Abstract
Type II mixed cryoglobulinemia without evidence of HCV infection but rather with renal involvement has been occasionally described. The pathogenesis of cryoglobulinemic kidney disease is most likely related to immune complex deposition including cryoglobulins, and cryoaggregation after cold exposure could play a pivotal role in clinical expression of cryoglobulinemia. In these cases, acute kidney injury and proteinuria remain the most frequent clinical expression of a cryoglobulinemic glomerulonephritis. Type II cryoglobulinemia with the laboratory finding of both monoclonal and polyclonal cryoglobulins is the most prevalent bio-humoral pattern among HCV-negative phenotypes with renal involvement, while type III cryoglobulinemia with polyclonal Ig is rare. Histological data in renal biopsies support the hypothesis that regardless of the HCV status cryoglobulinemia vasculitis share the same frequent pathological finding of membranoproliferative glomerulonephritides, but other histological patterns have also been observed in a minority of cases. In HCV-negative mixed cryoglobulinaemia, the paraneoplastic origin of the immune dysfunction should be ruled out and sporadic cases have been reported, while there is no cumulative evidence on the prevalence of these tumour-associated manifestations. Moving from the classification criteria and the etiopathogenesis of mixed cryoglobulinaemia, we provide a comprehensive review of the literature on the appearance of the disease with kidney injury in association with malignancies or autoimmune disorders without HCV coexistence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryoglobulinemia is an immunological disorder defined by the presence of serum cryoglobulins, which are immunoglobulins soluble at 37 °C and precipitating at lower temperatures and the presence of small-to-medium vessel vasculitis [1]. Usually, cryoglobulins show a characteristic pattern, as they undergo reversible precipitation if exposed to temperature below 37 °C and re-dissolve as re-warming is applied [2].
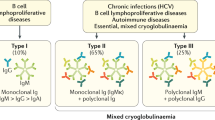
In 1974, Brouet et al. [3] proposed a classification of cryoglobulinemia into three patterns which correlated the immunochemical characteristics of cryoglobulinemias with clinical features of patients, as illustrated in Table 1. Type I cryoglobulinemia accounts for almost 10–15% of cases and include monoclonal immunoglobulins (Igs), mostly IgM, but also IgG (G1 and G2) or rarely IgA, with the latter being usually due to a lymphoproliferative process and almost exclusively found in the context of malignancies, such as multiple myeloma and Waldenström macroglobulinemia. Type I cryoglobulinemia manifests with the clinical signs typical of the underlying lymphoproliferative disease, with cryoglobulinemia being often a serendipitous encounter; nevertheless, vascular occlusion in association with hyperviscosity syndrome and purpuric/dystrophic lesions of the skin (usually affecting the lower limbs) is not uncommon. Type II and type III cryoglobulinemias account for 50–60% and 25–30% of all cases, respectively, and are usually referred to as mixed cryoglobulinemias, because a chemical bond between different cryoglobulins is involved [4]. In fact, type II cryoglobulinemias exhibit monoclonal and polyclonal Igs pattern, while type III only polyclonal Igs. Classically, in mixed cryoglobulinemia, the precipitation phenomenon is due to interactions occurring between involved Igs rather than to specific characteristics of single Igs themselves [5]. Type II mixed cryoglobulinemia is a rare disease characterized by the presence of a blend of polyclonal IgG in association with a monoclonal Ig, typically IgM or IgA. The IgM component of type II cryoglobulinemia usually shows rheumatoid factor (RF) activity. Type III cryoglobulinemia comprises immune complexes containing polyclonal rheumatoid factor (RF), but without a monoclonal component, and usually includes complexes such as IgM–IgG, IgM–IgG–IgA or IgG–IgA–FLCs (free light chains). The improvement in laboratoristic analysis such as immunofixation, immunoblotting, and two-dimensional polyacrylamide gel electrophoresis has raised the diagnostic sensitivity and specificity for Igs identification in patients affected by mixed cryoglobulinemia [6]. According to these developments, cryoglobulinemia microheterogeneity has been recognized and pointed out as an innovative taxonomic element that consists in the presence of two or more oligoclonal bands in mixed cryoglobulinemia [7, 8]. This condition is considered as an intermediate stage between type II and type III cryoglobulinemia, explaining the potential link between the two conditions [8].
Interestingly, in 1997, a novel immunochemical profile was described. It was observed in a patient affected by Gougerot–Sjogren syndrome, which consisted of a biclonal IgM component and a polyclonal IgG [9]. The authors, therefore, suggested a further classification of the type II group of cryoglobulinemia into the following two subgroups: type IIa, characterized by the presence of only one monoclonal component and type IIb, characterized by the presence of several monoclonal components. Thus, these pointed out subtypes of mixed cryoglobulinemia may be included in a wide range between II and III types.
Until 1991, the majority of cases of mixed cryoglobulinemia were considered essential, when several evidences reported that mixed cryoglobulinaemia was associated with chronic hepatitis C virus (HCV) infection [10, 11]. Nowadays, HCV infection is the putative agent of nearly 90% of cases, and the contribution of genetic and environmental factors remains unsolved [12]. HCV has been also considered a risk factor even for the possible switch to an immunological type from another one, such as from type III to type II cryoglobulinemia [13, 14].
Nonetheless, in up to 10% of cryoglobulinemia cases there is no sign of ongoing (with negative viremia) or previous exposure (serum antibodies) to HCV, with these cases being generally associated with a severe course and suboptimal response to conventional therapies [15]. In approximately 5% of patients, connective tissue diseases (primarily systemic lupus erythematous, Sjögren’s syndrome, and systemic sclerosis) or lymphoproliferative disorders (in most cases B cell non-Hodgkin lymphoma) are diagnosed [13, 16]. Cumulatively, cryoglobulinemia without an associated and recognizable disease or viral infection is coined essential mixed cryoglobulinemia (EMC) and accounts for 5% of cases [17] and has been reported mostly in areas where the overall HCV prevalence is almost negligible, such as Northern Europe [17].
The physiological basis of cryoprecipitation
The biochemical basis of cold insolubility remains incompletely understood and maybe not similar in monoclonal and mixed cryoglobulinemia. The solubility of proteins depends upon concentration, hydrophobicity, size and surface charge, as well as the solution temperature, pH and ionic strength [18]. The net charge depends on the hydrophobic amino acids, decreased tyrosine and sialic acid residues in the carbohydrate content. Abnormal Igs exhibit reduced concentrations of sialic acid and galactose in the Fc region, and this may lead to decreased solubility in cold. The aggregation is often the result of electrostatic interactions, which in turn depend on the structural characteristics of cryoglobulinemia, as an altered glycosylation with reduction in sialic acid content [19]. According to this point, Levo assumed that the existence of an impoverishment of sialic acid would make immunogenic Igs favouring cryoprecipitation, especially during persistence and intense immune stimulation [20]. This stimulation would lead to the manifestation of a secretory defect with production of Ig without sialic acid. The absence of sialic acid in the structure of Ig could generate an immune response to the epitope exposure before being masked; the immune complex thus formed would acquire the capacity to precipitate. Furthermore, the author extended his theory by assuming that the liver injury due to cryoglobulins deposits may promote the permanence of Igs with reduced sialic acid by affecting the capacity of hepatocytes to remove them.
Differently, cryoprecipitation in type I cryoglobulinemia recognizes other reasons behind the interaction between cryoglobulins and solvent at low temperatures [21]. In detail, cryoprecipitation in monoclonal cryoglobulins appears to be characterized by particular amino acid sequences that may create a structural change at the level of the quaternary structure of the protein, causing a pathological autoaggregation. This phenomenon starts with a slow phase (lag phase) and the formation of small aggregates of monoclonal Igs followed by rapid and extensive aggregation, due to a combination of weak nonionic and hydrophobic interactions which culminate in the precipitation [22]. A study of IgG cryoglobulinemia structure showed that it can produce amorphous, gelatinous and or crystalline precipitates [23]. Most of the factors that influence the cryoprecipitation of monoclonal cryoglobulinemia are also present in mixed forms, in which, however, the lag phase is typical missing. In fact, mixed cryoglobulinemia precipitation is the consequence of the rapid and progressive increase in the size of IgG–IgM immune complexes at low temperatures in the absence of lag phase. Mixed cryoglobulinemias also show typical biological properties of immune complexes, such as the ability to activate complement [24].
HCV-negative cryoglobulinemia: epidemiology and etiology
Renal involvement has been reported in 30–40% of patients with mixed cryoglobulinemia, regardless of HCV status. In HCV-negative cases, autoimmune disorders, particularly primary Sjögren’s syndrome, and hematologic malignancies, such as lymphoproliferative diseases, represent more than one quarter of cases each, whereas almost half of patients are classified as EMC [12, 25], while the prevalence cases associated with solid tumors is still unknown [26].
In the largest database of HCV-negative cryoglobulinaemia, the CryoVas Survey, that included 242 patients [27] the most common pattern described was type II mixed cryoglobulinaemia in 84% of patients. In this survey, 48% of cases were defined “essential” because no putative causal agent was recognized, 30% of cases were related to connective tissue disorders, mostly to primary Sjogren syndrome, and 22% were related to different grade of B-non-Hodgkin lymphomas. At the univariate analysis male gender, age > 65 years, the presence of renal involvement, GFR < 60 ml/min, hematuria, proteinuria > 1 g/die, lung and gastrointestinal involvement were associated to a worse prognosis. However, the survival rates at 5 and 10 years did not differ significantly from those in patients with HCV-related cryoglobulinaemia: 79% and 65%, respectively, p 0.70. In multivariate analysis using Cox proportional HR model only age > 65 years, male gender, GFR < 60 ml/min, lung and gastrointestinal involvement remained significantly independently associated with mortality at 5 years. According to the result of the multivariate analysis, the authors proposed a CryoVas prognostic score using the previous variables and reported a positive relation with death both in 1 and 5 years. Of note, the causes of HCV-negative cryoglobulinaemia did not affect significantly the survival and this result should be considered in the decision-making process of therapeutical strategies such as chemiotherapy and/or B cell depletion therapy agents. Recently, Galli et al. [28] performed a prospective observational multicentric study of 175 cases of HCV-negative cryoglobulinaemic vasculitis. The related conditions were primary Sjogren’s sindrome (21.1%), systemic erythematosus lupus (10.9%), other autoimmune disorders (10.9%), lymphoproliferative diseases (6.8%), solid tumors (2.3%) and HBsAg positivity (8.6%), whereas 69 patients (39.4%) had essential mixed cryoglobulinaemia. Type II mixed cryoglobulinaemia was diagnosed in 54.9% of cases, while type III in 45.1%. Older age, male gender, Type II mixed cryoglobulinaemia and HbsAg were independently associated with greater mortality at Cox multivariate analysis. According to Terrier’s evidence, also Galli et al. reported similar survival rates and in both studies older age and male gender were related to worse prognosis. It is worth to report that four patients of cases were associated with solid tumors: two follicular thyroid carcinomas, one lung cancer and one liver HBV-related cancer, respectively. All these patients showed mixed cryoglobulinemia (50% type II and 50% type III), and two of these patients exhibited renal abnormalities proteinuria and serum creatinine ≥ 1.5 mg/dl.
The patient affected by the liver cancer was HBC-Ab positive and this result suggested the hypothesis of a potential role of HBV occult infection as a trigger for mixed cryoglobulinemia. In this setting, HBV may promote mixed CG by protracting the antigenic stimulation of VH1-69-expressing B-cells [29].
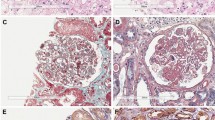
Further, Rullier et al. [30] provided a single-center inventory of cryoglobulinemia-associated solid cancers in their Internal Medicine Unit across a ten-year interval. The study included nine patients with HCV-related hepatocellular carcinoma and only 2 HCV-negative cases, chronologically compatible with a paraneoplastic phenomenon; these patients included a woman with treated breast cancer and type III cryoglobulinemia and a man with metastatic bladder and lung cancer with unknown etiology and undefined cryoglobulinemia. An additional case of HCV-negative cryoglobulinemia in association with solid cancer was reported in a Caucasian patient [31] with gastric adenocarcinoma and immunoelectrophoresis revealed type II cryoglobulinemia. Most recently, our group reported a case of mixed type II cryoglobulinemia associated to a relapse of a prostate cancer with acute kidney injury [26]. Renal biopsy showed a pattern of cryoglobulinemic MPGN at light microscopy (Fig. 1). Unfortunately, the limited patient compliance and obesity did not allow us to get samples also for immunofluorescence and electronic microscopy. To the best of our knowledge, no previous association of HCV-related mixed cryoglobulinemia and prostate cancer has been previously reported, even if an independent coexistence should be always carefully evaluated due to the high prevalence of prostate malignancy. The last evidences highlighted a potential link between solid tumor, HCV-negative cryoglobulinemia and renal involvement. The pathogenesis of cryoglobulinemia in malignances remains largely undefined [30,31,32], and it is still not known if it may be similar to those cases related to autoimmune disorders such as Sjogren’s syndrome leading to abnormal proliferation of abnormal clone B-cells [9]. The stimulus for monoclonal and/or polyclonal cryoglobulin may involve antibodies produced in response to tumor-associated antigens (cross-reacting with autologous antigens), antibodies produced by lymphoid elements in cancer, or systemic immunity alterations induced by the tumor in the presence of different antibodies, as illustrated by Lundberg et al. [32]. However, it is still unclear the “primum movens” as well as if some solid tumors are effectively more associated with paraneoplastic cryoglobulinemia.

Adapted from Spatola et al. [26]
Kidney histology at light microscopy showed a pattern of membranoproliferative glomerulonephritis characterized by variable degrees of mesangial proliferation, intracapillary leukocytes and focal/segmental extracapillary proliferation (crescents). There is also a diffuse basement membrane duplication (double contour) mostly due to leukocytes. Interstitium presents focal fibrosis with tubular atrophy. (PAS stain; ×200).
Of note, Visentini et al. [33] recently reported four cases of late relapses of HCV-positive mixed crioglobulinaemia, occurring in coincidence of infections or cancer. These conditions can both promote immune complexes production, which in turn may stimulate and activate persistent B cell activation with anti-HCV and rheumatoid factor (RF) production, leading to cryoglobulinaemia relapse. In this case series, one patient developed mixed cryoglobulinaemia after 13 years since achieving sustained viral response and was concomitantly diagnosed with unresectable lung carcinoma. In this contest, even years after the clearance of virus, persistent B cell clones producing cryoglobulin-related RF may remain susceptible to stimulation by immune complexes antigens related to the lung cancer. According to this evidence, some cancers may be more associated to paraneoplastic mixed cryoglobulinaemia than others, but this needs to be further investigated with prospective studies.
Renal involvement in HCV-related and HCV-unrelated cryoglobulinaemia
The most common renal histologic finding in HCV-associated type II mixed cryoglobulinemia is generally the membranoproliferative glomerulonephritis (MPGN), usually with nephritic or nephrotic syndrome [34]. The finding of a cryoglobulinemic vasculitis with renal involvement and a histological pattern of MPGN awards specific features on light, immunofluorescence, and electron microscopy [35, 36], as illustrated in Table 2 and Fig. 1. Indeed, light microscopy examination leads to typical histological findings: endocapillary “proliferation” due to an infiltration of monocytes; double-contoured appearance of the glomerular basement membrane, due to the peripheral interposition of monocytes, while mesangial matrix and cell interposition is less evident than in lupus nephritis and in idiopathic MPGN; intraluminal thrombi in the inner side of the glomerular capillary wall and sometimes filling the lumen completely. Lobular glomerulonephritis, with prevalent mesangial cell proliferation (but also monocyte infiltration), mesangial matrix expansion and areas of centrolobular sclerosis may also be present. Classically, on immunofluorescence IgM and IgG are the prevailing Igs in mixed cryoglobulinemia MPGN, suggesting that the deposits are locally trapped or precipitated cryoglobulins. Staining for IgM is generally more intense in comparison with IgG in idiopathic MPGN. On electronic microscopy, electron-dense deposits can be found in subendothelial and mesangial regions, which are characterized by thick-walled microtubular or annular structures, measuring 30 nm in diameter. Less frequently observed kidney lesions include MPGN without cryoglobulins and membranous nephropathy, focal segmental glomerulosclerosis, fibrillary or immunotactoid glomerulopathies, and thrombotic microangiopathy [37]. In addition, vasculitis and interstitial nephritis have been associated with HCV. The natural history of these HCV-associated nephropathies is characterized by remission and relapsing phases according to the HCV infection; however, the long-term outcome is not well known.
In non-HCV cryoglobulinemia, including EMC, the evidence of renal manifestations is mostly confined to case reports [26, 38] and surveys [17, 34] usually reported in areas, such as Northern Europe, where the overall HCV prevalence is almost negligible. In a multicenter French study comprised of 20 HCV-negative patients with renal manifestations, 50% of the patients had essential cryoglobulinemia [34]. In this study, renal involvement was characterized by microscopic hematuria in all patients, nephrotic range proteinuria in 75% of patients, hypertension in 80% of patients, and renal failure in 85% of patients (mean glomerular filtration rate, 46 ml/min per 1.73 m). Cryoglobulinemic MPGN type I was the only histologic pattern observed. Mild to moderate mesangial hypercellularity, moderate mesangial matrix expansion, and few to several double contours of the glomerular capillary walls were observed. The glomeruli were infiltrated by a large number of monocytes and few polymorph nuclear cells. “Protein thrombi” were detected in 82% of patients. Cryoglobulinemia was classified as type II MC in all patients, characterized by monoclonal IgM k and polyclonal IgG. According to this pathological achievements, in a recent survey conducted by Zaidan et al. [39] involving 80 HCV-negative mixed CG patients with renal presentation, type II mixed cryoglobulinemia was the most frequent syndrome (97.4%) with a monoclonal IgM k component in 81% of cases and cryoglobulinemic MPGN was the main histological pattern, seen in 92.5% of cases. Mesangial hypercellularity, capillary wall remodeling with double contours, and focal to diffuse endocapillary leukocyte infiltration characterized this entity at pathology. Intraluminal thrombi were also observed in 47.8% of biopsy specimens, mostly in patients with primary Sjögren’s syndrome. Other less frequent lesions included mesangial proliferative glomerulonephritis (7.5%), characterized by predominant mesangial hypercellularity without endocapillary proliferation and with no or mild capillary wall abnormalities; lobular accentuation of the glomerular tuft (14.9%) and crescentic extracapillary proliferation (13.4%). Interstitial fibrosis and tubular atrophy were reported in 61.2% of cases. Acute tubular necrosis was seen in 31.3% but was moderate-to-severe in 10% of cases. Inflammatory renal interstitial infiltration was observed in 56.7% of case.
According to previous evidence and to the best of our knowledge both HCV-related and HCV-unrelated mixed cryoglobulinemias seem to share the same frequent appearance of a cryoglobulinemic MPGN, due to the deposits of monoclonal and/or polyclonal cryoaggregates. The formation of cryoaggregates upon exposure to cold may be the triggering factor for vasculitis and their clinical expression in organs that are quite distant from the site of exposure to cold as kidneys. Alterations of chloride [40] and calcium [41] concentration in the renal interstitium have been hypothesized as potential risk factors for cryoglobulin aggregation.
Conclusions
HCV-negative mixed cryoglobulinemic glomerulonephritis, as a potential paraneoplastic event, remains a controversial topic for research and clinical management. A multidisciplinary and extensive clinical appraisal should be carried out in the presence of anti-HCV-negative mixed cryoglobulinemia and acute kidney injury, primarily to rule out a putative immunoproliferative disease and/or occult solid tumor. In fact, a correct therapeutic approach to cryoglobulinemia and its related disease as crioglobulinemic glomerulonephritis is conditioned by the coexistence of malignancy and its prognosis. The dysregulation of immunological system leading to the onset of cryoglobulinemia in case of autoimmune disease and/or malignancies needs further comprehension of the underlying mechanisms and additional clinical evidences in order to better define the epidemiology and the clinical spectrum of this condition.
References
Desbois AC, et al. Cryoglobulinemia vasculitis: how to handle. Curr Opin Rheumatol. 2017;29(4):343–7.
Sargur R, White P, Egner W. Cryoglobulin evaluation: best practice? Ann Clin Biochem. 2010;47(Pt 1):8–16.
Brouet JC, et al. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57(5):775–88.
Gulli F, et al. Cryoglobulin test and cryoglobulinemia hepatitis C-virus related. mediterr J Hematol Infect Dis. 2017;9(1):e2017007.
Trendelenburg M, Schifferli JA. Cryoglobulins in chronic hepatitis C virus infection. Clin Exp Immunol. 2003;133(2):153–5.
Motyckova G, Murali M. Laboratory testing for cryoglobulins. Am J Hematol. 2011;86(6):500–2.
Musset L, et al. Characterization of cryoglobulins by immunoblotting. Clin Chem. 1992;38(6):798–802.
Tissot JD, et al. Clinical implications of the types of cryoglobulins determined by two-dimensional polyacrylamide gel electrophoresis. Haematologica. 1998;83(8):693–700.
Pontet F, et al. Biclonal immunoglobulin M dysglobulinaemia: evolving aspects in a case of primary Sjogren’s syndrome. Eur J Clin Chem Clin Biochem. 1997;35(4):287–90.
Casato M, et al. Cryoglobulinaemia and hepatitis C virus. Lancet. 1991;337(8748):1047–8.
Durand JM, et al. Cutaneous vasculitis and cryoglobulinaemia type II associated with hepatitis C virus infection. Lancet. 1991;337(8739):499–500.
Cacoub P, et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment virus C. Arthritis Rheum. 1999;42(10):2204–12.
Trejo O, et al. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore). 2001;80(4):252–62.
Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25.
Galli M, Sollima S, Monti G. HCV-negative mixed cryoglobulinemia: facts and fancies. HCV infection and cryoglobulinemia. Milan: Springer; 2012. p. 239–43.
Saadoun D, et al. Increased risks of lymphoma and death among patients with non-hepatitis C virus-related mixed cryoglobulinemia. Arch Intern Med. 2006;166(19):2101–8.
Tervaert JW, Van Paassen P, Damoiseaux J. Type II cryoglobulinemia is not associated with hepatitis C infection: the Dutch experience. Ann N Y Acad Sci. 2007;1107:251.
Andersen BR, et al. Biological and physical properties of a human m-cryoglobulin and its monomer subunit. Clin Exp Immunol. 1971;9(6):795–807.
Kallemuchikkal U, Gorevic PD. Evaluation of cryoglobulins. Arch Pathol Lab Med. 1999;123(2):119–25.
Levo Y. Nature of cryoglobulinaemia. Lancet. 1980;1(8163):285–7.
Middaugh CR, et al. Molecular basis of the temperature dependent insolubility of cryoglobulins—IV. Structural studies of the IgM monoclonal cryoglobulin. Immunochemistry. 1987;15:171–87.
Lawson EQ, et al. Kinetics of the precipitation of cryoimmunoglobulins. Mol Immunol. 1987;24(9):897–905.
Podell DN, et al. Characterization of monoclonal IgG cryoglobulins: fine-structural and morphological analysis. Blood. 1987;69(2):677–81.
Sansonno D, Dammacco F. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect Dis. 2005;5(4):227–36.
Terrier B, et al. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood. 2012;119(25):5996–6004.
Spatola L, et al. HCV-negative mixed cryoglobulinemic glomeruolonephritis and solid malignancy: a case report and review of the literature. Nephro-Urol Mon. 2017. https://doi.org/10.5812/numonthly.58142.
Terrier B, et al. Prognostic factors of survival in patients with non-infectious mixed cryoglobulinaemia vasculitis: data from 242 cases included in the CryoVas survey. Ann Rheum Dis. 2013;72(3):374–80. https://doi.org/10.1136/annrheumdis-2012-201405.
Galli M, et al. HCV-unrelated cryoglobulinaemic vasculitis: the results of a prospective observational study by the Italian Group for the Study of Cryoglobulinaemias (GISC). Clin Exp Rheumatol. 2017;35 Suppl 103(1):67–76.
Visentini M, et al. Hepatitis B virus causes mixed cryoglobulinaemia by driving clonal expansion of innate B-cells producing a VH1-69-encoded antibody. Clin Exp Rheumatol. 2016;34(3 Suppl 97):S28–32.
Rullier P, Le Quellec A, Cognot C. Cryoglobulins not HCV-related and solid tumors: retrospective analysis from a series of 493 patients. Eur J Intern Med. 2009;20(8):e158.
Milas-Ahic J, et al. Cryoglobulinemicvasculitis as a manifestation of paraneoplastic syndrome—a case report. Reumatizam. 2015;62(1):27–30.
Lundberg WB, Mitchell MS. Transient warm autoimmune hemolytic anemia and cryoglobulinemia associated with seminoma. Yale J Biol Med. 1977;50(4):419–27.
Visentini M, et al. Late relapses of hepatitis C virus-cured mixed cryoglobulinaemia associated with infection or cancer. Rheumatology (Oxford). 2018. https://doi.org/10.1093/rheumatology/key157.
Matignon M, et al. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Medicine (Baltimore). 2009;88(6):341–8.
D’Amico G, et al. Renal involvement in essential mixed cryoglobulinemia. Kidney Int. 1989;35(4):1004–14.
El-Serag HB, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36(6):1439–45.
Fabrizi F, et al. Kidney and liver involvement in cryoglobulinemia. Semin Nephrol. 2002;22(4):309–18.
Okura T, et al. Case of membranoproliferative glomerulonephritis due to essential cryoglobulinemia without hepatitis C virus infection. Geriatr Gerontol Int. 2009;9(1):92–6.
Zaidan M, et al. Spectrum and prognosis of noninfectious renal mixed cryoglobulinemic GN. J Am Soc Nephrol. 2016;27(4):1213–24.
Di Stasio E, et al. Cl-regulates cryoglobulin structure: a new hypothesis for the physiopathological mechanism of temperature non-dependent cryoprecipitation. Clin Chem Lab Med. 2004;42(6):614–20.
Qi M, Steiger G, Schifferli JA. A calcium-dependent cryoglobulin IgM kappa/polyclonal IgG. J Immunol. 1992;149(7):2345–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights statement and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors and informed consent is not a standard required.
Rights and permissions
About this article
Cite this article
Spatola, L., Generali, E., Angelini, C. et al. HCV-negative mixed cryoglobulinemia and kidney involvement: in-depth review on physiopathological and histological bases. Clin Exp Med 18, 465–471 (2018). https://doi.org/10.1007/s10238-018-0514-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-018-0514-5