Abstract
Objectives
To evaluate the association between inflammatory back pain (IBP) features, acute and structural MRI findings suggestive of sacroiliitis, and diagnosis of spondyloarthritis (SpA).
Methods
Data from 224 patients who underwent MRI for suspected sacroiliitis (2005–2015) was retrospectively reviewed by an expert rheumatologist for the presence of IBP features and for clinical standard of reference diagnosis. A telephone questionnaire was performed in cases of missing data. Acute and structural MRI parameters were scored by an experienced radiologist for the presence of sacroiliitis using the Assessment of Spondyloarthritis International Society (ASAS) criteria, Berlin score, and observer’s global impression (GI) scores. Association between IBP features and MRI scores, and odds ratio for SpA diagnosis, were calculated.
Results
One hundred ninety-three subjects were included (119 F:74 M, mean age 39.7 ± 15.6, mean follow-up 49 ± 18 months). Fifty-two (26.9%) subjects were diagnosed with SpA. IBP scores were significantly higher in SpA patients (p < 0.001). IBP, ASAS, and GI MRI scores were significantly associated with the SpA diagnosis (p < 0.001 for all). The presence of night pain and morning stiffness was significantly associated with sacroiliac-joints’ bone marrow edema (BME, p < 0.05). Sensitivity for diagnosis of SpA was high for IBP (96%) and low for the MRI parameters (26.9–57.4%), and specificity was low for IBP (32%) and high for the MRI parameters (88.3–94.3%).
Conclusions
The presence of IBP features is highly associated with diagnosis of SpA and correlates with MRI BME, all probably reflect inflammation. The combination of IBP and MRI should be the cornerstone in the clinician’s final diagnosis of SpA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Axial spondyloarthropathy (axSpA) is a group of chronic inflammatory rheumatic diseases; the hallmark of which is inflammatory back pain (IBP) [1]. IBP is characterized by pain that begins insidiously in a person younger than 40, continues for more than 3 months, is associated with morning stiffness and night pain, and improves with physical activity [2]. These IBP features are included in different diagnostic criteria sets, used both in research and in clinical practice [3, 4]. Typically, the initial manifestation of axSpA on imaging is sacroiliitis, which may be evident on plain radiographs. The presence of sacroiliitis on plain radiographs is the basis of the modified New York criteria of 1984 for the diagnosis of ankylosing spondylitis (AS). The presence of sacroiliitis on radiographs is also part of the diagnostic criteria for SpA according to European Spondyloarthropathy Study Group (ESSG) criteria and the Amor criteria [5]. While these criteria have been used by rheumatologists in everyday practice for diagnosing SpA, they are not optimal mainly due to a delay of years in diagnosing sacroiliitis on radiographs [6]. MRI has been shown to be more sensitive in detecting sacroiliitis than radiographs [7]. Indeed, osteitis, seen as periarticular bone marrow edema (BME), is evident on MRI several years prior to the development of structural changes visible on radiograph, thus facilitating early diagnosis and treatment [7]. To this end, ASAS (Assessment of Spondyloarthritis International Society) introduced the new axSpA classification criteria in 2009, whereby MRI of the sacroiliac joints was incorporated alongside radiological examination. MRI findings suggestive of SpA can be divided into two groups: acute inflammatory and chronic, structural lesions. Acute lesions include BME, enthesitis, and capsulitis, while structural lesions include subchondral sclerosis, erosions, fat metaplasia, and ankylosis [8].
The pathogenesis underlying IBP is not fully understood; however, it is presumed that acute inflammation, as evident in and around the sacroiliac joints (SIJs) on MRI, contributes to this central clinical manifestation of SpA. Albeit, the association between acute and structural lesions on MRI of the SIJs with IBP has not been fully investigated. Herein, we aimed to retrospectively evaluate the association between IBP features and acute and structural lesions of sacroiliitis detected on MRI. We evaluate the association between the different imaging and IBP scores and the standard of reference of clinical diagnosis given by a rheumatologist, in order to encounter the best predictors for SpA among the different clinical and imaging features.
Materials and methods
Ethical considerations
The study was conducted according to the guidelines of the Declaration of Helsinki and approval of the institute’s review board (9192-11-SMC), allowing for the retrospective retrieval of data, analysis of MRI examinations, and performance of telephone interviews. Due to the retrospective nature of this study, informed consent was waived.
Patients
Inclusion criteria
-
A.
Subjects who were referred for and performed MRI examination of the sacroiliac joints for suspected sacroiliitis in our institution during the years 2005 to 2015
-
B.
Subjects who were whether attainable by telephone, or had a close and updated follow-up in our institution at the time of data retrieving
Exclusion criteria
Subjects who could not be contacted and for whom data regarding symptoms was incomplete were excluded.
MRI examinations and clinical data were retrospectively retrieved from the hospital’s computerized archives. A telephone interview was conducted in order to verify data and to obtain missing or incomplete information when needed.
Clinical parameters
IBP parameters included pain duration of more than 3 months, the presence of pain during the second half of the night, morning stiffness, improvement of pain with exercise, and symptom onset before the age of 40. IBP features were given a binary score (present = 1, absent = 0) and were summed up to a final IBP score of 0–5. Demographic data such as gender, age, and follow-up time were registered.
Patients were classified into different diagnostic groups: SpA, other autoimmune diseases, mechanical back pain, malignancy, infection, and fibromyalgia. Classification was performed by an expert rheumatologist with 6 years of experience, based on integrated information from medical files and patients’ interviews. This classification was used as the standard of reference for diagnosis in this study.
MRI examinations and evaluation
MRI examinations were carried out on a 1.5 T unit (Signa HDX, GE Healthcare, Milwaukee, USA) or a 3.0 T unit (Inginia, Philips Healthcare, Amsterdam, The Netherlands) with a phased-array 8/16-element coil. The examination was performed in the semicoronal orientation (along the long axis of the sacrum and perpendicular to the S2 vertebral body) in T1-W (TR = 534, TE = 8.9) and T2-W with fat saturation (TR = 6079, TE = 80).
All MRI examinations were retrospectively evaluated by a musculoskeletal radiologist with 16 years of experience in SIJ MRI reading. MRI examinations were scored according to the Berlin scoring system [9]. Briefly, parameters including BME, subchondral sclerosis, erosions, fat metaplasia, and ankylosis were analyzed in each quarter (upper ilium, lower ilium, upper sacrum, lower sacrum). All MRI examinations were evaluated using the ASAS criteria for a positive MRI, with an active sacroiliac inflammation defined as either one BME lesion on two consecutive slices or two BME lesions on the same slice, with a pattern suggestive of axSpA [10]. Minimal, questionable BME lesions not suggestive of SpA were not considered positive using these criteria. A global impression (GI) for the presence of definite sacroiliitis, possible/indecisive sacroiliitis, and no sacroiliitis on MRI was given using information from the entire MRI study regarding both active and structural lesions.
Statistics
The correlation between the Berlin score and the standard of reference diagnosis was tested using binomial logistic hierarchal regression. The first step of the regression used only the BME score, and the second step used the rest of the Berlin score (e.g., the structural lesion). The five components of the IBP score were separately correlated to the active and structural aspects of the Berlin score using point biserial correlation. In order to compare the accuracy of the Berlin score with the GI score, a one-way ANOVA test was used.
In order to determine the correlations of the MRI parameter of the ASAS score with the two main parts of the Berlin score (BME and the rest of the Berlin score), a point biserial correlation was calculated.
Comparison was made in order to detect the best predictor for axSpA. Since several of the predictors (ASAS, Berlin, GI) are based on the same imaging information, and since these predictors may thus be highly correlated, multicollinearity may be a weak point in trying to base a prediction on these predictors. In order to try and find the strongest predictor among the scores in this study, four different hierarchical binomial regression models were calculated using all scores as predictors, but with a different score entered as a last block. The Nagelkerke R2 change between the first and second block were examined for each model to determine the effectiveness of each predictor beyond the predictive power of the other scores. Association between the categorical measures (IBP, ASAS, GI) and the standard of reference diagnosis was calculated using Fisher’s exact test. Odds ratio, negative predictive value (NPV), and positive predictive value (PPV) were calculated.
One-way ANOVA was used in order to compare IBP scores between different diagnostic groups (SpA, other autoimmune diseases, mechanical back pain, malignancy, infection, and fibromyalgia), with Dunnett’s post hoc analysis for multiple comparisons.
For all calculations, only an IBP score of 2 and above was considered as positive. In order to validate the findings, ANOVA tests were used in examining demographic details submitted by test subjects, age in particular.
All calculations were made using IBM SPSS 23 and GraphPad Prism software (San Diego, CA, version 6.01) Figures were created using Prism GraphPad.
Results
Epidemiology and clinical features
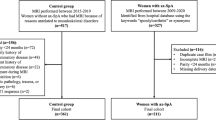
Two hundred twenty-four subjects had undergone an MRI for suspected sacroiliitis between 2005 and 2015. A total of 193 subjects (119 F: 74 M, mean age 39.7 ± 15.6) were included in the final analysis after excluding 31 subjects due to insufficient clinical data. Mean clinical follow-up period for these patients was 49 months ± 17.5 (range 17–110 months).
Distribution of patients by different diagnoses is present in Table 1. Other autoimmune diseases included two patients with rheumatoid arthritis, one with SAPHO, one with transient synovitis, and one with Behcet’s disease. Mean age was significantly lower in SpA compared in mechanical back pain group (36.1 ± 11 vs. 42.1 ± 16.9, years, p < 0.01).
IBP vs. Berlin score, ASAS, and GI
Association of the different IBP features with the two main parts of the Berlin score (BME and structural) is detailed in Table 2. Association of the IBP features with the ASAS MRI criteria and the GI is detailed in Table 3. Night pain and morning stiffness were the only IBP features with significant association to BME alone (p = 0.03, p = 0.01 respectively). While analyzing the association of all the Berlin criteria together (including both acute and structural lesion), only night pain remained significant (p = 0.01).
Night pain and morning stiffness were also significantly associated with positive ASAS MRI criteria (p < 0.01 for both) and with positive GI score (p < 0.01 for both). Beginning of the pain before the age of 40 and improvement with exercise were significantly associated only with the positive ASAS MRI criteria (p = 0.013, p = 0.026 respectively).
When comparing the different scores with each other, among patients with positive IBP (defined as 2 and above), 12 (27.3%) patients had a positive GI (p = 0.0002, OR = 4.5) and 18 (41.8%) had a positive ASAS (p = 0.0007, OR = 3.7).
IBP, Berlin, ASAS, GI scores, and diagnosis of SpA
The IBP, Berlin, ASAS, and GI scores utilized in this study were all found to be viable predictors for SpA, as diagnosed by a rheumatologist (p < 0.001, Table 4). The calculated sensitivity of IBP was 96%, and specificity was 32%, with NPV of 95% and PPV of 34%. The Nagelkerke R2 change for IBP was the highest (0.231, Table 4). Sensitivity of the MRI parameters (Berlin, ASAS, GI) was lower (26.9–57.4%) with higher specificity (88.3–94.3%).
Subjects in the SpA group had significantly higher IBP scores compared to mechanical back pain, infections, and fibromyalgia (3.6 ± 1.2 vs. 1.9 ± 1.2, 1.08 ± 0.9, 2.3 ± 1.1, respectively, p < 0.01 for all groups) (Fig. 1a).
Inflammatory back pain score, Berlin score, positive global impression and ASAS MRI for sacroiliitis in different diagnoses. a Inflammatory back pain score in different diagnoses. b Berlin score in different diagnoses. Data is shown as average ± SD. c Positive global impression and ASAS MRI in different diagnoses. ASAS Assessment of Spondyloarthritis International Society, IBP inflammatory back pain, SpA spondyloarthropathy, AID autoimmune diseases, GI global impression, **p < 0.001
Berlin scores were significantly higher in the SpA group compared in the mechanical back-pain and fibromyalgia (13.44 ± 15.9, 3.3 ± 7.1, 1.6 ± 2.5, respectively, p < 0.01 for both groups, Fig. 1b). Berlin score was higher in the infections group compared in the mechanical back pain and fibromyalgia as well (13.08 ± 11.8, p < 0.01, p = 0.012 respectively).
GI and ASAS MRI scores were not significantly different between the diagnostic groups (Fig. 1c).
Discussion
In this report, we evaluated the association between features of inflammatory back pain and MRI of the sacroiliac joint, in order to find the best predictors for SpA. Our results show a strong association of night pain and morning stiffness with bone marrow edema seen on MRI. Night pain was associated with the rest of the parameters in the Berlin score (including fat metaplasia, erosions, sclerosis, and ankyloses). Night pain and morning stiffness were associated with positive ASAS MRI and positive MRI global impression as well. All scores (IBP, Berlin, ASAS, and GI) were predictors for diagnosis of SpA, with IBP having the greatest contribution in the prediction model.
Association of specific anamnestic features and MRI lesions was previously reported, describing association between morning stiffness, sclerosis of the SIJ, and night pain with the MRI finding of BME [11]. BME most likely represent the active inflammatory process of the disease and is related to inflammatory infiltrates in other autoimmune joint diseases [12]. The connection between complains of night pain, morning stiffness, and inflammation may direct the clinician in the decision to perform an MRI, and to consider therapy against inflammation, in comparison to late, non-active disease.
Night pain and morning stiffness were significantly associated with positive ASAS MRI and with positive global impression as well. It is interesting to note that positive ASAS MRI was associated with improvement of pain with excretion and beginning of the pain before the age of 40 as well, again substantiating the role of BME lesions in the sacroiliac joints in pain induction.
IBP scores were significantly higher in the SpA group compared in the majority of the other diagnoses. Among the US population, 6% meet the criteria for IBP, whereas only 1% are diagnosed with SpA [13]. IBP is known to be a sensitive but rather a non-specific measure for SpA diagnosis [14]. The literature shows a range of values for sensitivity and specificity of IBP, varying from sensitivity of 70–95% and specificity of 76–81% [2, 15, 16]. Our results are indeed in agreement with the literature showing high sensitivity of 96%, but lower specificity of 32%. The lower specificity of IBP seen in the current study may be due to its retrospective nature and different cohort selection; however, it may also indicate an inborn weakness of this parameter precluding its use as a standalone tool for SpA diagnosis. Diagnostic abilities are improved by adding MRI in the diagnostic workup, substantiating its important role in the diagnosis of SpA.
Berlin score was significantly elevated in both SpA and infection groups. The high Berlin score in patients suffering infections represents false positive results due to shared inflammatory features such as osteomyelitis which is represented by BME on MRI [17, 18]. These MRI similarities stress the need to consider this differential diagnosis in selected patients, and warrants caution in MRI interpretation.
Our results should be interpreted with caution due to limitations of this study, including its retrospective nature and the heterogeneous group of patients in different disease stages involved. Although this heterogeneity may have complicated the conclusion drawing, it closely resembles the admixture of patients encountered in clinical practice. Indeed, this heterogeneity manifested in the cohort’s female predominance (28 F: 24 M) contrast to the known axSpA male predominance. These results are in agreement with the literature, which shows no male predominance in non-radiographic disease [19].
Our study showed that relatively low percentage of patients that were referred for MRI for suspected SpA were given this final diagnosis. Most of them had a high IBP score prior to MRI completion. These results should encourage the clinician to choose the patients referred for MRI carefully, keeping in mind the limitations of this powerful tool.
In conclusion, the presence of BME reflected by positive ASAS criteria and the relevant Berlin score is closely related to night pain and morning stiffness, all probably resulting from active inflammation. The presence of IBP features is highly associated with diagnosis of SpA, and in combination with MRI of the sacroiliac joints, may be the cornerstone in the clinician’s final diagnosis. Further research is needed in order to associate specific MRI features with clinical picture and underlying pathophysiology.
References
Sieper J, Braun J, Franklin B (2001) New treatment options in ankylosing spondylitis : a role for anti-TNF therapy. Ann Rheum Dis 60:58–61
Rudwaleit M, Metter A, Listing J, Sieper J, Braun J (2006) Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum 54:569–578. https://doi.org/10.1002/art.21619
Van Der LS, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum 27:361–368. https://doi.org/10.1002/art.1780270401
Moltó A, Paternotte S, Van Der Heijde D et al (2015) Evaluation of the validity of the different arms of the ASAS set of criteria for axial spondyloarthritis and description of the different imaging abnormalities suggestive of spondyloarthritis: data from the DESIR cohort. Ann Rheum Dis 74:746–751. https://doi.org/10.1136/annrheumdis-2013-204262
Dougados M, Van Der LS, Juhlin R et al (1991) The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 34:1218–1227. https://doi.org/10.1002/art.1780341003
Dincer U, Cakar E, Kiralp MZ, Dursun H (2008) Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol 27:457–462. https://doi.org/10.1007/s10067-007-0727-6
Sudoł-Szopińska I, Kwiatkowska B, Włodkowska-Korytkowska M et al (2015) Diagnostics of sacroiliitis according to ASAS criteria: a comparative evaluation of conventional radiographs and MRI in patients with a clinical suspicion of spondyloarthropathy. Preliminary results. Polish J Radiol 80:266–276. https://doi.org/10.12659/PJR.892529
Weber U, Pedersen SJ, Østergaard M et al (2012) Can erosions on MRI of the sacroiliac joints be reliably detected in patients with ankylosing spondylitis? - a cross-sectional study. Arthritis Res Ther 14:R124. https://doi.org/10.1186/ar3854
Hededal P, Østergaard M, SØrensen IJ et al (2018) Development and validation of MRI sacroiliac joint scoring methods for the semiaxial scan plane corresponding to the Berlin and SPARCC MRI scoring methods, and of a new global MRI sacroiliac joint method. J Rheumatol 45:70–77. https://doi.org/10.3899/jrheum.161583
Lambert RGW, Bakker PAC, Van Der Heijde D et al (2016) Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 75:1958–1963. https://doi.org/10.1136/annrheumdis-2015-208642
Arnbak B, Jurik AG, Jensen TS, Manniche C (2018) Association between inflammatory back pain characteristics and magnetic resonance imaging findings in the spine and sacroiliac joints. Arthritis Care Res (Hoboken) 70:244–251. https://doi.org/10.1002/acr.23259
Jimenez-Boj E, Nöbauer-Huhmann I, Hanslik-Schnabel B et al (2007) Bone erosions and bone marrow edema as defined by magnetic resonance imaging reflect true bone marrow inflammation in rheumatoid arthritis. Arthritis Rheum 56:1118–1124. https://doi.org/10.1002/art.22496
Weisman MH (2012) Inflammatory back pain: the United States perspective. Rheum Dis Clin 38:501–512
Poddubnyy D, Spiller I, Listing J et al (2016) The diagnostic value of the symptom of inflammatory back pain in axial spondyloarthritis in the rheumatology setting. Ann Rheum Dis suppl 2(75):578–579
Calin A, Porta J, Fries JF, Schurman DJ (1977) Clinical history as a screening test for ankylosing spondylitis. JAMA J Am Med Assoc 237:2613–2614. https://doi.org/10.1001/jama.1977.03270510035017
Rudwaleit M, van der Heijde D, Khan M, Et A (2004) How to diagnose axial spondyloarthritis early. Ann Rheum Dis 63:35–34
Sandrasegaran K, Saifuddin A, Coral A, Butt WP (1994) Magnetic resonance imaging of septic sacroiliitis. Skelet Radiol 23:289–292. https://doi.org/10.1007/BF02412363
Dunbar JAT, Sandoe JAT, Rao AS, Crimmins DW, Baig W, Rankine JJ (2010) The MRI appearances of early vertebral osteomyelitis and discitis. Clin Radiol 65:974–981. https://doi.org/10.1016/j.crad.2010.03.015
de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL (2016) Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther 18:196. https://doi.org/10.1186/s13075-016-1093-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was conducted according to the guidelines of the Declaration of Helsinki and approval of the institute’s review board (9192-11-SMC), allowing for the retrospective retrieval of data, analysis of MRI examinations, and performance of telephone interviews. Due to the retrospective nature of this study, informed consent was waived.
Disclosures
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kivity, S., Gofrit, S.G., Baker, F.a. et al. Association between inflammatory back pain features, acute and structural sacroiliitis on MRI, and the diagnosis of spondyloarthritis. Clin Rheumatol 38, 1579–1585 (2019). https://doi.org/10.1007/s10067-019-04432-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04432-5