Abstract
The aim of this study was to determine whether the functional major histocompatibility complex II transactivator (MHC2TA) −168 A/G polymorphism is associated with susceptibility to rheumatoid arthritis (RA). A meta-analysis was conducted to estimate the association between the MHC2TA−168 A/G polymorphism and RA. A total of 15 comparative studies, which included 14,158 patients and 13,642 controls, were included in the meta-analysis. Based on the meta-analysis, there was no association between RA and the MHC2TA −168 G allele in the study subjects (OR = 1.046, 95 % CI = 0.987–1.108, p = 0.130) or Caucasians (OR = 1.027, 95 % CI = 0.986–1.070, p = 0.193). However, the country-specific meta-analysis revealed an association between the MHC2TA −168 G allele and RA in the Swedish population (OR = 1.131, 95 % CI = 1.023–1.250, p = 0.016). A direct comparison between rheumatoid factor (RF)-positive and RF-negative patients revealed that the frequency of the G allele was significantly lower in RF-positive patients (OR = 0.783, 95 % CI = 0.628–0.975, p = 0.029) than in RF-negative patients. This meta-analysis demonstrated that the MHC2TA −168 A/G polymorphism is not associated with susceptibility to RA in Caucasians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease predominantly involving the synovial joints and affects up to 1 % of adults worldwide. Major histocompatibility complex (MHC) class II molecules have been shown to be strongly associated with RA, but family studies suggest that this association accounts for only one-third of the total genetic susceptibility [1]. Furthermore, significant familial aggregation, convincing demonstrations of multiple genetic linkages, and its genetic associations demonstrate that the etiology of RA is genetic in nature [2].
MHC molecules play key roles in the induction and regulation of immune responses as well as the maintenance of self-tolerance and affect the breakdown of tolerance in autoimmune diseases [3]. MHC class II molecules are cell surface glycoproteins and present peptides to the antigen receptors of CD4+ T cells. MHC class II-mediated peptide presentation is critical in T cell-dependent immunity and in inflammatory responses [3]. The MHC class II transactivator (MHC2TA) is a transcription factor required for the expression of MHC class II molecules, and MHC2TA is mapped to chromosome 16p13, which is a linkage locus of RA based on genome-wide linkage scans [2, 4]. The MHC2TA gene regulates MHC class II expression. The MHC2TA −168 A/G polymorphism (rs3087456) is located in the third promoter, which determines the expression of antigen-presenting cells and is associated with MHC2TA expression [5]. The G allele at this locus is known to be associated with decreased expression of the gene [5]. Thus, MHC2TA is a candidate gene for RA, considering chromosomal location and the function of the gene.
However, previous studies of the association between the MHC2TA −168 A/G polymorphism and RA have produced inconsistent results [5–16], which may reflect inadequate statistical power, racial/ethnic differences in the frequencies of alleles, or publication bias. To overcome the limitations of individual studies, resolve inconsistencies, and reduce the likelihood that random errors were responsible for false-positive or false-negative associations, we performed a meta-analysis [17–19]. The aim of this study was to determine whether the MHC2TA −168 A/G polymorphism contributes to susceptibility to RA in various populations.
Methods
Identification of eligible studies and data extraction
We performed a search for studies that examined the association between the MHC2TA −168 A/G polymorphism and RA. The literature was searched using the MEDLINE and EMBASE citation databases to identify available articles in which the MHC2TA −168 A/G polymorphism was analyzed in RA patients. Combinations of keywords such as “MHC2TA,” “polymorphism,” “rheumatoid arthritis,” and “RA” were entered as Medical Subject Heading (MeSH) components and as text words. References in the identified studies were also investigated to identify additional studies not indexed by MEDLINE or EMBASE. No language or country restrictions were applied. Studies were included if: (1) they were case–control studies, (2) the data were original (independence among studies), (3) they provided enough data to calculate the odds ratios (OR), and (4) the distribution of the MHC2TA −168 A/G polymorphism in normal controls was consistent with the Hardy–Weinberg equilibrium. We excluded the following: (1) studies that contained overlapping data, (2) studies in which the number of null and wild-type genotypes could not be ascertained, and (3) studies in which family members were included, as these analyses are based on linkage considerations. The following information was extracted from each study: author, year of publication, ethnicity of the study population, demographics, the number of cases and controls, and genotype and allele frequency for the MHC2TA promoter −168 A/G polymorphism. The allele frequencies were calculated from the corresponding genotype distributions.
Evaluations of statistical associations
Meta-analyses were performed using: (1) allelic contrast, (2) recessive models, (3) dominant models, and (4) homozygote contrast. Point estimates of risks, ORs, and 95 % confidence intervals (CIs) were estimated for each study. Cochran’s Q statistic was also used to assess within- and between-study variation and heterogeneity. This heterogeneity test assesses the null hypothesis that all studies evaluated the same effect. The effect of heterogeneity was also quantified using I 2, which ranges from 0 to100 % and represents the proportion of between-study variability attributable to heterogeneity rather than chance [20]. I 2 values of 25, 50,, and 75 % were nominally considered low, moderate, and high estimates, respectively. When a significant Q statistic (p < 0.10) indicated heterogeneity across studies, the random effects model was used for meta-analysis, and if heterogeneity across studies was not indicated, the fixed effects model was used. The fixed effects model assumes that a genetic factor has a similar effect on RA susceptibility across all studies investigated and that the observed variation among studies is caused by chance alone [21]. On the other hand, the random effects model assumes that studies show substantial diversity and assesses both within-study sampling errors and between-study variances [22]. When study groups are homogeneous, the two models are similar. However, if this is not the case, the random effects model usually provides wider CIs than does the fixed effects model. Thus, the random effects model is best used if there is a significant between-study heterogeneity [22]. Statistical manipulations were performed using the Comprehensive Meta-Analysis program (Biostat, Englewood, NJ, USA). The power of each study was computed as the probability of detecting an association between the MHC2TA −168 A/G polymorphism and RA using a significance level of 0.05 and assuming a small effect size (Cohen effect size, w = 0.1). A power analysis was performed using the statistical program G*Power (http://www.gpower.hhu.de/).
Evaluation of publication bias
Funnel plots are often used to detect publication bias. However, due to the limitations of funnel plotting, which requires a range of studies of varying sizes involving subjective judgments, we evaluated publication bias using Egger’s linear regression test [23], which measures funnel plot asymmetry using a natural logarithm scale of ORs.
Results
Studies included in the meta-analysis
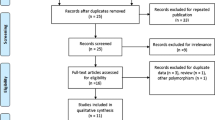
Forty-seven studies were identified by electronic and manual searching, and 16 were selected for a full-text review based on the title and abstract details. Four studies were excluded because three did not contain genotype data for the MHC2TA polymorphism [8] and one was a review. Thus, 12 studies met the inclusion criteria [5–16]. One of the eligible studies contained data on two different country populations [6] and one contained data on three ethnically different populations [13], and these were treated independently (Fig. 1). Thus, a total of 15 separate comparisons were considered in the meta-analysis, and these contained 14,158 patients and 13,642 controls in total (Table 1). These 15 studies included 1 Asian, 1 Latin American, and 13 Caucasian populations. An ethnicity-specific meta-analysis was conducted for the Caucasian population, and a country-specific meta-analysis was also performed. A meta-analysis was performed if two comparisons were included in the meta-analysis of the association between the MHC2TA polymorphism and rheumatoid factor (RF) positivity. Selected characteristics of the relationships found between the MHC2TA −168 A/G polymorphism and RA are summarized in Table 1. The statistical power for these 17 studies ranged from 66.8 to 100 %. Nine of the studies had a statistical power exceeding 90 %.
Frequency of the C allele of the MHC2TA −168 A/G polymorphism in various ethnic groups
The mean frequency of the G allele of the MHC2TA −168 A/G polymorphism was 27.5 % among all normal controls, and Caucasians had a lower G allele prevalence than did the other ethnic groups (25.3 %). Among normal controls, the frequencies of the G allele in the Caucasian, Latin American, and Asian populations were 25.3, 37.8, and 81.7%, respectively (Table 2).
Meta-analysis of the relationship between the MHC2TA −168 A/G polymorphism and RA
A summary of meta-analysis findings concerning the association between the MHC2TA −168 A/G polymorphism and RA is shown in Table 2. Based on the meta-analysis, there was no association between RA and the MHC2TA −168 G allele in the study subjects (OR = 1.046, 95 % CI = 0.987–1.108, p = 0.130) (Table 3, Fig. 2). After grouping the data by ethnicity, there was no association between the MHC2TA −169 C allele and RA in Caucasians (OR = 1.027, 95 % CI = 0.986–1.070, p = 0.193) (Table 3). We divided the Caucasian population into populations from Sweden, UK, and Spain. The country-specific meta-analysis revealed an association between the MHC2TA −169 C allele and RA in the Swedish population (OR = 1.131, 95 % CI = 1.023–1.250, p = 0.016), but not in the UK and Spain populations (Table 3, Fig. 2) Furthermore, analyses assuming the recessive model and homozygote contrast showed the same pattern for the MHC2TA −168 G allele in the Swedish population (OR = 1.371, 95 % CI = 1.067–1.760, p = 0.013; OR = 1.394, 95 % CI = 1.061–1.799, p = 0.010) (Table 3).
Meta-analysis of the relationship between the MHC2TA −168 A/G polymorphism and seropositive or seronegative RA
A summary of the meta-analysis findings concerning the association between the MHC2TA −168 A/G polymorphism and seropositive or seronegative RA is shown in Table 4. There were two studies considering seropositive RA and seronegative RA patients [12, 14] (Table 4). A direct comparison between RF-positive and RF-negative patients revealed that the frequency of the G allele was significantly lower in RF-positive patients (OR = 0.783, 95 % CI = 0.628–0.975, p = 0.029) (Table 4, Fig. 3). A meta-analysis of the GG + AA genotype showed the same result as that observed for the G allele of the MHC2TA −168 A/G polymorphism (Table 4).
Heterogeneity and publication bias
Between-study heterogeneity was found for all subjects and Caucasian groups. However, heterogeneity was not found in the meta-analysis of the Sweden and UK populations and in the meta-analysis that used the presence of RF as a factor (Table 3). Publication bias results in their being a disproportionate number of positive studies and poses a problem for the interpretation of meta-analyses. However, Egger’s regression test showed no evidence of publication bias (Egger’s regression test p > 0.1) (Fig. 4).
Discussion
It is well known that a breakdown of self-tolerance contributes to autoimmune diseases including RA [3]. MHC class II plays a key role in maintaining self-tolerance [3]. The expression of MHC class II molecules requires MHC2TA, which is a transcription factor, and MHC2TA production depends on transcription of the MHC2TA gene [4]. An MHC2TA −168 A/G polymorphism is associated with MHC2TA gene expression [5].
Previous studies on the association between the MHC2TA −168 A/G polymorphism and RA have produced inconsistent results [5–16]. Therefore, we performed a meta-analysis to clarify the association. Genetic factors may predispose a person to produce autoantibodies such as RF that contribute to RA. Sorting the data according to autoantibody status is important for genetic association tests. Thus, we performed further meta-analyses based on RF status. Our meta-analysis showed no association between the MHC2TA −168 G allele and RA in Caucasians. The prevalence of the MHC2TA −168 G allele differed among the Caucasian, Asian and Latin American populations. In addition, the frequency of the MHC2TA −168 G allele was lower in Sweden control individuals than in UK and Spain control individuals (23 vs 25.6 %). Thus, we divided the Caucasian population into populations from Sweden, UK, and Spain. A country-specific meta-analysis revealed an association between the MHC2TA −168 G allele and RA in the Swedish population, but not in the UK and Spain populations. Furthermore, analyses assuming a recessive model and homozygote contrast showed the same pattern for the MHC2TA −168 G allele in the Swedish population, i.e., an association between the MHC2TA −168 A/G polymorphism and RA. The difference between the three populations may be explained by the variation in the G allele frequencies among the control populations. Considering that the prevalence of RA is not uniform across Europe, with a higher prevalence in Northern Europe than in Southern European countries, it is likely that this is a true population-specific genetic effect. Findings regarding the relationship between MHC2TA −168 A/G and RF remain unclear. Our meta-analysis showed that RF-positive RA patients had a lower frequency of the MHC2TA −168 G allele than did RF-negative patients. Although our data suggest that the presence of RF is influenced by the MHC2TA −168 G allele, we cannot make conclusions in meta-analysis based only on two studies. We should take into account limited number of studies when interpreting results because only two studies were included for the comparison between RF-positive and RF-negative patients [12, 14].
Our data could result from linkage disequilibrium or indicate a functional role of the polymorphism. The functional MHC2TA −168 A/G polymorphism associated with susceptibility to RA in the Swedish population may be due to a difference in linkage disequilibrium with the causal polymorphism among populations. The finding that RF positivity is associated with the MHC2TA −168 A/G polymorphism could be due to the function of the MHC2TA −168 A/G polymorphism. However, we could not rule out the possibility that it results from linkage disequilibrium with the causative polymorphism. There is the impact of the results obtained by this meta-analysis in RA epidemiology. The results from our meta-analysis provide the evidence that genetic susceptibility to RA may differ among countries even if there is no difference in genetic susceptibility among same ethnic groups.
The present study has some limitations. First, the relative importance of the MHC2TA −168 A/G polymorphism during the development of RA may vary between ethnic groups, but we were only able to perform ethnic-specific meta-analyses for RA in Caucasians. Thus, our results are applicable only to Caucasians. Second, it would have been interesting to examine associations between the MHC2TA polymorphism and disease activity and clinical features, but such studies were not possible due to limited or unavailable data. Third, heterogeneity and confounding factors might have distorted the meta-analysis results. HLA-DRB1 is located in MHC class II, the expression of which is regulated by MHC2TA. Studies with data sorted according to HLA-DB1 shared epitope, gender, or ACPA status are needed.
In summary, this meta-analysis found that the functional MHC2TA −168 A/G polymorphism is not associated with susceptibility to RA in Caucasians. However, the MHC2TA −168 A/G polymorphism is associated with susceptibility to RA in the Swedish population, suggesting that the association is population-dependent. Further studies are warranted to clarify the role of the MHC2TA gene in the pathogenesis of RA in various ethnic groups because the effect of the MHC2TA polymorphism may vary among ethnic populations.
References
MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K, Silman AJ (2000) Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 43:30–37
Choi SJ, Rho YH, Ji JD, Song GG, Lee YH (2006) Genome scan meta-analysis of rheumatoid arthritis. Rheumatology (Oxford) 45:166–170
Friese MA, Jones EY, Fugger L (2005) MHC II molecules in inflammatory diseases: interplay of qualities and quantities. Trends Immunol 26:559–561
Ting JP, Trowsdale J (2002) Genetic control of MHC class II expression. Cell 109(Suppl):S21–S33
Swanberg M, Lidman O, Padyukov L, Eriksson P, Akesson E, Jagodic M, Lobell A, Khademi M, Borjesson O, Lindgren CM, Lundman P, Brookes AJ, Kere J, Luthman H, Alfredsson L, Hillert J, Klareskog L, Hamsten A, Piehl F, Olsson T (2005) MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat Genet 37:486–494
Eike MC, Skinningsrud B, Ronninger M, Stormyr A, Kvien TK, Joner G, Njolstad PR, Forre O, Flato B, Alfredsson L, Padyukov L, Undlien DE, Lie BA (2012) CIITA gene variants are associated with rheumatoid arthritis in Scandinavian populations. Genes Immun 13:431–436
Dieguez-Gonzalez R, Akar S, Calaza M, Gonzalez-Alvaro I, Fernandez-Gutierrez B, Lamas JR, de la Serna AR, Caliz R, Blanco FJ, Pascual-Salcedo D, Velloso ML, Perez-Pampin E, Pablos JL, Navarro F, Narvaez J, Lopez-Longo FJ, Herrero-Beaumont G, Gomez-Reino JJ, Gonzalez A (2009) Lack of association with rheumatoid arthritis of selected polymorphisms in 4 candidate genes: CFH, CD209, eotaxin-3, and MHC2TA. J Rheumatol 36:1590–1595
Plant D, Barton A, Thomson W, Ke X, Eyre S, Hinks A, Bowes J, Gibbons LJ, Wilson AG, Marinou I, Morgan AW, Steer S, Hocking LJ, Reid DM, Wordsworth P, Harrison P, Worthington J (2009) A re-evaluation of three putative functional single nucleotide polymorphisms in rheumatoid arthritis. Ann Rheum Dis 68:1373–1375
O’Doherty C, Hawkins S, Rooney M, Vandenbroeck K (2007) The MHC2TA-168A/G and +1614G/C polymorphisms and risk for multiple sclerosis or chronic inflammatory arthropathies. Tissue Antigens 70:247–251
Eyre S, Bowes J, Spreckley K, Potter C, Ring S, Strachan D, Worthington J, Barton A (2006) Investigation of the MHC2TA gene, associated with rheumatoid arthritis in a Swedish population, in a UK rheumatoid arthritis cohort. Arthritis Rheum 54:3417–3422
Martinez A, Sanchez-Lopez M, Varade J, Mas A, Martin MC, de Las Heras V, Arroyo R, Mendoza JL, Diaz-Rubio M, Fernandez-Gutierrez B, de la Concha EG, Urcelay E (2007) Role of the MHC2TA gene in autoimmune diseases. Ann Rheum Dis 66:325–329
Harrison P, Pointon JJ, Farrar C, Harin A, Wordsworth BP (2007) MHC2TA promoter polymorphism (−168*G/A, rs3087456) is not associated with susceptibility to rheumatoid arthritis in British Caucasian rheumatoid arthritis patients. Rheumatology 46:409–411
Orozco G, Robledo G, Linga Reddy MV, Garcia A, Pascual-Salcedo D, Balsa A, Gonzalez-Gay MA, Eimon A, Paira S, Scherbarth HR, Pons-Estel BA, Petersson IF, Alarcon-Riquelme M, Martin J (2006) Study of the role of a functional polymorphism of MHC2TA in rheumatoid arthritis in three ethnically different populations. Rheumatology 45:1442–1444
Yazdani-Biuki B, Brickmann K, Wohlfahrt K, Mueller T, Marz W, Renner W, Gutjahr M, Langsenlehner U, Krippl P, Wascher TC, Paulweber B, Graninger W, Brezinschek HP (2006) The MHC2TA -168A > G gene polymorphism is not associated with rheumatoid arthritis in Austrian patients. Arthritis Res Ther 8:R97
Akkad DA, Jagiello P, Szyld P, Goedde R, Wieczorek S, Gross WL, Epplen JT (2006) Promoter polymorphism rs3087456 in the MHC class II transactivator gene is not associated with susceptibility for selected autoimmune diseases in German patient groups. Int J Immunogenetics 33:59–61
Iikuni N, Ikari K, Momohara S, Tomatsu T, Hara M, Yamanaka H, Okamoto H, Kamatani N (2007) MHC2TA is associated with rheumatoid arthritis in Japanese patients. Ann Rheum Dis 66:274–275
Lee YH, Rho YH, Choi SJ, Ji JD, Song GG (2007) PADI4 polymorphisms and rheumatoid arthritis susceptibility: a meta-analysis. Rheumatol Int 27:827–833
Lee YH, Woo JH, Choi SJ, Ji JD, Song GG (2010) Associations between osteoprotegerin polymorphisms and bone mineral density: a meta-analysis. Mol Biol Rep 37:227–234
Lee YH, Bae SC, Choi SJ, Ji JD, Song GG (2011) Associations between vitamin D receptor polymorphisms and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Mol Biol Rep 38:3643–3651
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Smith GD, Phillips AN (1997) Meta-analysis: principles and procedures. BMJ 315:1533–1537
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Acknowledgements
This study was supported in part by a grant of the Korea Healthcare technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI13C2124).
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, Y.H., Bae, SC. Association between the functional MHC2TA −168 A/G polymorphism and susceptibility to rheumatoid arthritis: a meta-analysis. Clin Rheumatol 35, 901–909 (2016). https://doi.org/10.1007/s10067-015-3089-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3089-5