid arthritis. Bosn J Basic Med Sci 10(2):121–124. https://doi.org/10.17305/bjbms.2010.2706
Diaz JC, Vallejo S, Canas CA (2012) Drug-induced lupus in anti-TNF-alpha therapy and its treatment with rituximab. Rheumatol Int 32(10):3315–3317. https://doi.org/10.1007/s00296-011-2137-y
Oldroyd AGS, Symmons DPM, Sergeant JC, Kearsley-Fleet L, Watson K, Lunt M, Hyrich KL (2018) Long-term persistence with rituximab in patients with rheumatoid arthritis. Rheumatology (Oxford) 57(6):1089–1096. https://doi.org/10.1093/rheumatology/key036
Tavakolpour S (2017) Towards personalized medicine for patients with autoimmune diseases: opportunities and challenges. Immunol Lett 190:130–138. https://doi.org/10.1016/j.imlet.2017.08.002
Kłak A, Paradowska-Gorycka A, Kwiatkowska B, Raciborski F (2016) Personalized medicine in rheumatology. Reumatologia 54(4):177–186. https://doi.org/10.5114/reum.2016.62472
Tavakolpour S, Darvishi M, Ghasemiadl M (2018) Pharmacogenetics: a strategy for personalized medicine for autoimmune diseases. Clin Genet 93(3):481–497. https://doi.org/10.1111/cge.13186
Stradner MH, Dejaco C, Brickmann K, Graninger WB, Brezinschek HP (2016) A combination of cellular biomarkers predicts failure to respond to rituximab in rheumatoid arthritis: a 24-week observational study. Arthritis Res Ther 18:190. https://doi.org/10.1186/s13075-016-1091-1
Piantoni S, Scarsi M, Tincani A, Airo P (2015) Circulating CD4+ T-cell number decreases in rheumatoid patients with clinical response to rituximab. Rheumatol Int 35(9):1571–1573. https://doi.org/10.1007/s00296-015-3295-0
Lurati A, Marrazza MG, Re KA, Scarpellini M (2009) Relationship between NK cell activation and clinical response in rheumatoid arthritis treated with rituximab. Int J Biomed Sci 5(2):92–95
Lurati A, Bertani L, Marrazza M, Re KA, Bompane D, Scarpellini M (2012) NK cell count as predictor of clinical response in patients with rheumatoid arthritis treated with rituximab. Biologics 6:83–87. https://doi.org/10.2147/btt.s29079
Parietti V, Chifflot H, Sibilia J, Muller S, Monneaux F (2010) Rituximab treatment overcomes reduction of regulatory iNKT cells in patients with rheumatoid arthritis. Clin Immunol 134(3):331–339. https://doi.org/10.1016/j.clim.2009.11.007
Sellam J, Hendel-Chavez H, Rouanet S, Abbed K, Combe B, Le Loet X, Tebib J, Sibilia J, Taoufik Y, Dougados M, Mariette X (2011) B cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: a six-month, national, multicenter, open-label study. Arthritis Rheum 63(4):933–938. https://doi.org/10.1002/art.30233
Sellam J, Riviere E, Courties A, Rouzaire PO, Tolusso B, Vital EM, Emery P, Ferraciolli G, Soubrier M, Ly B, Hendel Chavez H, Taoufik Y, Dougados M, Mariette X (2016) Serum IL-33, a new marker predicting response to rituximab in rheumatoid arthritis. Arthritis Res Ther 18(1):294. https://doi.org/10.1186/s13075-016-1190-z
Couderc M, Mathieu S, Pereira B, Glace B, Soubrier M (2013) Predictive factors of rituximab response in rheumatoid arthritis: results from a French university hospital. Arthritis Care Res (Hoboken) 65(4):648–652. https://doi.org/10.1002/acr.21865
Pyrpasopoulou A, Douma S, Triantafyllou A, Simoulidou E, Samara M, Parapanisiou E, Aslanidis S (2010) Response to rituximab and timeframe to relapse in rheumatoid arthritis patients: association with B-cell markers. Mol Diagn Ther 14(1):43–48. https://doi.org/10.2165/11530570-000000000-00000
Khan A, Scott DL, Batley M (2012) Smoking, rheumatoid factor status and responses to rituximab. Ann Rheum Dis 71(9):1587–1588. https://doi.org/10.1136/annrheumdis-2012-201758
Gardette A, Ottaviani S, Tubach F, Roy C, Nicaise-Roland P, Palazzo E, Gill G, Meyer O, Dieude P (2014) High anti-CCP antibody titres predict good response to rituximab in patients with active rheumatoid arthritis. Joint Bone Spine 81(5):416–420. https://doi.org/10.1016/j.jbspin.2014.06.001
Jimenez Morales A, Maldonado-Montoro M, Martinez de la Plata JE, Perez Ramirez C, Daddaoua A, Alarcon Payer C, Exposito Ruiz M, Garcia Collado C (2019) FCGR2A/FCGR3A gene polymorphisms and clinical variables as predictors of response to tocilizumab and rituximab in patients with rheumatoid arthritis. J Clin Pharmacol 59(4):517–531. https://doi.org/10.1002/jcph.1341
Fabris M, Quartuccio L, Lombardi S, Benucci M, Manfredi M, Saracco M, Atzeni F, Morassi P, Cimmino MA, Pontarini E, Fabro C, Pellerito R, Sarzi-Puttini P, Cutolo M, Carletto A, Bambara LM, Fischetti F, Curcio F, Tonutti E, De Vita S (2010) Study on the possible role of the -174G>C IL-6 promoter polymorphism in predicting response to rituximab in rheumatoid arthritis. Reumatismo 62(4):253–258
Daien CI, Fabre S, Rittore C, Soler S, Daien V, Tejedor G, Cadart D, Molinari N, Daures JP, Jorgensen C, Touitou I (2012) TGF beta1 polymorphisms are candidate predictors of the clinical response to rituximab in rheumatoid arthritis. Joint Bone Spine 79(5):471–475. https://doi.org/10.1016/j.jbspin.2011.10.007
Ruyssen-Witrand A, Rouanet S, Combe B, Dougados M, Le Loet X, Sibilia J, Tebib J, Mariette X, Constantin A (2013) Association between -871C>T promoter polymorphism in the B-cell activating factor gene and the response to rituximab in rheumatoid arthritis patients. Rheumatology (Oxford) 52(4):636–641. https://doi.org/10.1093/rheumatology/kes344
Juge PA, Gazal S, Constantin A, Mariette X, Combe B, Tebib J, Dougados M, Sibilia J, Le Loet X, Dieude P (2017) Variants of genes implicated in type 1 interferon pathway and B-cell activation modulate the EULAR response to rituximab at 24 weeks in rheumatoid arthritis. RMD Open 3(2):e000448. https://doi.org/10.1136/rmdopen-2017-000448
Ruyssen-Witrand A, Rouanet S, Combe B, Dougados M, Le Loet X, Sibilia J, Tebib J, Mariette X, Constantin A (2012) Fcgamma receptor type IIIA polymorphism influences treatment outcomes in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis 71(6):875–877. https://doi.org/10.1136/annrheumdis-2011-200337
Sarsour K, Greenberg J, Johnston JA, Nelson DR, O'Brien LA, Oddoux C, Ostrer H, Pearlman A, Reed G (2013) The role of the FcGRIIIa polymorphism in modifying the association between treatment and outcome in patients with rheumatoid arthritis treated with rituximab versus TNF-alpha antagonist therapies. Clin Exp Rheumatol 31(2):189–194
Quartuccio L, Fabris M, Pontarini E, Salvin S, Zabotti A, Benucci M, Manfredi M, Biasi D, Ravagnani V, Atzeni F, Sarzi-Puttini P, Morassi P, Fischetti F, Tomietto P, Bazzichi L, Saracco M, Pellerito R, Cimmino M, Schiavon F, Carraro V, Semeraro A, Caporali R, Cavagna L, Bortolotti R, Paolazzi G, Govoni M, Bombardieri S, De Vita S (2014) The 158VV Fcgamma receptor 3A genotype is associated with response to rituximab in rheumatoid arthritis: results of an Italian multicentre study. Ann Rheum Dis 73(4):716–721. https://doi.org/10.1136/annrheumdis-2012-202435
Pal I, Szamosi S, Hodosi K, Szekanecz Z, Varoczy L (2017) Effect of Fcgamma-receptor 3a (FCGR3A) gene polymorphisms on rituximab therapy in Hungarian patients with rheumatoid arthritis. RMD Open 3(2):e000485. https://doi.org/10.1136/rmdopen-2017-000485
Raterman HG, Vosslamber S, de Ridder S, Nurmohamed MT, Lems WF, Boers M, van de Wiel M, Dijkmans BA, Verweij CL, Voskuyl AE (2012) The interferon type I signature towards prediction of non-response to rituximab in rheumatoid arthritis patients. Arthritis Res Ther 14(2):R95. https://doi.org/10.1186/ar3819
de Jong TD, Sellam J, Agca R, Vosslamber S, Witte BI, Tsang ASM, Mantel E, Bijlsma JW, Voskuyl AE, Nurmohamed MT, Verweij CL, Mariette X (2017) A multi-parameter response prediction model for rituximab in rheumatoid arthritis. Joint Bone Spine 85:219–226. https://doi.org/10.1016/j.jbspin.2017.02.015
Julia A, Barcelo M, Erra A, Palacio C, Marsal S (2009) Identification of candidate genes for rituximab response in rheumatoid arthritis patients by microarray expression profiling in blood cells. Pharmacogenomics 10(10):1697–1708. https://doi.org/10.2217/pgs.09.99
Duroux-Richard I, Pers YM, Fabre S, Ammari M, Baeten D, Cartron G, Touitou I, Jorgensen C, Apparailly F (2014) Circulating miRNA-125b is a potential biomarker predicting response to rituximab in rheumatoid arthritis. Mediat Inflamm 2014:342524–342529. https://doi.org/10.1155/2014/342524
Tchetina EV, Pivanova AN, Markova GA, Lukina GV, Aleksandrova EN, Aleksankin AP, Makarov SA, Kuzin AN (2016) Rituximab downregulates gene expression associated with cell proliferation, survival, and proteolysis in the peripheral blood from rheumatoid arthritis patients: a link between high baseline autophagy-related ULK1 expression and improved pain control. Arthritis 2016:4963950–4963912. https://doi.org/10.1155/2016/4963950
Valleala H, Kauppi MJ, Kouri VP, Korpela M (2015) Epstein-Barr virus in peripheral blood is associated with response to rituximab therapy in rheumatoid arthritis patients. Clin Rheumatol 34(8):1485–1488. https://doi.org/10.1007/s10067-015-2992-0
Chatzidionysiou K, Lukina G, Gabay C, Hetland ML, Hauge EM, Pavelka K, Nordstrom D, Canhao H, Tomsic M, Rotar Z, Lie E, Kvien TK, van Vollenhoven RF, Saevarsdottir S (2019) Smoking and response to rituximab in rheumatoid arthritis: results from an international European collaboration. Scand J Rheumatol 48(1):17–23. https://doi.org/10.1080/03009742.2018.1466363
Dass S, Rawstron AC, Vital EM, Henshaw K, McGonagle D, Emery P (2008) Highly sensitive B cell analysis predicts response to rituximab therapy in rheumatoid arthritis. Arthritis Rheum 58(10):2993–2999. https://doi.org/10.1002/art.23902
Breedveld F, Agarwal S, Yin M, Ren S, Li NF, Shaw TM, Davies BE (2007) Rituximab pharmacokinetics in patients with rheumatoid arthritis: B-cell levels do not correlate with clinical response. J Clin Pharmacol 47(9):1119–1128. https://doi.org/10.1177/0091270007305297
Ramwadhdoebe TH, van Baarsen LGM, Boumans MJH, Bruijnen STG, Safy M, Berger FH, Semmelink JF, van der Laken CJ, Gerlag DM, Thurlings RM, Tak PP (2019) Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis. Rheumatology (Oxford) 58(6):1075–1085. https://doi.org/10.1093/rheumatology/key428
Lioger B, Edupuganti SR, Mulleman D, Passot C, Desvignes C, Bejan-Angoulvant T, Thibault G, Gouilleux-Gruart V, Melet J, Paintaud G, Ternant D (2017) Antigenic burden and serum IgG concentrations influence rituximab pharmacokinetics in rheumatoid arthritis patients. Br J Clin Pharmacol 83(8):1773–1781. https://doi.org/10.1111/bcp.13270
Sellam J, Marion-Thore S, Dumont F, Jacques S, Garchon HJ, Rouanet S, Taoufik Y, Hendel-Chavez H, Sibilia J, Tebib J, Le Loet X, Combe B, Dougados M, Mariette X, Chiocchia G (2014) Use of whole-blood transcriptomic profiling to highlight several pathophysiologic pathways associated with response to rituximab in patients with rheumatoid arthritis: data from a randomized, controlled, open-label trial. Arthritis Rheumatol 66(8):2015–2025. https://doi.org/10.1002/art.38671
Tony HP, Roll P, Mei HE, Blumner E, Straka A, Gnuegge L, Dorner T (2015) Combination of B cell biomarkers as independent predictors of response in patients with rheumatoid arthritis treated with rituximab. Clin Exp Rheumatol 33(6):887–894
Brezinschek HP, Rainer F, Brickmann K, Graninger WB (2012) B lymphocyte-typing for prediction of clinical response to rituximab. Arthritis Res Ther 14(4):R161. https://doi.org/10.1186/ar3901
Fabris M, De Vita S, Blasone N, Visentini D, Pezzarini E, Pontarini E, Fabro C, Quartuccio L, Mazzolini S, Curcio F, Tonutti E (2010) Serum levels of anti-CCP antibodies, anti-MCV antibodies and RF IgA in the follow-up of patients with rheumatoid arthritis treated with rituximab. Auto Immun Highlights 1(2):87–94. https://doi.org/10.1007/s13317-010-0013-5
Roodenrijs NMT, de Hair MJH, Wheater G, Elshahaly M, Tekstra J, Teng YKO, Lafeber F, Hwang CC, Liu X, Sasso EH, van Laar JM (2018) The multi-biomarker disease activity score tracks response to rituximab treatment in rheumatoid arthritis patients: a post hoc analysis of three cohort studies. Arthritis Res Ther 20(1):256. https://doi.org/10.1186/s13075-018-1750-5
Sellam J, Rouanet S, Hendel-Chavez H, Abbed K, Sibilia J, Tebib J, Le Loet X, Combe B, Dougados M, Mariette X, Taoufik Y (2011) Blood memory B cells are disturbed and predict the response to rituximab in patients with rheumatoid arthritis. Arthritis Rheum 63(12):3692–3701. https://doi.org/10.1002/art.30599
M'Barek RB, Dupre T, Tubach F, Dieude P, Palazzo E, Hayem G, Dawidowicz K, Ottaviani S, Alfaiate T, Lecon-Malais V, Boutten A, Meyer O (2014) 25-hydroxyvitamin D status does not affect the clinical rituximab response in rheumatoid arthritis. Joint Bone Spine 81(1):96–97. https://doi.org/10.1016/j.jbspin.2013.04.017
Riviere E, Sellam J, Pascaud J, Ravaud P, Gottenberg JE, Mariette X (2018) Serum IL-33 level is associated with auto-antibodies but not with clinical response to biologic agents in rheumatoid arthritis. Arthritis Res Ther 20(1):122. https://doi.org/10.1186/s13075-018-1628-6
Nakayamada S, Tanaka Y (2016) BAFF- and APRIL-targeted therapy in systemic autoimmune diseases. Inflammation and Regeneration 36(1):6. https://doi.org/10.1186/s41232-016-0015-4
Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A (2013) A critical review of the role of fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol 6:1–1. https://doi.org/10.1186/1756-8722-6-1
Canestri S, Totaro MC, Serone E, Tolusso B, Frezza D, Gremese E, Ferraccioli G (2012) Association between the response to B cell depletion therapy and the allele*2 of the HS1,2A enhancer in seropositive rheumatoid arthritis patients. Reumatismo 64(6):368–373. https://doi.org/10.4081/reumatismo.2012.368
Viecceli D, Garcia MP, Schneider L, Alegretti AP, Silva CK, Ribeiro AL, Brenol CV, Xavier RM (2017) Correlation between cellular expression of complement regulatory proteins with depletion and repopulation of B-lymphocytes in peripheral blood of patients with rheumatoid arthritis treated with rituximab. Rev Bras Reumatol Engl Ed 57(5):385–391. https://doi.org/10.1016/j.rbre.2016.09.007
Henry C, Deschamps M, Rohrlich PS, Pallandre JR, Remy-Martin JP, Callanan M, Traverse-Glehen A, GrandClement C, Garnache-Ottou F, Gressin R, Deconinck E, Salles G, Robinet E, Tiberghien P, Borg C, Ferrand C (2010) Identification of an alternative CD20 transcript variant in B-cell malignancies coding for a novel protein associated to rituximab resistance. Blood 115(12):2420–2429. https://doi.org/10.1182/blood-2009-06-229112
Gamonet C, Deschamps M, Marion S, Herbein G, Chiocchia G, Auger I, Saas P, Ferrand C, Toussirot E (2015) The alternative CD20 transcript variant is not a surrogate marker for resistance to rituximab in patients with rheumatoid arthritis. Rheumatology (Oxford) 54(9):1744–1745. https://doi.org/10.1093/rheumatology/kev210
Schroder C, Azimzadeh AM, Wu G, Price JO, Atkinson JB, Pierson RN (2003) Anti-CD20 treatment depletes B-cells in blood and lymphatic tissue of cynomolgus monkeys. Transpl Immunol 12(1):19–28. https://doi.org/10.1016/s0966-3274(03)00059-5
Kane SV, Acquah LA (2009) Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol 104(1):228–233. https://doi.org/10.1038/ajg.2008.71
Chakravarty EF, Murray ER, Kelman A, Farmer P (2011) Pregnancy outcomes after maternal exposure to rituximab. Blood 117(5):1499–1506. https://doi.org/10.1182/blood-2010-07-295444
Gualtierotti R, Ingegnoli F, Meroni PL (2013) Pre-conceptional exposure to rituximab: comment on the article by Ojeda-Uribe et al. Clin Rheumatol 32(5):727–728. https://doi.org/10.1007/s10067-013-2241-3
Gotestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, da Silva J, Nelson-Piercy C, Cetin I, Costedoat-Chalumeau N, Dolhain R, Forger F, Khamashta M, Ruiz-Irastorza G, Zink A, Vencovsky J, Cutolo M, Caeyers N, Zumbuhl C, Ostensen M (2016) The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 75(5):795–810. https://doi.org/10.1136/annrheumdis-2015-208840
Flint J, Panchal S, Hurrell A, van de Venne M, Gayed M, Schreiber K, Arthanari S, Cunningham J, Flanders L, Moore L, Crossley A, Purushotham N, Desai A, Piper M, Nisar M, Khamashta M, Williams D, Gordon C, Giles I (2016) BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford) 55(9):1693–1697. https://doi.org/10.1093/rheumatology/kev404
Pham T, Fautrel B, Gottenberg JE, Goupille P, Hachulla E, Masson C, Morel J, Mouthon L, Saraux A, Schaeverbeke T, Wendling D, Mariette X, Sibilia (2008) Rituximab (MabThera) therapy and safety management. Clinical tool guide. Joint Bone Spine 75 Suppl 1:S1–99. https://doi.org/10.1016/s1297-319x(08)73620-0
De Cock D, Birmingham L, Watson KD, Kearsley-Fleet L, Symmons DP, Hyrich KL (2017) Pregnancy outcomes in women with rheumatoid arthritis ever treated with rituximab. Rheumatology (Oxford) 56(4):661–663. https://doi.org/10.1093/rheumatology/kew493
Ojeda-Uribe M, Afif N, Dahan E, Sparsa L, Haby C, Sibilia J, Ternant D, Ardizzone M (2013) Exposure to abatacept or rituximab in the first trimester of pregnancy in three women with autoimmune diseases. Clin Rheumatol 32(5):695–700. https://doi.org/10.1007/s10067-012-2156-4
Bae SC, Lee YH (2019) Comparative efficacy and safety of biosimilar rituximab and originator rituximab in combination with methotrexate in patients with active rheumatoid arthritis: a Bayesian network meta-analysis. Int J Clin Pharmacol Ther 57(4):188–196. https://doi.org/10.5414/cp203360
Tony HP, Kruger K, Cohen SB, Schulze-Koops H, Kivitz AJ, Jeka S, Vereckei E, Cen L, Kring L, Kollins D (2019) Brief report: safety and immunogenicity of rituximab biosimilar GP 2013 after switch from reference rituximab in patients with active rheumatoid arthritis. Arthritis Care Res (Hoboken) 71(1):88–94. https://doi.org/10.1002/acr.23771
Park W, Bozic-Majstorovic L, Milakovic D, Berrocal Kasay A, El-Khouri EC, Irazoque-Palazuelos F, Molina FFC, Shesternya P, Miranda P, Medina-Rodriguez FG, Wiland P, Jeka S, Chavez-Corrales J, Garmish O, Linde T, Rekalov D, Hrycaj P, Krause A, Fomina N, Piura O, Abello-Banfi M, Suh CH, Shim SC, Lee SJ, Lee SY, Kim SH, Yoo DH (2018) Comparison of biosimilar CT-P10 and innovator rituximab in patients with rheumatoid arthritis: a randomized controlled phase 3 trial. MAbs 10(6):934–943. https://doi.org/10.1080/19420862.2018.1487912
Suh CH, Yoo DH, Berrocal Kasay A, Chalouhi El-Khouri E, Cons Molina FF, Shesternya P, Miranda P, Medina-Rodriguez FG, Wiland P, Jeka S, Chavez-Corrales J, Linde T, Hrycaj P, Abello-Banfi M, Hospodarskyy I, Jaworski J, Piotrowski M, Brzosko M, Krogulec M, Shevchuk S, Calvo A, Andersone D, Park W, Shim SC, Lee SJ, Lee SY (2019) Long-term efficacy and safety of biosimilar CT-P10 versus innovator rituximab in rheumatoid arthritis: 48-week results from a randomized phase III trial. BioDrugs 33(1):79–91. https://doi.org/10.1007/s40259-018-00331-4
Shim SC, Bozic-Majstorovic L, Berrocal Kasay A, El-Khouri EC, Irazoque-Palazuelos F, Cons Molina FF, Medina-Rodriguez FG, Miranda P, Shesternya P, Chavez-Corrales J, Wiland P, Jeka S, Garmish O, Hrycaj P, Fomina N, Park W, Suh CH, Lee SJ, Lee SY, Bae YJ, Yoo DH (2019) Efficacy and safety of switching from rituximab to biosimilar CT-P10 in rheumatoid arthritis: 72-week data from a randomized phase 3 trial. Rheumatology (Oxford). https://doi.org/10.1093/rheumatology/kez152
Cohen SB, Burgos-Vargas R, Emery P, Jin B, Cronenberger C, Vazquez-Abad MD (2018) Extension study of PF-05280586, a potential rituximab biosimilar, versus rituximab in subjects with active rheumatoid arthritis. Arthritis Care Res (Hoboken) 70(11):1598–1606. https://doi.org/10.1002/acr.23586
Author information
Authors and Affiliations
Contributions
Soheil Tavakolpour and Samira Alesaeidi contributed substantially to the study conception and design; Mojtaba Ghasemiadl, Sahar Darabi Monadi, Somayeh Elkaei Behjati, and Meisam Akhlaghdoust contributed to the literature review, acquisition and analysis of data; Soheil Tavakolpour drafted the manuscript; Samira Alesaeidi, Mohammad Darvishi, and Arash Jafarieh contributed to the revision of the manuscript. All authors approved the final version and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tavakolpour, S., Alesaeidi, S., Darvishi, M. et al. A comprehensive review of rituximab therapy in rheumatoid arthritis patients. Clin Rheumatol 38, 2977–2994 (2019). https://doi.org/10.1007/s10067-019-04699-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04699-8