Abstract
Psoriatic arthritis (PsA) is an inflammatory rheumatic disorder occurring in patients with psoriasis. Several studies have shown an association between Psa and traditional atherosclerotic risk factors. We evaluated the relationship between small dense low-density lipoproteins particles (sd-LDL) a risk marker for atherosclerosis, sub-clinical atherosclerosis and PsA in a group of 50 patients with PsA and in 100 controls. Cholesterol, high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides, insulin, homeostasis model assessment (HOMA), Apo B, and sd-LDL have been measured. LDL particle separation was performed and seven LDL subfractions were obtained, LDL score (percentage of sd-LDL) and mean LDL particle size were calculated. PsA patients and control group differ significantly (p < 0.001) in triglycerides values (119.3 ± 52.0 vs 90.7 ± 40.7 mg/dL), Apo B (1.1 ± 0.2 vs 0.9 ± 0.1 g/L), insulin (8.9 ± 4.9 vs 5.8 ± 3.2 mU/L), HOMA (2.2 ± 1.7 vs 1.3 ± 0.8), BMI (27.7 ± 3.3 vs 25.8 ± 3.8). LDL score is significantly higher in PsA as compared to control (9.0 ± 10.7 vs 2.9 ± 4.7 mg/dL); and mean LDL size is significantly lower in PsA than control (268.1 ± 4.6 vs 271.2 ± 2.7 Å). These differences were confirmed when stratifying PsA patients for treatment and for disease activity. LDL score and LDL diameter significantly were correlated with the carotid IMT in patients with PsA. These findings show a novel relationship between LDL score and mean LDL size with PsA diagnosis and with sub-clinical atherosclerosis. Sd-LDL gives potentially useful information in the risk assessment for atherosclerotic disease in PsA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is a chronic arthropathy associated with psoriasis, marked by an axial and/or peripheral joint involvement [1]. In addition to joint manifestations, subjects with PsA exhibit an abnormally high cardiovascular (CV) risk and, in turn, of the metabolic syndrome (MetS), of its major vascular risk factors (VRFs) [2]. Accordingly, as compared with healthy controls, PsA patients exhibit a higher than normal platelet reactivity [3], and a higher than normal prevalence of hepatic steatosis (HS) [4, 5] and of carotid plaques (CPs) [6, 7]. All these conditions are well-known markers of atherosclerosis and of CV risk [8–10].
Several large, prospective studies in general populations, using gradient gel electrophoresis to determine LDL particle size, confirmed the relationship of small dense LDL (sd-LDL) with coronary events [11, 12]. Moreover, metabolic syndrome is usually accompanied by increased prevalence of sd-LDL [13–15]. Although some studies examined the lipid profile in PsA patients [16], no data are currently available on a possible relationship of high levels of sd-LDL with PsA. The aim of the present study was to evaluate the concentration of sd-LDL in PsA patients and to assess the relationship with carotid intima–media thickness.
Methods
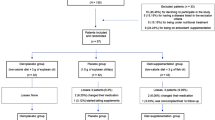
During a 3-month period (January 2010–April 2010), consecutive patients with a diagnosis of PsA (according to CASPAR criteria) [1] referred to the Regional Reference Center for the treatment of spondyloarthropathies of the Federico II University of Naples were evaluated for the enrollment in this study. For all enrolled subjects, exclusion criteria were: lack of informed consent signature, age <18 years, malignancy, hematologic diseases, autoimmune diseases other than PsA, unstable medical conditions, ongoing pregnancy, history of venous and/or arterial thrombosis, known alterations of lipid metabolism and active treatment with statins or other drugs modifying lipid profile. After approval of the study by the local Ethics Committee and after informed consent signature, data about age, gender, height, weight, disease duration, disease activity, previous and/or current treatments, and vascular risk factors were collected from all patients as previously described [17]. All PsA subjects were classified into different clinical subsets according to Moll and Wright criteria [18]. Briefly, PsA patients were classified as having axial disease if they had at least grade 2 unilateral sacroiliitis in the presence of a combination of inflammatory back pain plus back stiffness. The PsA patients were classified as pure axial if they had no peripheral joint involvement, mixed if they had both peripheral joint arthritis and axial disease, or as having only peripheral joint involvement. The rare mutilans form was diagnosed in the presence of distal interphalangeal joint bone resorption (osteolysis), “pencil-in-cup” radiographic finding or telescoping motion of the digit.
At enrollment, all PsA subjects underwent a complete clinical rheumatologic and laboratory evaluation that included: tender joint count (TJC, n = 78), swollen joint count (SJC, n = 76), tender entheseal count (according to the Maastricht Ankylosing Spondylitis Enthesitis Score), psoriasis area severity index (PASI), health assessment questionnaire (HAQ), visual analog scale for pain (VAS), patient global disease activity VAS score, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). PsA subjects were classified as in minimal disease activity (MDA) when fulfilling five or more of the following seven outcome measures at T1: TJC ≤1, SJC ≤1, PASI ≤1 or body surface area ≤3, VAS for pain ≤15, patient global disease activity VAS score of ≤20, HAQ ≤0.5, and tender entheseal points ≤1 [19]. Otherwise, they were considered in active disease (AD).
According to the NCEP criteria [20], abdominal obesity was defined as a waist circumference ≥102 cm for men and ≥88 cm for women; hypertriglyceridemia, as triglycerides (TG) levels ≥150 mg/dL; hypercholesterolemia as a total cholesterol ≥200 mg/dL with high-density lipoprotein cholesterol (HDL-C) <40 mg/dL for men and <50 mg/dL for women; hypertension as a blood pressure ≥130 and/or 85 mmHg; impaired fasting glucose (IFG) as a fasting glucose ≥100 mg/dL. Patients were defined as having the MetS if three or more of these VRFs were present.
In addition, all patients underwent a carotid artery ultrasound examination for the assessment of the intima–media thickness (IMT). After 5-min rest in supine position, the subjects underwent a bilateral carotid ultrasonography (US) with a 7.5- to 12-MHz linear-array transducer and a duplex scanner (ESAOTE MyLab 25Gold, Genoa, Italy). The ultrasound evaluation was performed longitudinally and transversally by using gray-scale and color-Doppler US imaging. The scan protocol requires the full-length visualization of the near and far wall of the right and left common carotid artery (CCA) and of the bulb in three different projections (anterior, lateral, and posterior). The intima–media thickness (IMT) was measured in each of the three projections in CCA and bulb and, the presence of CPs was defined for IMT ≥1.3 mm. All the examinations were performed by the same operator blind as to the presence of PsA. Control subjects without PsA (or other rheumatic diseases), comparable with PsA cases for age and gender, have been selected from the registries of three different General Practitioners in the Naples province. Subjects comparable for age and gender with PsA patients were enrolled as controls groups using the same exclusion criteria as cases. Major clinical and demographic characteristics of these subjects have been recorded according to same criteria used for the PsA population.
For both cases and controls, blood was drawn by puncture of antecubital vein after a 12-h fast and withdrawal for at least 5 weeks from any lipid-lowering treatment.
Total cholesterol and TG concentrations were measured using standard enzymatic methods [21, 22]. HDL-C was measured after the precipitation of very-low-density lipoproteins (VLDL) and low-density-lipoproteins (LDL-C) with phosphotungstic acid [23], and LDL-C was calculated according to the Friedewald formula. Fasting glucose levels were enzymatically determined by the peroxidase method. Fasting insulin levels were determined by enzyme immunoassay (Ultrasensitive ELISA, Mercodia, Uppsala, Sweden). The homeostasis model assessment (HOMA) index was used to estimate insulin resistance and calculated as fasting serum insulin (in microunits per milliliter) × fasting serum glucose (in millimolar)/22.5 [24]. Apolipoprotein B (apo B) was measured by turbidimetric assay with an automated method (ABX Pentra 400, Horiba Medical, Italy).
LDL particle size determination
LDL particles separation was performed by Lipoprint System (Quantimetrix Inc., Redondo Beach, CA USA). This method is based on electrophoresis of lipid stained serum (Sudan black) in non-denaturing gel gradient of polyacrylamide [13]. The proportion of sd-LDL particles to the whole LDL area was calculated in our sample (LDL score). The diameter of the LDL particles at the cut-off point separating subfractions 1–2 from subfractions 3–7 was 251 Å.
The present study protocol has been approved by the Federico II University Local Ethic Committee.
Statistical analysis
Statistical analysis was performed with the SPSS 16 system (SPSS Inc., Chicago, IL, USA). Continuous data were expressed as mean ± SD or as median values; categorical variables were expressed as percent. The T test was performed to compare continuous variables; the chi-square test was employed to analyze categorical data. When the minimum expected value was <5, the Fisher’s exact test was used. Continuous variables were described as mean and standard deviation. Comparisons between Control subjects and PsA patients were performed using Student’s t test for independent samples and non parametric test, the Mann–Whitney U test, for variables (triglycerides, HOMA, hypertension, LDL score, median LDL particle diameter, metabolic syndrome diagnosis) not normally distributed. Correlation was assessed using the Pearson linear correlation coefficient (r). Logistic regression analysis at univariate and multivariate level was used to test the association between the LDL score (dependent discrete variable: above or below the upper 75th percentile cut points of control group) with age, gender, PsA duration, metabolic syndrome diagnosis (independent continuous variables). Odds ratio (OR) for the presence of LDL score were calculated by unconditional logistic regression and 95 % confidence intervals (CI) of the odds ratio were computed. All the results are presented as two-tailed values with statistical significance if p values <0.05.
Results
After excluding those already under treatment with statins and those with a known diagnosis of alterations in lipid metabolism (n = 33), 50 PsA subjects and 100 matched controls were enrolled in this study. Among the 50 PsA patients, 23 were receiving DMARDs and 27 were under TNF-α blockers. None of PsA patients were receiving combined treatment with DMARDs + TNF-α blockers. Minimal disease activity was found in 16 PsA patients (seven under DMARDs and nine under TNF-α blockers).
Major clinical, biochemical, and demographic characteristics of the two study populations are reported in Table 1. Triglycerides, Apo B, BMI, HOMA, and the prevalence of MS and carotid plaques were significantly lower in the control group as compared to PsA patients, 21 (42.00 %) out of 50 PsA patients had axial and 28 (58.00 %) had peripheral subset, among these 15 (30.00 %) had oligoarticular, and 14 (28.00 %) polyarticular involvement.
Median LDL score was significantly higher in patient with PsA as compared to control group (median 5.6 vs. 1.4, p < 0.001; Fig. 1). Median LDL particle diameter was lower in PsA patients as compared to control group (median 269 vs. 272, p < 0.001; Fig. 2).
LDL score values above the 75th and 90th percentiles of the values of the control group (4.0 and 9.6, respectively) were found in 66 and 28 %, respectively, of PsA patients.
All results were confirmed when stratifying analyses according to the type of treatment [disease-modifying anti-rheumatic drugs (DMARDs) vs TNF-α blockers] or according to disease activity [minimal disease activity (MDA) vs active disease (AD)]. In detail, as showed in Fig. 3, LDL score and LDL diameter were significantly higher both in PsA patient receiving DMARDs and in those under TNF-α blockers as compared with controls. Interestingly, LDL score and LDL diameter resulted increased in PsA compared with controls, regardless the disease activity status (Fig. 4).
LDL score and LDL diameter in PsA subjects, stratified for type of treatment, and controls. PsA: psoriatic arthritis; DMARDs: traditional disease-modifying anti-rheumatic drugs; TNF-α blockers: tumor necrosis factor-α blockers. Circumflex accent (^), medians (interquartile ranges). Asterisk (*), Mann–Whitney U
As reported in Table 2, univariate logistic analysis shows a positive and significant relationship of the LDL score (below or above the 75th percentiles of the control values), with Metabolic Syndrome Diagnosis and PsA diagnosis (OR 9.68; 95 % CI 3.03–30.86; p < 0.001 for MS diagnosis; OR 4.14; 95 % CI 2.01–8.52; p < 0.001 for PsA diagnosis). Negative and significant relationship between LDL score and gender was found (OR 0.40; 95 % CI 0.20–0.79; p = 0.009 for gender).
Multivariate logistic analysis between LDL score, PsA diagnosis, MS diagnosis, and other covariates is reported in Table 3. After controlling for age and gender LDL score (below or above the 75th percentiles of the control values) is independently related to PsA diagnosis (OR 3.94; 95 % CI 1.86–8.32; p < 0.001 for PsA diagnosis). This relation was confirmed after controlling for age, gender, PsA diagnosis and MS diagnosis (OR 3.22; 95 % CI 1.47–7.03; p = 0.003 for PsA diagnosis).
Carotid atherosclerosis ultrasound assessment
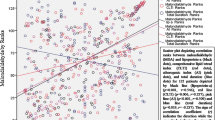
The carotid ultrasound examination showed that carotid plaques were reported by 38 % of PsA patients and 14 % of controls (OR 3.93, 95% CI 1.61–9.60, p = 0.003; Table 1). Moreover, as showed in Fig. 5, LDL score and LDL diameter significantly and directly correlated with the carotid IMT in patients with PsA.
Discussion
The present study shows a novel relationship between sd-LDL and PsA diagnosis and sub-clinical atherosclerosis. This relationship is independent of the presence of MS. Almost 26 % of studied PsA patients were diagnosed as having MS; recent experimental evidence have demonstrated that sd-LDL were highly prevalent in both men and women with MS [13, 15]. Such background prompted us to adjust the relationship between sd-LDL and PsA status for the presence of MS. The independent role of PsA status as predictors of elevated sd-LDL was confirmed after this adjustment. A number of studies [25–27] have found that sd-LDL are more easily oxidized, higher affinity for extracellular matrix, reduced binding to LDL receptor, and a longer residence time in the circulation compared to large LDL. These characteristics are linked to the increased cardiovascular risk [11, 12]. PsA is frequently associated with obesity, diabetes, dyslipidemia, hypertension, early atherosclerosis, and increased cardiovascular morbidity and mortality [28]. Patients with PsA without cardiovascular risk factors or clinically evident cardiovascular disease have a increased carotid artery IMT compared with matched controls, this data suggesting that PsA is associated to accelerated atherosclerosis [6, 29]. Another study conducted by Raychaudhuri [30] has reported high prevalence of the metabolic syndrome in patients with PsA.
Only one study [31] was conducted to investigate the presence of small dense LDL in patients with PsA but LDL size analysis was performed by a different method (ultracentrifugation) and the sample size was only of 13 patients. In this study, the authors carried out that patients with PsA have raised levels of subfraction LDL3 and low levels of HDL as compared to controls group, no statistical differences were reported in TG values and Apo B values. The impact of MS was not reported.
Our study indicates that patients with PsA have an increased concentration of sd-LDL independently of the presence of metabolic syndrome; this data suggests a possible link between PsA and the development of atherosclerosis mediated by sd-LDL. LDL size measurement gives potentially useful information in the risk assessment for atherosclerotic disease in these patients and could be useful in identifying a subsample of high-risk patients, with prominent lipoprotein abnormality, among those with the PsA diagnosis, deserving lipid-lowering intervention. This hypothesis has, however, to be further tested with prospective epidemiological studies.
References
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR study group (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 54:2665–2673
Di Minno MN, Iervolino S, Lupoli R, Russolillo A, Coppola A, Peluso R, Scarpa R, Di Minno G (2012) Cardiovascular risk in rheumatic patients: the link between inflammation and atherothrombosis. Semin Thromb Hemost 38:497–505
Di Minno MN, Iervolino S, Peluso R, Scarpa R, Di Minno G (2012) Platelet reactivity and disease activity in subjects with psoriatic arthritis. J Rheumatol 39:334–6
Seitz M, Reichenbach S, Möller B, Zwahlen M, Villiger PM, Dufour JF (2010) Hepatoprotective effect of tumour necrosis factor alpha blockade in psoriatic arthritis: a cross-sectional study. Ann Rheum Dis 69:1148–50
Di Minno MN, Iervolino S, Peluso R, Russolillo A, Lupoli R, Scarpa R, Di Minno G, Tarantino G, CaRRDS Study Group (2012) Hepatic steatosis and disease activity in subjects with psoriatic arthritis receiving tumor necrosis factor-α blockers. J Rheumatol 39:1042–6
Gonzalez-Juanatey C, Llorca J, Amigo-Diaz E, Dierssen T, Martin J, Gonzalez-Gay MA (2007) High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum 57:1074–1080
Di Minno MN, Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, Tremoli E (2015) Cardiovascular risk markers in patients with psoriatic arthritis: a meta-analysis of literature studies. Ann Med 47:346–53
Targher G, Day CP, Bonora E (2010) Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 363:1341–50
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M (2007) Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115:459–467
Di Minno MN, Peluso R, Iervolino S, Lupoli R, Russolillo A, Tarantino G, Scarpa R (2012) Hepatic steatosis, carotid plaques and achieving MDA in psoriatic arthritis patients starting TNF-α blockers treatment: a prospective study. Arthritis Res Ther 14:R211
St-Pierre AC, Cantin B, Dagenais GR, Mauriege P, Bernard PM, Despres JP et al (2005) Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol 25:553–9
Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ et al (1997) Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Circulation 95:69–75
Gentile M, Panico S, Jossa F, Mattiello A, Ubaldi S, Marotta G et al (2008) Small dense LDL particles and metabolic syndrome in a sample of middle-aged women. Findings from Progetto Atena. Clin Chim Acta 388:179–83
Rizzo M, Berneis K (2007) Small, dense low density lipoproteins and metabolic syndrome. Diabetes Metab Res Rev 23:14–20
Hulthe J, Bokemark L, Wikstrand J, Fagerberg B (2000) The metabolic syndrome, LDL particle size, and atherosclerosis: the Atherosclerosis and Insulin Resistance (AIR) study. Arterioscler Thromb Vasc Biol 20:2140–7
Di Minno MN, Ambrosino P, Peluso R, Di Minno A, Lupoli R, Dentali F, CaRRDs Study Group (2014) Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-α blockers: a meta-analysis of prospective studies. Ann Med 46:73–83
Di Minno MND, Iervolino S, Peluso R, Scarpa R, Di Minno G (2011) Carotid intima-media thickness in psoriatic arthritis. Differences between tumor necrosis factor-alpha-blockers and traditional disease modifying anti-rheumatic drugs. Arterioscler Thromb Vasc Biol 31:705–712
Moll JM, Wright V (1973) Psoriatic arthritis. Semin Arthritis Rheum 3:55–78
Coates LC, Helliwell PS (2010) Validation of minimal disease activity criteria for psoriatic arthritis using interventional trial data. Arthritis Care Res (Hoboken) 62:965–9
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–52
Siedel J, Schlumberger H, Klose S, Ziegenhorn J, Wahlefeld AW (1981) Improved reagent for enzymatic determination of serum cholesterol. J Clin Chem Biochem 19:838–9
Wahlefeld AW (1974) Triglyceride determination after enzymatic hydrolysis. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol. 4, 2nd edn. Academic, New York, p 1831
Lopes-Virella MF, Stone P, Ellis S, Colwell JA (1977) Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem 23:882–4
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–9
Packard CJ (2006) Small dense low-density lipoprotein and its role as an independent predictor of cardiovascular disease. Curr Opin Lipidol 17:412–7
Gardner CD, Fortmann SP, Krauss RM (1996) Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA 276:875–81
Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH (1996) A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA 276:882–8
Tam LS, Tomlinson B, Chu TT, Li M, Leung YY, Kwok LW, Li TK, Yu T, Zhu YE, Wong KC, Kun EW, Li EK (2008) Cardiovascular risk profile of patients with psoriatic arthritis compared to controls—the role of inflammation. Rheumatology (Oxford) 47:718–23
Kimhi O, Caspi D, Bornstein NM, Maharshak N, Gur A, Arbel Y, Comaneshter D, Paran D, Wigler I, Levartovsky D, Berliner S, Elkayam O (2007) Prevalence and risk factors of atherosclerosis in patients with psoriatic arthritis. Semin Arthritis Rheum 36:203–9
Raychaudhuri SK, Chatterjee S, Nguyen C, Kaur M, Jialal I, Raychaudhuri SP (2010) Increased prevalence of the metabolic syndrome in patients with psoriatic arthritis. Metab Syndr Relat Disord 8:331–4
Jones SM, Harris CP, Lloyd J, Stirling CA, Reckless JP, McHugh NJ (2000) Lipoproteins and their subfractions in psoriatic arthritis: identification of an atherogenic profile with active joint disease. Ann Rheum Dis 59:904–9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Marco Gentile and Rosario Peluso contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gentile, M., Peluso, R., Di Minno, M.N.D. et al. Association between small dense LDL and sub-clinical atherosclerosis in patients with psoriatic arthritis. Clin Rheumatol 35, 2023–2029 (2016). https://doi.org/10.1007/s10067-016-3344-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3344-4