Abstract
Macrophages play an important role in the ankylosing spondylitis (AS) auto-inflammatory responses and fibrocartilage destruction. Adenosine is a key modulator of inflammatory conditions. The various effects of adenosine are mediated by its interaction with adenosine receptors (AR). In this study, we investigated the mRNA expression of A1, A2A, A2B, and A3 adenosine receptors, ectonucleoside triphosphate diphosphohydrolase-1 (CD39), and ecto-5′-nucleotidase (CD73) in the monocyte-derived macrophages from AS patients in comparison to healthy controls. We also explored the correlation between analyzed gene expression and patients’ clinical manifestations. Whole blood-separated monocytes from 23 healthy controls and 23 active AS patients were stimulated by macrophage colony-stimulating factor (M-CSF) for 7 days and differentiated to macrophages. Monocyte and macrophage markers were analyzed by flow cytometry. Analysis of adenosine receptors (ADORA1، ADORA2A، ADORA2B، ADORA3), CD39 and CD73 gene expression was performed by SYBR green real-time PCR. Our results demonstrated monocyte-derived macrophages from AS patients expressed increased level of A2AAR and reduced level of A1, A2BAR, and CD39 mRNA compared to healthy controls. We found an inverse correlation between A2AAR mRNA expression and Bath Ankylosing Spondylitis Functional Index (BASFI) score in AS patients. According to our results, altered expression level of adenosine-relying system would be involved in AS macrophage dysfunction and inflammation and correlated with functional status in AS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is the prototypic and most common member of a group of inflammatory rheumatic diseases named spondyloarthritis (SpA) [1]. The disease is characterized by inflammatory type of low back pain and progressive spinal stiffness [2]. Inflammation of the enthesis, erosion, and new bone (syndesmophyte) formation are the major clinicoradiographic features of AS [3, 4]. The pro-inflammatory cytokine, tumor necrosis factor alpha (TNF-α), contributed to the inflammatory process in AS [5]. Several TNF-α inhibitors have been developed to block spinal pain and inflammation in the disease [6]. Activated macrophages play a crucial role in inflammatory and immune responses through the secretion of some pro-inflammatory cytokines such as TNF-α [7, 8]. Previous studies demonstrated that macrophages are the predominant cells in the inflammatory lesions, enthesis, and overlaying synovium of SpA and involved in the enthesis and joint inflammation and fibrocartilage destruction [9,10,11,12].
The endogenous purine nucleoside adenosine has a range of anti-inflammatory and immunomodulatory properties and involved in the pathogenesis of inflammatory rheumatic diseases [13, 14]. Adenosine is a major innate regulator of function and activation of macrophage and other phagocytic mononuclear cells [15]. The various effects of adenosine are mediated by its interaction with four specific cell surface G-protein-coupled receptors: A1, A2A, A2B, and A3 adenosine receptors that are expressed on macrophages and nearly all cell types [16]. Activation of these receptors suppresses the initiation of NF-κB pathways and the production of pro-inflammatory molecules [17,18,19]. According to pervious reports, A2A, A2B, and A3 adenosine receptor activation can inhibit the secretion of pro-inflammatory TNF-α cytokine via macrophages [20,21,22,23,24].
Adenosine accumulates in the extracellular space under conditions of stress, cell damage, or inflammation [25, 26]. It is generated by degradation of adenosine triphosphate (ATP) by soluble and membrane-bound ectonucleotidases [27]. Following the release into the extracellular space in response to cellular inflammation, ATP breaks down to ADP and AMP by ectonucleoside triphosphate diphosphohydrolase-1 (E-NTPDase1, CD39) and then dephosphorylates into adenosine by ecto-5′-nucleotidase (CD73) [28, 29]. There are other nucleotide-metabolizing enzymes, however, CD39 and CD73 are the major ectonucleotidases that hydrolase pro-inflammatory ATP into anti-inflammatory adenosine and regulate inflammatory responses and immunity [30, 31].
Previous results indicate that lymphocytes from SpA patients express higher level of A2A and A3 adenosine receptors [32]. However, the expression and function of adenosine receptors and ectonucleotidases in macrophages from patients with ankylosing spondylitis is still unclear. In this study, we investigated the messenger RNA (mRNA) expression of all adenosine receptors and CD39 and CD73 ectonucleotidases in the monocyte-derived macrophages from AS patients in comparison to healthy controls. We also explored the correlation between adenosine receptors and ectonucleotidases mRNA expression with patient’s clinical manifestations.
Materials and methods
Patients and controls
The study population consisted of 23 active AS patients (4 females and 19 males with a mean age of 32 ± 10 years), who fulfilled the modified New York classification criteria for AS [33], and 23 age- and sex-matched healthy volunteers (4 females and 19 males with a mean age of 35 ± 11 years) without family history of any rheumatic disease. Patients were consecutively recruited from the outpatient clinic of the Rheumatology Research Center (RRC), Shariati Hospital, Tehran University of Medical Sciences (TUMS). Patients with Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ≥ 4 were selected. Prior to participation, written informed consent was obtained from all participants. This study was approved by the ethics committee of Tehran University of Medical Sciences.
Monocyte separation and macrophage derivation
Fifty milliliters of peripheral blood were collected into Ethylenediaminetetraacetic acid (EDTA) contained tubes from each subject and processed no longer than 5 h after had been taken. Samples were then diluted 1:2 with phosphate buffered saline (PBS; GIBCO Invitrogen) at pH 7.2. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll (Lymphodex, Inno-Train) density gradient centrifugation. Mononuclear cells were washed with PBS and then incubated with magnetic beads. Monocytes separated using positive selection for CD14 using MACS CD14 microbeads and magnetic-activated cell sorter columns (all from Miltenyi Biotec). Immunofluorescence staining of isolated cells was performed by phycoerythrin (PE)-conjugated anti-CD14 antibody (BD bioscience), and flow cytometry analysis showed that the purity was 92–95% (Supplementary Fig. 1). Monocytes were cultured in 24-well plates at 500,000 cells per well in complete Roswell Park Memorial Institute (RPMI) media containing 2 mM L glutamine (Biosera), 10% fetal bovine serum (FBS; Gibco BRL), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma), with 50 ng/ml recombinant human macrophage-colony stimulating factor (M-CSF; eBioscience) for 7 days.
Flow cytometry analysis of macrophage surface markers
Monocyte-derived macrophages (2 × 105) were immunostained with fluorochrome-labeled antibodies after 7 days of stimulation by M-CSF. Cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-human CD163, PE-conjugated anti-human CD206 (BD bioscience) and appropriate isotype-matched antibodies for 30 min avoiding exposure to the light.
macrophages were analyzed on a CyFlow ML flow cytometer (Partec, GmbH, Munster, Germany) and FASC data analysis was performed using the FlowJo software (Tree Star, Ashland, OR, USA). The cells expressed macrophage markers CD163 and CD206 (97 and 95%, respectively) (Supplementary Fig. 2a and b).
Analysis of gene expression using real-time quantitative PCR
After 7 days of stimulation by M-CSF, total RNA from monocyte-derived macrophages were extracted using the High Pure RNA Isolation Kit (Roche). The Transcriptor First Strand synthesis kit (Roche) was used for complementary DNA (cDNA) synthesis from the equal amount of total RNA. The relative expression levels of ADORA1, ADORA2A, ADORA2B, ADORA3, ENTPD1 (CD39), and NT5E (CD73) genes were performed using StepOnePlus™ real-time polymerase chain reaction (PCR) system (Applied Biosystems) and SYBR green master mix (Ampliqon). Reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. The specific primer sequences are shown in Table 1. The relative differences in expression of selected mRNAs between healthy controls, and patients were analyzed using the comparative CT method (2-ΔCT).
Statistical data analysis
Quantitative variables were assessed for normality assumption by Kolmogorov-Smirnov normality test. Statistical analysis of the relative gene expression between the patients and control groups with normal distribution was performed by one-way analysis of variance (ANOVA) and independent t tests and for those with non-normal distribution was performed by Kruskal-Wallis H and Mann-Whitney U tests. A simple liner regression model was used to analyze the dependence of clinical variables on gene expression levels. The differences were considered statistically significant with a P value less than 0.05. All of the statistical analysis was done by using SPSS version 22 and GraphPad Prism 6. All data are presented as mean ± SD.
Results
Demographic and clinical features
Clinical characteristics were extracted at the time patients fulfilled the criteria of AS. Recorded demographic and clinical characteristics of healthy donors and AS patients are described in Table 2.
AS macrophages express altered mRNA level of adenosine receptors
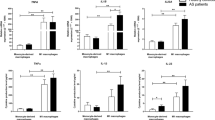
To determine the role of adenosine receptors in AS macrophages, the mRNA expression level of A1, A2A, A2B, and A3 adenosine receptors was assessed in human monocyte-generated macrophages from healthy volunteers and AS patients by qPCR. Our results indicated that monocyte-derived macrophages preferentially expressed A3, A2B, and A2A adenosine receptors, and only weakly expressed A1 adenosine receptor. The ratios of expression of A3:A2B:A2A:A1 receptors by macrophages were approximately 4:1:1:0.03 (Fig. 1). The A2AAR mRNA expression in macrophages from AS patients was significantly increased by 2.03-fold in comparison to healthy subjects (Fig. 2; P < 0.05). Interestingly, A1 and A2BAR mRNA expressions were significantly down regulated (− 2.5-fold and − 1.4-fold, respectively) in macrophages from AS patients compared to normal macrophages (Fig. 3a, b; P < 0.05 for all). Our results showing that the mRNA expression level of A3AR was not significantly different in patient’s macrophages compared to healthy subjects (Supplementary Fig. 3a).
Adenosine receptor mRNA expression in monocyte-derived macrophages. The ratios of expression of A3: A2B: A2A: A1 receptors by macrophages were approximately 4:1:1:0.03. Monocytes from 23 healthy controls were differentiated to macrophages with M-CSF for 7 days. The receptor mRNA was determined by real-time PCR and normalized to GAPDH. Data are expressed as the means ± SD
A2AAR mRNA expression in monocyte-derived macrophages from AS patients was significantly increased by 2.03-fold as compared to healthy controls. Isolated monocytes from 23 AS patients and 23 healthy donors were differentiated to macrophages with M-CSF. A2AAR mRNA was determined in monocyte-generated macrophages by real-time PCR and normalized to GAPDH. Data are expressed as the means ± SD (*P ≤ 0.05)
(a) A1AR and (b) A2BAR mRNA expression in M-CSF-generated macrophages from AS patients (n = 23) was significantly decreased by − 2.5-fold and − 1.4-fold, respectively, comparing to normal macrophages (n = 23). mRNA expression was assessed by real-time PCR and normalized to GAPDH. Data are expressed as the means ± SD (*P ≤ 0.05)
CD39 mRNA expression was decreased in AS macrophages
To study the enzymes contributed to adenosine production, we also analyzed the mRNA expression of CD39 and CD73 ectonucleotidases in human macrophages from AS patients and healthy controls. CD39 and CD73 are both expressed in monocyte-induced macrophages. Nevertheless, CD73 enzyme is weakly expressed on the cells. The results showed CD39 mRNA expression was significantly (− 1.3-fold) diminished in AS patient’s macrophages compared to normal controls (Fig. 4; P < 0.05). However, the expression of CD73 enzyme was not significantly different between normal and patients group (Supplementary Fig. 3b).
The mRNA expression of CD39 enzyme in monocyte-generated macrophages. Expression was significantly down regulated in macrophage from patients (n = 23) by − 1.3-fold as compared to normal individuals (n = 23). The expression was detected by qPCR and normalized to GAPDH. Data are presented as the means ± SD (*P ≤ 0.05)
A2A adenosine receptor expression inversely correlated with BASFI score in AS patients
We next investigated the association of the clinical manifestation of AS patients with analyzed mRNA expressions. Clinical data included Bath Ankylosing Spondylitis Metrology Index (BASMI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Disease Activity Index (BASDI), Bath Ankylosing Spondylitis Global Score (BAS-G), and patient’s disease global assessment (PDGA). We found an inverse correlation between A2AAR mRNA expression and BASFI score in AS patients (Fig. 5; P < 0.01). There was no other significant correlation between clinical manifestations and selected gene mRNA expression.
Discussion
To study the involvement of adenosine-relying system in ankylosing spondylitis, we primarily investigated the expression of adenosine receptors in AS macrophages compared to healthy controls. This study is the first to analyze the expression of adenosine receptors in macrophages from AS patients. All four adenosine receptors are expressed by monocyte-derived macrophages with A3AR mRNA predominance on the cells. The results of the study revealed an increase at mRNA level of A2AAR in monocyte-generated macrophages from AS patients. Our results are in line with the previous works demonstrated an upregulation of A2AAR in lymphocytes from systemic lupus erythematosus (SLE) [34], rheumatoid arthritis (RA), psoriatic arthritis (PsA), and AS patients [32]. Previous reports showed that lymphocytes from patients with RA, AS, and PsA inflammatory diseases [32] and PBMCs from patients with psoriasis, RA, and Crohn’s disease [35] express higher level of A3 adenosine receptor. However, we did not find a significant difference between A3AR mRNA level in macrophages from AS patients compared to healthy control group. We also observed a decrease at a transcriptional level of A1 and A2BAR in AS macrophages. Our finding is different from the previous reports, showing no significant differences between mRNA levels of A1 and A2B adenosine receptors in lymphocytes from AS and other arthritic patients comparing to normal lymphocytes [32, 34, 36]. The present study showed, for the first time, the involvement of A1 and A2BAR mRNA alternations in the most common form of SpA. According to our results, altered expression level of adenosine receptors would be involved in macrophage dysfunction and inflammation in AS.
We next verified whether the expression of adenosine receptors in macrophages was associated with patient’s clinical characteristics. Our findings, for the first time, demonstrated an inverse correlation between functional status of AS patients (BASFI score) and A2AAR mRNA expression in monocyte-derived macrophages. The lower level of BASFI scores were correlated with higher level of A2AAR mRNA expression in macrophages, showing a significant role for these receptors in AS pathogenesis. Former studies demonstrated that A2A adenosine receptor activation prevents differentiation of osteoclasts [37], suggesting a mechanism by which A2AAR could target bone erosion, formation, and functional status in AS. This finding supports the previous studies showing an inverse correlation between A2AAR density and Disease Activity Score (DAS) in RA patients [36], and clinical parameters in SLE patients [34].
Adenosine serves as a signaling molecule to limit inflammation. CD39 and CD73 membrane-bound ectonucleotidases degrade extracellular nucleotides to the adenosine [30]. Alterations in CD39 and CD73 expression correlate with disturbed adenosine signaling [30, 38]. Therefore, in the present work, we also analyzed the mRNA expression of CD39 and CD73 ecto-enzymes in AS macrophages in comparison to normal macrophages. Current study is the first to analyze the mRNA expression of CD73 and CD39 enzymes in macrophages from AS patients. Our results revealed that monocyte-derived macrophages from AS patients express diminished mRNA level of CD39 compared to controls. We did not find a significant difference in the mRNA level of CD73 between macrophages from AS patients and healthy individuals. We also did not observe a significant correlation between these enzymes expression and patient’s clinical manifestation.
In consistent with our study, Matthew et al. have reported T cell expression of the CD39 enzyme is defective in a subset of active lupus patients [39]. Nevertheless, our data is different from the former studies on regulatory T cells from other arthritic patients. It has been reported that CD4+ T cells from the synovial fluid of patients with RA [40], SpA [41], and juvenile arthritis (JIA) [42] express increased CD39 and reduced CD73 levels. There is no previous report about CD39 and CD73 expression in macrophages from AS and other rheumatic disorders. According to the anti-inflammatory role of adenosine, and the macrophage involvement in AS inflammation, it is suggested that diminished adenosine-generating enzyme CD39 in AS macrophages would be involved in the disease pathogenesis and joint inflammation.
The current study highlights, for the first time, the altered expression of ecto-enzyme CD39, A2A, A2B, and A1 adenosine receptors in monocyte-derived macrophages from AS patients. These data indicate the emerging role of adenosine signaling molecules in AS disease as therapeutic targets. Adenosine-relying system would be involved in AS macrophage dysfunction and inflammation and correlated with functional status in AS patients. Further studies on the function of adenosine signaling molecules in the macrophages, and inflammatory pathways should be done to fully distinguish the role of adenosine in the pathogenesis of AS.
References
Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet 369:1379–1390. https://doi.org/10.1016/S0140-6736(07)60635-7
Tam LS, Gu J, Yu D (2010) Pathogenesis of ankylosing spondylitis. Nat Rev Rheumatol 6:399–405. https://doi.org/10.1038/nrrheum.2010.79
Rudwaleit M, Baeten D (2006) Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol 20:451–471. https://doi.org/10.1016/j.berh.2006.03.010
Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, Landewe R, van ver Tempel H, Mielants H, Dougados M, van der Heijde D (2003) Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 62:127–132
Henderson C, Davis JC (2006) Drug insight: anti-tumor-necrosis-factor therapy for ankylosing spondylitis. Nat Clin Pract Rheumatol 2:211–218. https://doi.org/10.1038/ncprheum0157
Coates LC, Marzo-Ortega H, Bennett AN, Emery P (2010) Anti-TNF therapy in ankylosing spondylitis: insights for the clinician. Ther Adv Musculoskelet Dis 2:37–43. https://doi.org/10.1177/1759720X09359728
Fujiwara N, Kobayashi K (2005) Macrophages in inflammation. Curr Drug Targets Inflamm Allergy 4:281–286
Laria A, Lurati A, Marrazza M, Mazzocchi D, Re KA, Scarpellini M (2016) The macrophages in rheumatic diseases. J Inflamm Res 9:1–11. https://doi.org/10.2147/JIR.S82320
Melis L, Elewaut D (2009) Progress in spondylarthritis. Immunopathogenesis of spondyloarthritis: which cells drive disease? Arthritis Res Ther 11:233. https://doi.org/10.1186/ar2722
McGonagle D, Marzo-Ortega H, O'Connor P, Gibbon W, Hawkey P, Henshaw K, Emery P (2002) Histological assessment of the early enthesitis lesion in spondyloarthropathy. Ann Rheum Dis 61:534–537
Baeten D, De Keyser F (2004) The histopathology of spondyloarthropathy. Curr Mol Med 4:1–12
Bollow M, Fischer T, Reisshauer H, Backhaus M, Sieper J, Hamm B, Braun J (2000) Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis—cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis 59:135–140
Hasko G, Cronstein BN (2004) Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25:33–39
Cronstein BN, Sitkovsky M (2017) Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat Rev Rheumatol 13:41–51. https://doi.org/10.1038/nrrheum.2016.178
Hasko G, Pacher P (2012) Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol 32:865–869. https://doi.org/10.1161/ATVBAHA.111.226852
Buenestado A, Grassin Delyle S, Arnould I, Besnard F, Naline E, Blouquit-Laye S, Chapelier A, Bellamy JF, Devillier P (2010) The role of adenosine receptors in regulating production of tumour necrosis factor-alpha and chemokines by human lung macrophages. Br J Pharmacol 159:1304–1311. https://doi.org/10.1111/j.1476-5381.2009.00614.x
Hasko G, Deitch EA, Szabo C, Nemeth ZH, Vizi ES (2002) Adenosine: a potential mediator of immunosuppression in multiple organ failure. Curr Opin Pharmacol 2:440–444
Majumdar S, Aggarwal BB (2003) Adenosine suppresses activation of nuclear factor-kappaB selectively induced by tumor necrosis factor in different cell types. Oncogene 22:1206–1218. https://doi.org/10.1038/sj.onc.1206184
Hasko G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7:759–770. https://doi.org/10.1038/nrd2638
Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C (2000) Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J 14:2065–2074. https://doi.org/10.1096/fj.99-0508com
Kreckler LM, Wan TC, Ge ZD, Auchampach JA (2006) Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther 317:172–180. https://doi.org/10.1124/jpet.105.096016
Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I (2008) Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther 324:694–700. https://doi.org/10.1124/jpet.107.131540
Le Vraux V, Chen YL, Masson I, De Sousa M, Giroud JP, Florentin I, Chauvelot-Moachon L (1993) Inhibition of human monocyte TNF production by adenosine receptor agonists. Life Sci 52:1917–1924
Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS (1996) Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J Immunol 156:3435–3442
Blackburn MR, Vance CO, Morschl E, Wilson CN (2009) Adenosine receptors and inflammation. Handb Exp Pharmacol:215–269. https://doi.org/10.1007/978-3-540-89615-9_8
Fredholm BB (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14:1315–1323. https://doi.org/10.1038/sj.cdd.4402132
Hasko G, Cronstein B (2013) Regulation of inflammation by adenosine. Front Immunol 4:85. https://doi.org/10.3389/fimmu.2013.00085
Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783:673–694. https://doi.org/10.1016/j.bbamcr.2008.01.024
Jacob F, Perez Novo C, Bachert C, Van Crombruggen K (2013) Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal 9:285–306. https://doi.org/10.1007/s11302-013-9357-4
Antonioli L, Pacher P, Vizi ES, Hasko G (2013) CD39 and CD73 in immunity and inflammation. Trends Mol Med 19:355–367. https://doi.org/10.1016/j.molmed.2013.03.005
Cekic C, Linden J (2016) Purinergic regulation of the immune system. Nat Rev Immunol 16:177–192. https://doi.org/10.1038/nri.2016.4
Ravani A, Vincenzi F, Bortoluzzi A, Padovan M, Pasquini S, Gessi S, Merighi S, Borea PA, Govoni M, Varani K (2017) Role and function of A2A and A(3) adenosine receptors in patients with ankylosing spondylitis, psoriatic arthritis and rheumatoid arthritis. Int J Mol Sci 18. https://doi.org/10.3390/ijms18040697
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria Arthritis Rheum 27:361–368
Bortoluzzi A, Vincenzi F, Govoni M, Padovan M, Ravani A, Borea PA, Varani K (2016) A2A adenosine receptor upregulation correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Res Ther 18:192. https://doi.org/10.1186/s13075-016-1089-8
Ochaion A, Bar-Yehuda S, Cohen S, Barer F, Patoka R, Amital H, Reitblat T, Reitblat A, Ophir J, Konfino I, Chowers Y, Ben-Horin S, Fishman P (2009) The anti-inflammatory target A(3) adenosine receptor is over-expressed in rheumatoid arthritis, psoriasis and Crohn's disease. Cell Immunol 258:115–122. https://doi.org/10.1016/j.cellimm.2009.03.020
Varani K, Padovan M, Vincenzi F, Targa M, Trotta F, Govoni M, Borea PA (2011) A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res Ther 13:R197. https://doi.org/10.1186/ar3527
Mediero A, Perez-Aso M, Cronstein BN (2013) Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFkappaB nuclear translocation. Br J Pharmacol 169:1372–1388. https://doi.org/10.1111/bph.12227
Matyash M, Zabiegalov O, Wendt S, Matyash V, Kettenmann H (2017) The adenosine generating enzymes CD39/CD73 control microglial processes ramification in the mouse brain. PLoS One 12:e0175012. https://doi.org/10.1371/journal.pone.0175012
Loza MJ, Anderson AS, O'Rourke KS, Wood J, Khan IU (2011) T-cell specific defect in expression of the NTPDase CD39 as a biomarker for lupus. Cell Immunol 271:110–117. https://doi.org/10.1016/j.cellimm.2011.06.010
Herrath J, Chemin K, Albrecht I, Catrina AI, Malmstrom V (2014) Surface expression of CD39 identifies an enriched Treg-cell subset in the rheumatic joint, which does not suppress IL-17A secretion. Eur J Immunol 44:2979–2989. https://doi.org/10.1002/eji.201344140
Guo H, Zheng M, Zhang K, Yang F, Zhang X, Han Q, Chen ZN, Zhu P (2016) Functional defects in CD4+ CD25high FoxP3+ regulatory cells in ankylosing spondylitis. Sci Rep 6:37559. https://doi.org/10.1038/srep37559
Moncrieffe H, Nistala K, Kamhieh Y, Evans J, Eddaoudi A, Eaton S, Wedderburn LR (2010) High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J Immunol 185:134–143. https://doi.org/10.4049/jimmunol.0803474
Acknowledgements
We would like to thank all participants for their collaboration.
Funding
This work has been supported by a research grant from Tehran University of Medical Sciences (TUMS); grant no. 95-04-41-33869.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
None.
Electronic supplementary material
Supplementary Fig. 1
Flow cytometry analysis of CD14 expression on the cell surface of isolated monocyte. Histograms of PBMC-isolated monocyte staining with PE-conjugated anti-CD14 and isotype control antibodies. The results showed that monocytes were isolated by positive selection with 92–95% purity (GIF 21 kb)
Supplementary Fig. 2
Flow cytometry analysis of the cell surface macrophage markers CD163 and CD206. Histograms of monocyte-derived macrophage staining with a) FITC-conjugated anti-CD163 b) PE-conjugated anti-CD206, and appropriate isotype control antibodies. Results showed that Monocyte-derived macrophages with 7 days’ stimulation by M-CSF were a) 97% CD163 positive and b) 95% CD206 positive (GIF 17 kb)
Supplementary Fig. 3
There was not significant differences in a) A3AR and b) CD73 enzyme mRNA expression in monocyte-derived macrophages between AS patients and healthy controls. Isolated monocytes from 23 AS patients and 23 healthy donors were differentiated to macrophages with M-CSF. The expression was analyzed in monocyte-generated macrophages by real-time PCR and normalized to GAPDH. Data are expressed as the means ± SD (GIF 22 kb)
Rights and permissions
About this article
Cite this article
Akhtari, M., Zargar, S.J., Mahmoudi, M. et al. Ankylosing spondylitis monocyte-derived macrophages express increased level of A2A adenosine receptor and decreased level of ectonucleoside triphosphate diphosphohydrolase-1 (CD39), A1 and A2B adenosine receptors. Clin Rheumatol 37, 1589–1595 (2018). https://doi.org/10.1007/s10067-018-4055-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4055-9