Abstract
Objective
To evaluate the frequency of sacroiliitis in older subjects.
Materials and methods
Consecutive MRI examinations of the sacroiliac joints (SIJs) performed for suspected sacroiliitis (2005–2019) in patients ≥ 18 years were retrospectively evaluated for the presence of active/structural lesions and were categorized for the presence/absence of sacroiliitis. Clinical and imaging parameters were compared between subjects with sacroiliitis according to age groups < 40 years, 40–55, and > 55 years. Clinical parameters including inflammatory back pain (IBP) and other spondyloarthritis (SpA) features were retrieved from the medical records.
Results
A total of 431 patients with SIJs MRI were evaluated: median age, 44 [IQR 35–54]; female:male 267(62%):164(38%). Sacroiliitis was diagnosed in 89 (20.6%) subjects—median age, 41 years [IQR 32–54], 52% females— and was equally prevalent among the different age groups: > 40 years old, 23.6%; 40–55, 20%; and > 55 years old, 17%, p = 0.43, with active/structural lesions equally dispersed. Older patients (> 55) started suffering from back pain at an older age and had a longer delay in diagnosis. Gender distribution, the presence of IBP, and other SpA features were no different in patients < 45 and > 55 years of age.
Conclusions
The frequency of sacroiliitis on SIJs-MRI in subjects > 55 years is similar to its frequency in younger subjects and is associated with the same type and magnitude of active and structural MRI lesions. Clinical parameters such as IBP and additional SpA features are similarly prevalent in older and younger subjects suggesting they suffer from the same disease and differing only in age of presentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Axial spondyloarthritides (axSpA) are a group of chronic inflammatory diseases predominantly affecting the axial spine [1]. The central manifestation of axSpA is sacroiliitis [2], best detected on MRI of the sacroiliac joints (SIJs). MRI is the imaging modality of choice to detect sacroiliitis as it is highly sensitive to early, acute inflammatory changes which are not evident on X-rays or CT scans that depict only structural findings [3].
The clinical hallmark of sacroiliitis is inflammatory back pain (IBP) characterized by its insidious onset, association with nocturnal worsening and morning stiffness, relief with activity, and aggravation by immobility. The diagnosis of axSpA peaks in the third decade of life as approximately 80% of patients experience symptoms of IBP before 30 years of age [4]. Consequently, the prerequisite for inclusion of the widely accepted ASAS (Assessment of SpondyloArthritis international Society) classification criteria for axSpA [5] is that patients be younger than 45 years of age at back pain onset. Despite the reported low incidence of axSpA onset in subjects older than 45 years of age [4, 6,7,8,9,10], it was our impression that many patients older than 45 are not infrequently referred to an MRI of the SIJs for suspected sacroiliitis.
We therefore aimed to evaluate the frequency of sacroiliitis in this patient population, presuming findings would reinforce accepted diagnostic criteria and provide limited support for raising the upper age limit for radiologic referral.
Materials and methods
All consecutive MRI examinations of the sacroiliac joints performed in our institution, a tertiary medical center, for suspected sacroiliitis between the years 2005 and 2019 were retrospectively evaluated. Patients younger than 18 years old were excluded. Also excluded were patients referred for suspected infection or space-occupying lesions of the sacroiliac joints.
The study protocol received approval from the locally appointed ethics committee permitting a retrospective review of the MRI images and retrieval of the clinical data from the computerized patient charts. Informed consent was waived by the ethics committee due to the retrospective nature of the study.
MRI examinations and evaluations
MRI examinations were carried out on either a 1.5-T unit (Signa HDX, GE healthcare, Milwaukee, MN, USA) with a phased-array 8-element coil, or a 3.0-T unit (Inginia, Philips healthcare, Amsterdam, The Netherlands) with a phased-array 16-element coil. The examination was performed in the semicoronal orientation (along the long axis of the sacrum and perpendicular to the S2 vertebral body) in T1-W (TR = 534, TE = 8.9), and T2-W with fat saturation (TR = 6079, TE = 80).
All MRI examinations were anonymized prior to their evaluation.
MRI images were evaluated twice by an experienced musculoskeletal radiologist with 17 years of experience in reading MRI of the sacroiliac joints. The reader was not blinded to the age or the gender of the patient while performing the first read but blinded to both in the reliability, second read.
In addition, for inter-rater reliability evaluation, a second reader, a musculoskeletal radiologist with 7 years of experience in reading MRI examinations of the sacroiliac joints evaluated a random selection of 25% of the MRI examinations. Discrepancies between reads were resolved in a 3rd joined reading session.
The images were evaluated for structural changes (including erosions, fat metaplasia, back-fill, and ankylosis) and active changes (including bone marrow edema (BME), enthesitis, and capsulitis), of sacroiliitis correlating with the Assessment of SpondyloArthritis International Society (ASAS) definitions [11,12,13]. The presence/absence of each of the above features per patient was scored as 1/0 resulting in a maximum structural score of 4 and a maximal active score of 3. A sum score for the MRI findings (maximum of 7) was then calculated per patient. In addition, the reader gave a global impression for the presence of sacroiliitis (present = 1, absent = 0). This global impression took into account the presence of active and structural lesions highly suggestive of sacroiliitis following the revised ASAS definition [11]. Imaging findings of degenerative changes such as mild punctate BME, cortical irregularity, or subchondral sclerosis were registered but had no weight for diagnosis of sacroiliitis in the global impression. No detailed scoring such as the Berlin or SPARCC scores was performed since the aim of the study was not to evaluate the burden of sacroiliac disease but to rather to denote its presence.
Subjects positive for sacroiliitis as per global impression were subsequently divided into three groups according to age: younger than 40 years old, between 40 and 55 years, and older than 55 years old. This division was carried out in an attempt to validate our hypothesis that incidence of inflammatory sacroiliitis declines with age. Also, we chose to focus on patients older than 55, in order to avoid overlap of patients suffering from early onset disease (< 45) with delayed diagnosis from those with bona fide late-onset disease (> 55).
A second analysis comparing the MRI results of patients younger and older than 45 (the upper age limit set by the ASAS criteria) was also performed.
Clinical data
For each subject, the following clinical parameters were retrieved from the computerized medical records: age of onset of back pain, type of pain (mechanical or inflammatory), family history of SpA, and presence of SpA clinical features (uveitis, arthritis, enthesitis, dactylitis, psoriasis, IBD (inflammatory bowel disease) and/or FMF (familial Mediterranean fever)). The time to MRI diagnosis was calculated and defined as the interval between onset of back pain symptoms and diagnosis of positive sacroiliitis on MRI. Additionally, telephone visits were made to all subjects > 55 years of age in whom sacroiliitis was identified by a rheumatologist with > 15 years of experience (ML) in order to ascertain late onset of symptoms and additional SpA features.
Statistical analysis
Statistical analysis was performed using the JMP pro 15.1 software (SAS Institute Inc., Cary, NC, USA). Continuous parameters (scores for MRI changes, age at onset of back pain, and delay in radiologic diagnosis) were compared between groups using ANOVA and parametric parameters (gender, presence of different MRI findings, and clinical features) were compared between groups using a two-tailed Fisher exact or a Pearson X2 test. A p value of less than 0.05 was considered statistically significant. Odds ratios for sacroiliitis were calculated for age above 55, age above 45, the presence of IBP, the presence of SpA features, and the combined presence of both SpA features and IBP.
Intrareader and interreader reliabilities were assessed using the intraclass correlation coefficient (ICCs) analysis calculated by two-way mixed analysis for absolute agreement. An ICC is presented for each parameter evaluated (BME, subchondral sclerosis, etc.). ICC values were interpreted as follows: 0–0.2 = poor agreement, 0.3–0.4 = fair agreement, 0.5–0.6 = moderate agreement, 0.7–0.8 = strong agreement, and > 0.8 = almost perfect agreement.
Results
A total of 431 subjects underwent MRI of the sacroiliac joints for suspected SpA-related sacroiliitis. The median age of the cohort was 44 [IQR 35–54], 267 (62%) were females, and 164 (38%) were males.
Sacroiliitis was diagnosed in 89 (20.6%) subjects (38 (23.6% of entire age group) age: < 40 years, 33 (20% of entire age group) age: 40–55 years, 18 (17% of entire age group) age: > 55 years, Fig. 1). Patients with sacroiliitis had a median SIJ MRI score of 1 [IQR 1–1] for active changes, 2 [IQR 1–2] for structural changes, and 3 [IQR 2–3.5] for total (active and structural) changes, compared with a median of 0 [IQR 0–0] for all in patients without sacroiliitis. The median age of subjects with sacroiliitis was 41 years [IQR 32–54], 52% of them were females (n = 46).
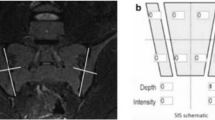
MRI of the sacroiliac joints of a 57-year-old male with 5 years of lower back pain. Bilateral, periarticular bone marrow edema on the iliac and sacral sides of the sacroiliac joints is present on the semicoronal T2-w with fat suppression image (A) along with bilateral multiple small erosions on the iliac side of the sacroiliac joints detected on the T1-w image (B)
The incidence of sacroiliitis was similar in the different age groups (23.6%, 20%, and 17% for patients younger than 40, 40–55 years old, and older than 55 respectively, p = 0.43). No significant statistical difference was seen in the frequency of each of the active (BME, enthesitis, capsulitis) or structural (erosions, fat metaplasia, back-fill, ankylosis) lesions between the different age groups (Table 1).
Analyzing the MRI results between patients younger and older than 45 (the age limit set by the ASAS criteria) resulted in similar outcome with no statistically significant difference between the two age groups (Table 2).
Table 3 compares the clinical features of patients with sacroiliitis on MRI according to age (< 40 years vs. > 55 years). The older patients’ group started suffering from back pain at an older age, and had a longer delay between the onset of back pain and the MRI diagnosis of sacroiliitis. Albeit, the two age groups did not statistically differ in gender distribution, presence of IBP, nor prevalence of different SpA features (data regarding the existence of IBP was available for 33/38 patients younger than 40 and for 11/18 patients older than 55). SpA features included either the presence of peripheral arthritis (in 13 patients younger than 40 and in 6 patients older than 55), IBD (in 4 patients younger than 40 and 3 patients older than 55), psoriasis (2 patients younger than 40 and 4 patients older than 55), uveitis (1 patient younger than 40 and 2 patients older than 55), FMF (in 4 patients younger than 40), positive HLA B27 (3 patients younger than 40 and 1 patient older than 55), or a family history of SpA (3 younger than 40, 1 older than 55). Repeating this analysis using a cutoff of 45 years again yielded similar results without statistically significant differences between the groups.
Figure 2 shows the odds ratios for sacroiliitis for age > 55, age > 45, the presence of IBP, the presence of SpA features, and the presence of both SpA features and IBP. Only the combined presence of both SpA features and IBP significantly increased the odds for sacroiliitis. Importantly, older age did not significantly decrease the odds for sacroiliitis.
Inter-reader and intra-reader reliabilities were respectively almost perfect for global impression of the presence of sacroiliitis (ICC = 0.93/0.90, p = 0.0001), strong for BME (ICC = 0.75/0.82, p = 0.0001) and ankylosis (ICC = 0.77/0.79, p = 0.0001), moderate for erosions (ICC = 0.55/0.47, p = 0.0001) and fat metaplasia (ICC = 0.70/0.67, p = 0.0001), and fair for backfill (ICC = 0.35/0.29, p = 0.02) and enthesitis (ICC = 0.46/0.30, p = 0.0001).
Discussion
In the current study, we aimed to evaluate patients with clinically suspected sacroiliitis for the frequency of sacroiliitis on MRI, in different age groups anticipating to find a relatively low frequency in subjects older than 55 years. Contrary to expectations, the rate of sacroiliitis in the older age group did not differ significantly from the rate observed among younger patients.
As life expectancy is on the rise, the incidence of late-onset sacroiliitis is expected to increase [10] and discrimination on the basis of age may lead to both under-diagnosis and delayed diagnosis in older patients who develop axial SpA in their 6th and 7th decades.
Preceding studies reported a significantly lower frequency of late onset sacroiliitis (3–8%) compared to us (17%) [4, 7,8,9,10]. This discrepancy may be attributed to the use of different imaging modalities for the diagnosis of sacroiliitis. Previously, subjects were classified using the modified New York criteria for sacroiliitis on pelvic radiographs [7, 9, 14], a method renowned for its low reliability and reproducibility [15, 16]. Herein, we assessed sacroiliitis on MRI, a method far more reliable for the characterization of structural lesions which has, in addition, the inherent additive value of detecting typical active lesions [17] that are indiscernible on radiographs.
Predictably, the notion that active sacroiliitis is rare after age 45 resulted in a longer delay in diagnosis among subjects over 55 compared with subjects younger than 40, although it still remained well within the boundaries of the standard 5–10 year diagnostic delay associated with axial SpA [18]. This is in concordance with previous data that showed that older age at diagnosis was independently associated with a longer diagnostic delay of this entity [19].
The ASAS classification criteria for sacroiliitis aim to select, with high sensitivity and specificity, subjects with sacroiliitis for clinical trials, and thus set 45 years as the upper age limit for inclusion, an age cutoff that is not necessarily applicable for clinical diagnosis [6]. However, due to the lack of diagnostic criteria for SpA, it is common practice to invalidly apply the ASAS classification criteria for diagnosis. When we disregard the arbitrary cutoff at 45, we show that the prevalence of active sacroiliitis is not significantly different among the younger and older groups, reinforcing that classification criteria should not be applied in clinical practice for diagnosis.
Neither age, IBP, nor SpA-associated features correlated individually with increased odds of sacroiliitis. This supports previous data showing that the diagnostic utility of IBP, regardless of age of onset, is rather low with a likelihood ratio of 3–4 at best for axial SpA [20, 21] and therefore insufficient for establishing a verifiable diagnosis. Following this line, Rudwaleit et al. [21] showed that the addition of a clinical parameter such as anterior uveitis, peripheral arthritis, good response to non-steroidal anti-inflammatory drugs, positive family history, dactylitis, enthesitis, elevated acute phase reactants, or HLA-B27 positivity increase the likelihood ratio for axial SpA. The presence of 3–4 parameters yields a 90% probability for the disease. This approach was evidently applied by the physicians who had referred older patients for an MRI of the SIJs in our center as the majority had a combination of IBP + SpA features which were associated with increased odds for sacroiliitis on MRI (OR 1.81 [1.05–3.12]).
The median age of onset of IBP in our > 55 group was 55 [51.75–62] with a 6-year [0.25–10] median delay in diagnosis. Although the diagnostic delay in the older age group was significantly higher than that in younger patients (Table 3), it was well within the commonly cited range [18]. As mentioned previously, the majority (72%) of older patients had additional SpA features on top of IBP which could have contributed to the relative rapidity of the diagnosis. Furthermore, the presence of ancillary SpA features emphasizes that the sacroiliitis detected in these older patients is not a mere radiological finding but rather occurs in a setting consistent with a clinical diagnosis of axSpA. Importantly, there were no differences in the prevalence of structural lesions between older and younger patients, further suggesting the notion that these are cases of sacroiliitis that developed de novo in older age.
Different referral strategies for individuals undergoing an evaluation for axSpA and sacroiliitis were previously formulated [22,23,24], all defining a “target population” of individuals younger than 45 with chronic back pain. Referring patients from this target population, in the presence of additional SpA features, to rheumatologists resulted in the diagnosis of axSpA in 33–45% of cases of which 41–62% had undiagnosed ankylosing spondylitis [25]. Again, the data presented here challenges the common paradigm which refrains from even considering a diagnosis of AxSpA in older individuals, a paradigm which results at the least in a delay in diagnosis and initiation of appropriate treatment.
Our study’s strength is the inclusion of a relatively large number of subjects and its’ main limitation is its retrospective nature precluding us from suggesting a structured referral strategy.
In conclusion, the study challenges the current concept that axial spondyloarthritis is a disease of the young as it shows that the incidence of sacroiliitis in subjects older than 55 is similar to its incidence in younger subjects and is associated with the same type and magnitude of active as well as structural MRI lesions. While the majority of patients with AxSpA develop symptoms and are diagnosed before the age of 45, an important minority, which was hitherto overlooked, develop clinical symptoms, supported by the presence of sacroiliitis on imaging, at an older age.
The clinical implication therefore is that age < 45 should not be a prerequisite for the diagnosis of sacroiliitis as is frequently the case when the ASAS classification criteria are erroneously applied for diagnosis.
References
Poddubnyy D. Axial spondyloarthritis: is there a treatment of choice? Therapeutic advances in musculoskeletal disease. 2013;5(1):45–54.
Slobodin G, Hussein H, Rosner I, Eshed I. Sacroiliitis - early diagnosis is key. J Inflamm Res. 2018;11:339–44.
Hermann KG, Bollow M. Magnetic resonance imaging of sacroiliitis in patients with spondyloarthritis: correlation with anatomy and histology. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2014;186(3):230–7.
Feldtkeller E, Bruckel J, Khan MA. Scientific contributions of ankylosing spondylitis patient advocacy groups. Curr Opin Rheumatol. 2000;12(4):239–47.
Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–83.
Olivieri I, D’Angelo S, Padula A, Leccese P, Palazzi C. Spondyloarthritis with onset after age 45. Curr Rheumatol Rep. 2013;15(12):374.
Chen HA, Chen CH, Liao HT, Lin YJ, Chen PC, Chen WS, et al. Clinical, functional, and radiographic differences among juvenile-onset, adult-onset, and late-onset ankylosing spondylitis. J Rheumatol. 2012;39(5):1013–8.
Karaarslan A, Yilmaz H, Aycan H, Orman M, Kobak S. Demographic, clinical, and laboratory features of Turkish patients with late onset ankylosing spondylitis. Bosn J Basic Med Sci. 2015;15(3):64–7.
Montilla C, Del Pino-Montes J, Collantes-Estevez E, Font P, Zarco P, Mulero J, et al. Clinical features of late-onset ankylosing spondylitis: comparison with early-onset disease. J Rheumatol. 2012;39(5):1008–12.
Toussirot E. Diagnosis and management of late-onset spondyloarthritis: implications of treat-to-target recommendations. Drugs Aging. 2015;32(7):515–24.
Lambert RG, Bakker PA, van der Heijde D, Weber U, Rudwaleit M, Hermann KG, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis. 2016;75(11):1958–63.
Maksymowych WP, Lambert RG, Ostergaard M, Pedersen SJ, Machado PM, Weber U, et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis. 2019;78(11):1550–8.
Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68(10):1520–7.
Skare TL, Leite N, Bortoluzzo AB, Goncalves CR, da Silva JA, Ximenes AC, et al. Effect of age at disease onset in the clinical profile of spondyloarthritis: a study of 1424 Brazilian patients. Clin Exp Rheumatol. 2012;30(3):351–7.
Christiansen AA, Hendricks O, Kuettel D, Horslev-Petersen K, Jurik AG, Nielsen S, et al. Limited reliability of radiographic assessment of sacroiliac joints in patients with suspected early spondyloarthritis. J Rheumatol. 2017;44(1):70–7.
van den Berg R, Lenczner G, Feydy A, van der Heijde D, Reijnierse M, Saraux A, et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs Results from the DESIR cohort. Arthritis & rheumatology. 2014;66(9):2403–11.
Diekhoff T, Hermann KG, Greese J, Schwenke C, Poddubnyy D, Hamm B, et al. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: results from the SIMACT study. Ann Rheum Dis. 2017;76(9):1502–8.
Sieper J, Rudwaleit M. How early should ankylosing spondylitis be treated with tumour necrosis factor blockers? Annals of the rheumatic diseases. 2005; 64 Suppl 4:iv61–64.
Masson Behar V, Dougados M, Etcheto A, Kreis S, Fabre S, Hudry C, et al. Diagnostic delay in axial spondyloarthritis: a cross-sectional study of 432 patients. Joint Bone Spine. 2017;84(4):467–71.
Poddubnyy D, Callhoff J, Spiller I, Listing J, Braun J, Sieper J, et al. Diagnostic accuracy of inflammatory back pain for axial spondyloarthritis in rheumatological care. RMD open. 2018; 4(2):e000825.
Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 2005;52(4):1000–8.
Jois RN, Macgregor AJ, Gaffney K. Recognition of inflammatory back pain and ankylosing spondylitis in primary care. Rheumatology. 2008;47(9):1364–6.
Poddubnyy D, Vahldiek J, Spiller I, Buss B, Listing J, Rudwaleit M, et al. Evaluation of 2 screening strategies for early identification of patients with axial spondyloarthritis in primary care. J Rheumatol. 2011;38(11):2452–60.
Sieper J, Srinivasan S, Zamani O, Mielants H, Choquette D, Pavelka K, et al. Comparison of two referral strategies for diagnosis of axial spondyloarthritis: the Recognising and Diagnosing Ankylosing Spondylitis Reliably (RADAR) study. Ann Rheum Dis. 2013;72(10):1621–7.
Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis. Nat Rev Rheumatol. 2012;8(5):262–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Grant(s) or any other financial supporter(s) of the study
None.
Competing interest
None declared.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eshed, I., Druyan, A., Stern, M. et al. The frequency of sacroiliitis on MRI in subjects over 55 years of age. Skeletal Radiol 51, 1595–1601 (2022). https://doi.org/10.1007/s00256-022-04001-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-022-04001-z