Abstract
Type I cryoglobulinemia is associated with B cell proliferative diseases, whereas essential mixed cryoglobulinemia is classically associated with infections, malignancy, and autoimmune diseases, but may be idiopathic. Prognosis in patients with grave manifestations and renal involvement is often poor. We report a case of a 40-year-old woman, 2 weeks post-partum for pre-eclampsia who was hospitalized with nephritic syndrome and acute renal failure. The patient harbored type I and type II cryoglobulinemia. Renal and cutaneous biopsies confirmed the diagnosis; however, an underlying etiology was not established. A bone marrow biopsy suggested monoclonal gammopathy of undetermined source (MGUS). Despite therapy with intravenous cyclophosphamide, rituximab, plasmapheresis, dialysis, and bortezomib, the patient succumbed after 8 months of hospitalization. We suggest that an overlap entity of types I and II cryoglobulinemia with severe multi-organ involvement not only is rare but also may be resistant to conventional therapy and fatal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryoglobulin-related clinical characteristics vary according to the pathogenesis, which is represented either by a hyperviscosity syndrome or by immune complex deposition–related manifestations.
Type I cryoglobulinemia consists of monoclonal immunoglobulins (Ig), typically IgG or IgM, and is associated in the majority of cases with B cell proliferative diseases, such as multiple myeloma (MM). Monoclonal gammopathy of unclear significance (MGUS) has also been documented with a lower incident rate of 10–15%. Clinical manifestations are usually secondary to hyperviscosity complications which can affect multiple organs of the body [1].
Essential mixed cryoglobulinemia is a rare disease and is described as idiopathic or secondary to infections, malignancy, or autoimmune diseases. Between 70 and 90% of reported cases are associated with hepatitis C (HCV) infection. Classically, the mixed cryoglobulinemia syndrome originates from type II or type III cryoglobulins. Pathogenesis includes deposition of a polyclonal IgG and a monoclonal IgM with rheumatoid factor (RF) activity directed against IgG, creating antigen-antibody complexes which deposit with complement in the vessel wall of small arterioles and capillaries. The most common manifestations are represented by purpura, peripheral neuropathy, and renal involvement [2].
To date, directed therapy for the different types of cryoglobulinemia is based on expert opinion due to disease rarity. As a rule, therapy is directed by the underlying etiology.
Conventional therapy consists of high-dose corticosteroids (1 mg/kg/day), intravenous cyclophosphamide (CYC), rituximab (RTX), bortezomib, and plasma exchange. Prognosis in patients with grave manifestations is often poor [1, 3, 4].
We present a rare case of an overlap cryoglobulinemic syndrome (types I+II) refractory to conventional therapy.
Patient description
A 40-year-old woman, 2 weeks post-partum, following pre-eclampsia, presented to our institution with dyspnea, central lower quadrant abdominal pain, and hematuria. She was hospitalized with nephritic syndrome and acute renal failure. Previously, she was healthy and without medication therapy.
On examination, abnormal findings included hypertension (178/70 mmHg), tachypnea with auxiliary breathing muscles, diffuse abdominal pain without rebound, and peripheral leg edema. Laboratory results indicated acute renal injury. Investigations for new-onset renal failure included 24-h urine protein collection which demonstrated nephritic range proteinuria (2.3 g/day); autoimmune serologic studies showed very low C4, normal C3, cryocrit − 0.2%, positive RF (× 600 the upper limit of the normal value), and negative serology: ANCA, ANA, anti-dsDNA and antibodies, and ACPA. Urinalysis showed light chains, followed by monoclonal peaks on serum immune electrophoresis. Infectious studies for HCV, hepatitis B (HBV), and HIV were negative.
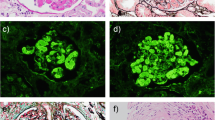
The patient underwent a renal biopsy that demonstrated mesangio-capillary glomerulonephritis (Fig. 1). On electron microscopy, no immune complex depositions were noted. The first interpretation of the kidney biopsy was not conclusive and two diagnostics were considered, lupus nephritis versus cryoglobulinemic glomerulonephritis. However, lupus serology was negative. Later, due to extensive cutaneous involvement, which developed during hospitalization and manifested as purpura, a skin punch biopsy was performed and showed thrombotic vasculopathy without accompanying obvious vasculitis, compatible with cryoglobulinemia, most likely type I. Direct immunofluorescence study for IgG, IgM, C3, and C4 was negative. Still, no underlying disease was established.
A bone marrow biopsy was later performed and showed light chain IgM kappa restricted monoclonal plasma cells comprising about 4% of the cellular population, findings compatible with monoclonal gammopathy of unknown source-MGUS. However, the low cryocrit and insignificant MGUS were not compatible with the progressive clinical manifestations of the patient which included an extended purpuric rash, progressive renal failure, and peripheral neuropathy commonly found in essential cryoglobulinemia.
At first, therapy was initiated with intravenous pulse steroid therapy (1 g methylprednisolone, for 3 days) and continued with oral high-dose prednisone (60 mg per day) and a regimen of intravenous cyclophosphmide (CYC) at a fixed dose of 500 mg every 2 weeks.
Without clinical response, worsening of kidney function, volume overload manifested clinically with anasarca, plasmapheresis (5 sessions every 2 days, instead of 10 sessions) and dialysis were initiated in addition to the existing therapy.
Treatment with IV CYC was discontinued after 2 doses due to pancytopenia, but also due to lack of clinical remission. Therapy for cryoglobulinemia with renal involvement was initiated with rituximab- (RTX) at a dose of 375 mg/m2, equivalent to 700 mg, once a week, for 4 weeks, without immediate clinical improvement. Later, the patient had a worsening of disease that manifested as severe extended purpuric rash and peripheral neuropathy and, on laboratory blood tests, increasing titers of cryoglobulins (cryocrit 3.2%), RF (× 5000 the upper limit of normal), and severe thrombocytopenia. Plasma exchange was initiated by protocol (10 sessions every 2 days). In the absence of clinical and immunologic response, therapy with RTX was discontinued (after 4 doses). Based on a second bone marrow biopsy, which supported the previous biopsy, a multidisciplinary consult decided that although the findings of light chain IgM kappa monoclonal plasma cells comprised less than 10% of the cellular population, it was the most probable cause of cryoglobulin disease exacerbation and treatment with bortezomib was initiated according to protocol (1.3 mg/m2, equivalent to 2 mg, initiated on days 1, 4, 8, and 11 every 21 days).
Under this treatment (total of 20 cycles), the patient had alternating periods of clinical and immunologic improvement along with disease flares, for a period of 3.5 months (Fig. 2).
During this period, the patient experienced repeated infections such as nosocomial pneumonia, line sepsis with pseudomonas, and candidemia and necessitated intensive care therapy. As a result, bortezomib therapy was discontinued and the patient continued therapy with plasma exchange (15 sessions every 2 days) and dialysis only.
Despite several intensive lines of therapy, the patient developed a massive intra-cerebral hemorrhage and succumbed in the intensive care unit (ICU) following 8 months of hospitalization.
Discussion
We present a rare case of a young patient which presented with a nephritic syndrome and new-onset renal failure. Renal biopsy showed cryoglobulinemic glomerulonephritis. Treatment was initiated with combined therapy of high-dose corticosteroids and IV CYC [5]. Repeated laboratory tests raised the suspicion of multiple myeloma, and cryoglobulins (IgM with RF activity) were positive. The most compatible underlying disease was MGUS.
It is important to mention that a therapeutic course with IV CYC in combination with high-dose corticosteroids is still a valid option for this condition, mostly in life-threatening manifestations such as were manifested in our patient [6]. Due to pancytopenia and supported by clinical trials, treatment was replaced accordingly to an immunosuppression therapy with RTX.
One trial which used a data base from a cohort of 242 patients demonstrated that combined therapy with high-dose corticosteroids had the best clinical and immunologic response compared to monotherapy with corticosteroids or combined therapy of corticosteroids with an alkylator such as CYC. The disadvantage of the combination therapy is the increased risk of severe infections [7].
On the contrary, there are reports of disease relapses after RTX therapy. One study documented disease relapse in half the patients treated with RTX after a median of 1 year (a cohort of 23 patients). Clinical factors associated with disease relapse include purpura, articular and cutaneous involvement, and immunologic response defined as poor response to previous immunosuppressive therapy [8].
The efficacy and safety of RTX were tested in several studies, the biggest data base consisted of 191 patients [9]. Conclusions were unanimous—RTX has the greatest efficacy in noninfectious cryoglobulinemia, though its safety should be further evaluated carefully due to severe secondary infections [8,9,10,11]. Low-dose RTX may be beneficial for cryoglobulinemia of an infectious origin [3].
In addition, survival prognostic factors were identified and mentioned in a few studies including age > 65 years old, pulmonary and gastrointestinal vasculitis, and renal failure. A higher score represented a poor outcome. Acute renal failure in particular was associated with a very poor prognosis [12,13,14,15,16,17].
In our case, while the patient was treated with RTX, she developed peripheral neuropathy and an extensive skin rash; laboratory blood tests showed high cryocrit (maximum of 15%) and high titers of RF (maximum of × 5000 the upper limit of normal). The skin biopsy excluded vasculitis and was compatible with cryoglobulinemia type I.
A repeat bone marrow biopsy was performed due to the absence of clinical remission on RTX therapy. The possibility of cryoglobulinemia induced by MGUS was speculated and treatment with plasma exchange and bortezomib was initiated.
Increasing numbers of case reports documented the benefit of plasma exchange in patients with cryoglobulinemia, hyperviscosity syndrome, and life-threatening manifestations such as rapidly progressive glomerulonephritis. In addition, plasma exchange can be used in disease flares with high IgM titers after rituximab therapy [6, 18,19,20]. However, plasma exchange can induce cryoglobulin rebound with secondary increased cryoglobulin production after therapy discontinuation [21].
Bortezomib, a proteasome inhibitor, is administered as first-line therapy in patients with multiple myeloma. Recently, it has been used for some patients with type I cryoglobulinemia associated with MGUS, refractory to treatment and with good outcomes after a median of 1.5 therapy courses [22].
To date, 4 cases were described with good clinical and immunological response after 2 cycles of combination therapy with bortezomib and high-dose corticosteroids (Table 1). All patients presented with skin involvement and cutaneous vasculitis, 2 patients had also purpura. Only one case presented with renal involvement, which is a less common manifestation in patients with type I cryoglobulinemia and one patient developed significant side effects represented by peripheral neuropathy [22,23,24].
It is important to mention that bortezomib therapy has a delayed clinical response, with a median of 40 days and its administration in a setting of life-threatening conditions has been poorly explored. In this situation, a combination of bortezomib, high-dose corticosteroids, and plasmapheresis is the recommended treatment [25].
Two cases of type II cryoglobulinemia associated with MGUS were reported (Table 1). The first case described a patient which presented with rapidly progressive glomerulonephritis. High-dose corticosteroids, plasma exchange, CYC, and RTX were initiated, and disease remission ensued. After 2 months, the patient had a disease exacerbation, which manifested with purpura and an increased cryocrit. The purpura resolved after one dose of RTX. Later on, the patient continued to have recurrence of clinical manifestations and bortezomib therapy was initiated with clinical and immunologic remission after 2 cycles of therapy, similar to previously documented case reports [22,23,24, 26].
The second case described a patient which presented with membranoproliferative glomerulonephritis and negative HCV. CYC and steroids (prednisone 20 mg for 3 months) were initiated with good clinical and laboratory response. This patient continued low-dose steroids (prednisone 5 mg daily) for 4 years, with nearly full resolution of her kidney function [27].
Our case is extremely intriguing. According to the findings on laboratory blood tests and biopsies performed, a clear-cut diagnosis whether the patient had type I or type II cryoglobulinemia could not be established. Blood laboratory tests were consistent with type II cryoglobulinemia with RF activity, in the absence of HCV infection or any evidence of an underlying autoimmune disease. The clinical presentation with renal involvement, which was identified as a bad prognostic factor, is associated in the majority of cases with type II cryoglobulinemia, but was reported in one case with MGUS-associated type I cryoglobulinemia [22]. Skin and renal biopsies of our patient were consistent with type I cryoglobulinemia, and bone marrow biopsy showed MGUS, which are consistent with type I cryoglobulinemia. Only one case was described with MGUS and type II cryoglobulinemia [26].
To date, no official classification criteria were published for the hyperviscosity syndrome which is associated with type I cryoglobulinemia. In contrast, a preliminary classification criterion for cryoglobulinemic vasculitis which is associated with type II cryoglobulinemia exists. The classification includes major and minor criteria for serologic tests and clinical manifestations as follows: major criteria for serologic tests include mixed-type cryoglobulins, low C4. Minor criteria include positive RF, HCV, and HBV. Major clinical manifestations include purpura. Minor criteria include chronic hepatitis, MPGN, peripheral neuropathy, and skin ulcers. A diagnosis of mixed-type cryoglobulinemic syndrome is established by the presence of the classic triad of mixed-type cryoglobulins (in the presence or absence of low C4), purpura, and leukocytoclastic vasculitis or by the presence of mixed-type cryoglobulins (in the presence or absence of low C4) plus 2 minor clinical criteria plus 2 minor serological findings [26].
Based on this classification, our patient was not fully compatible with a type II cryoglobulinemia. Despite this fact, therapy was initiated with recommended protocols with high-dose corticosteroids, CYC, RTX, and plasma exchange as for essential cryoglobulinemia. The patient had no clinical resolution and she was admitted to the ICU several times due to severe infections which necessitated mechanical ventilation.
Following the finding of MGUS, it was decided to start therapy with bortezomib. Contrary to what was described in the current literature, the patient did not have an adequate clinical and immunologic response and had only minor serologic improvement with decreased titers of cryoglobulins and RF which rapidly increased in a short period of time.
Recent literature recommends the initiating of bortezomib as soon as the diagnosis is recognized. There is no data on patients in whom bortezomib therapy was initiated later in their course of disease or on patients with life-threatening conditions. Considering our patient’s presentation and refractory disease, could we expect disease remission after a median of 1.5 cycles of therapy as was previously suggested in 5 case reports similar to ours? Our patient started therapy with bortezomib after 2.5 months of persistent active disease and therapy failure with high-dose corticosteroids, CYC, RTX, and plasma exchange. In total, our patient underwent 20 cycles of bortezomib for 3 months without significant disease remission and seemed to manifest with continuous disease activity. Was the therapy initiated too late? Or was the patient too ill in order to respond to therapy, taking in consideration periods of severe infections which were treated with wide-spectrum antibiotics without immunosuppressive medications.
We speculate that an overlap entity of different types of cryoglobulin (type I+II) with severe multi-organ involvement may be more resistant to conventional therapy and even fatal.
References
Damoiseaux J (2014) The diagnosis and classification of the cryoglobulinemic syndrome. Autoimmun Rev 13:359–362
Trejo O, Ramos-Casals M, García-Carrasco M et al (2001) Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore) 80:252–262
Visentini M, Tinelli C, Colantuono S, Monti M, Ludovisi S, Gragnani L, Mitrevski M, Ranieri J, Fognani E, Piluso A, Granata M, de Silvestri A, Scotti V, Mondelli MU, Zignego AL, Fiorilli M, Casato M (2015) Efficacy of low-dose rituximab for the treatmen of mixed cryoglobulinemic vasculitis: phase II clinical trial and systematic review. Autoimmun Rev 14:889–896
Cacoub P, Comarmond C, Domont F, Savey L (2015) Cryoglobulinemia vasculitis. Am J Med 128:950–955
Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido ER, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R (2002) Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 46:2121–2131
Muchtar E, Magen H, Gertz MA (2017) How I treat cryoglobulinemia. Blood 129:289–298
Terrier B, Krastinova E, Marie I, Launay D, Lacraz A, Belenotti P, de Saint-Martin L, Quemeneur T, Huart A, Bonnet F, le Guenno G, Kahn JE, Hinschberger O, Rullier P, Diot E, Lazaro E, Bridoux F, Zenone T, Carrat F, Hermine O, Leger JM, Mariette X, Senet P, Plaisier E, Cacoub P (2012) Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood 119:5996–6004
Terrier B, Launay D, Kaplanski G, Hot A, Larroche C, Cathébras P, Combe B, de Jaureguiberry JP, Meyer O, Schaeverbeke T, Somogyi A, Tricot L, Zénone T, Ravaud P, Gottenberg JE, Mariette X, Cacoub P (2010) Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French Autoimmunity and Rituximab registry. Arthritis Care Res 62:1787–1795
Wink F, Houtman PM, Jansen TL (2001) Rituximab in cryoglobulinaemic vasculitis, evidence for its effectivity: a case report and review of literature. Clin Rheumatol 30:293–300
Colantuono S, Mitrevski M, Yang B, Tola J, Carlesimo M, de Sanctis GM, Fiorilli M, Casato M, Visentini M (2017) Efficacy and safety of long-term treatment with low-dose rituximab for relapsing mixed cryoglobulinemia vasculitis. Clin Rheumatol 36:617–623
Perez-Alamino R, Espinoza LR (2014) Non-infectious cryoglobulinemia vasculitis (CryoVas): update on clinical and therapeutic approach. Curr Rheumatol Rep 16:420
Terrier B, Carrat F, Krastinova E, Marie I, Launay D, Lacraz A, Belenotti P, de Saint Martin L, Quemeneur T, Huart A, Bonnet F, le Guenno G, Kahn JE, Hinschberger O, Rullier P, Hummel A, Diot E, Pagnoux C, Lzaro E, Bridoux F, Zenone T, Hermine O, Leger JM, Mariette X, Senet P, Plaisier E, Cacoub P (2013) Prognostic factors of survival in patients with non-infectious mixed cryoglobulinaemia vasculitis: data from 242 cases included in the CryoVas survey. Ann Rheum Dis 72:374–380
Mazzaro C, Maso LD, Mauro E et al (2018) Survival and prognostic factors in mixed cryoglobulinemia: data from 246 cases. Diseases 3:6
Cordonnier D, Martin H, Groslambert P et al (1975) Mixed IgG-IgM cryoglobulinemia with glomerulonephritis. Immunochemical, fluorescent and ultrastructural study of kidney and in vitro cryoprecipitate. Am J Med 59:867–872
Frankel AH, Singer DR, Winearls CG, Evans DJ, Rees AJ, Pusey CD (1992) Type II essential mixed cryoglobulinaemia: presentation, treatment and outcome in 13 patients. Q J Med 82:101–124
Terrier B, Marie I, Launay D, Lacraz A, Belenotti P, de Saint-Martin L, Quemeneur T, Huart A, Bonnet F, le Guenno G, Kahn JE, Hinschberger O, Rullier P, Diot E, Lazaro E, Bridoux F, Zénone T, Carrat F, Hermine O, Léger JM, Mariette X, Senet P, Plaisier E, Cacoub P (2014) Predictors of early relapse in patients with non-infectious mixed cryoglobulinemia vasculitis: results from the French nationwide CryoVas survey. Autoimmun Rev 13:630–634
Ghetie D, Mehraban N, Sibley CH (2015) Cold hard facts of cryoglobulinemia: updates on clinical features and treatment advances. Rheum Dis Clin N Am 41:93–108
Rockx MA, Clark WF (2010) Plasma exchange for treating cryoglobulinemia: a descriptive analysis. Transfus Apher Sci 42:247–251
Madore F, Lazarus JM, Brady HR (1996) Therapeutic plasma exchange in renal diseases. J Am Soc Nephrol 7:367–386
Guillevin L, Pagnoux C (2003) Indications of plasma exchanges for systemic vasculitides. Ther Apher Dial 7:155–160
De Vita S, Quartuccio L, Isola M et al (2012) A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum 64:843–853
Ramirez GA, Campochiaro C, Salmaggi C, Pagliula G, D’Aliberti T, Marcatti M, Tresoldi M, Praderio L (2015) Bortezomib in type I cryoglobulinemic vasculitis: are we acting too late? Intern Med 54:1119–1123
Spizzo G, Mitterer M, Gunsilius E (2010) Bortezomib for the treatment of refractory type I cryoglobulinaemia. Br J Haematol 150:235–237
Talamo G, Claxton D, Tricot G, Fink L, Zangari M (2008) Response to bortezomib in refractory type I cryoglobulinemia. Am J Hematol 83:883–884
Besada E, Vik A, Koldingsnes W, Nossent JC (2013) Successful treatment with bortezomib in type I cryoglobulinemic vasculitis patien after rituximab failure: a case report and literature review. Int J Hematol 97:800–803
Bazari H, Mahindra AK, Farkash EA (2014) Case records of the Massachusetts General Hospital. A 61-year-old woman with gastrointestinal symptoms, anemia, and acute kidney injury. N Engl J Med 370:362–373
Hsu JH, Fang YW, Yang AH, Tsai MH (2018) Mixed cryoglobulinemic membranoproliferative glomerulonephritis due to monoclonal gammopathy of undetermined significance: a case report. Medicine (Baltimore) 97(37):e12416
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tocut, M., Rozman, Z., Biro, A. et al. The complexity of an overlap type resistant cryoglobulinemia: a case report and review of the literature. Clin Rheumatol 38, 1257–1262 (2019). https://doi.org/10.1007/s10067-018-04423-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-04423-y