Abstract
Pain in RA is multifaceted and complex. Measuring instruments are inadequate. Rheumatoid Arthritis Pain Scale (RAPS) (Arthritis Care Res 45:317–323, 2001) was designed to measure pain comprehensively but has been sparsely reported. We decided to validate a suitable version for our community. Post translation (contextual), RAPS was administered (face to face interview) to 172 consenting patients of moderately severe RA (mean pain visual analogue scale (VAS) 5.4 cm) in a cross-sectional study using standard rheumatology case record form. RAPS contained 24 questions (numeric score, anchored at 0 (never) and 6 (always); range 0–144). Fifty-seven cohort patients on supervised rheumatology care were followed for 16 weeks. SPSS (v16) was used for statistical analysis, significant p < 0.05. RAPS showed good face and content validity (consensus). Construct/criterion validity was demonstrated for subclass domains and total RAPS (Cronbach’s alpha 0.91, test–retest interclass correlation (Pearson) 0.71). Fair to modest correlation (p < 0.05) was seen with swollen joint count (0.16), Indian health assessment questionnaire (0.23), medical outcome short form (SF), 36 physical score (−0.35), SF 36 mental score (−0.21) and C-reactive protein (0.25), not with pain VAS. Similar results were shown for subclass domains (physiologic, affective, sensory, cognitive), except low alpha for affective. Age, disease duration and SF 36 were significant predictors (linear regression). In factor analysis, RAPS loaded with SF 36. The standardized response mean (0.6) was equal to pain VAS and DAS 28. RAPS was found to be a valid and clinically relevant instrument for measuring pain in Indian patients suffering from RA. It merits more widespread clinical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
RA is a prototypic painful inflammatory disease. Despite great strides in therapeutic management, pain management in RA continues to be a challenge. RA is a lifelong disease. Patients often seek medical consultation because of pain [1–3]. And directly or indirectly, pain influences several disease expressions and outcomes [2, 4]. Treat pain or all else will fail is an overarching therapeutic goal. Effective control of pain encourages adherence to medications and in particular disease-modifying anti-rheumatic drug (DMARD). Pain relief encourages participation in physical therapy and rehabilitation. It is prudent to add that effective control of pain is critical to long-term compliance to drugs [5]. Pain is a core set efficacy measures in clinical research [6].
Chronic pain is multifaceted and more so in chronic arthritis [2, 7]. Though the onset of pain is usually due to inflammatory synovitis, there are complex interactions between body and mind. This seems to lead to some kind of a vicious cycle wherein pain begets pain. Several mechanical sequels (articular deformities) further contribute to increasing pain. The pain persona in RA is indeed unique and complex and difficult to understand. To begin with, we need a suitable measure.
Comprehensive measure of pain is difficult [1, 8–11]. Several instruments are used in patients suffering from arthritis to measure function and quality of life and may also contain questions pertaining to pain [11–16]. However, few are developed to measure generic pain [9, 11, 17, 18]. During a search for instruments to measure pain in RA, we were impressed with the Rheumatoid Arthritis Pain Scale (RAPS) developed by Anderson [19]. And we were surprised to find very little published literature (RAPS).
In the current study, we decided to develop a translated version of RAPS and validate it for clinical use in Indian (Asian) patients suffering from RA.
Patients and methods
This single-centre study was carried out in the Centre for Rheumatic Disease (CRD), Pune [20]. CRD is a private community-based rheumatology outpatient facility and is attended by urban and rural patients. Patients mostly belong to middle socioeconomic class and are referred from all over the West India state of Maharashtra. The average daily attendance is about 75 patients.
The study was non-commercial and investigator initiated (TK) and a part of a larger research project on diet and pain in RA [21]. It was cleared by the ethics committee of CRD. The design was prospective, and consenting eligible patients were selected on first come first served basis in a consecutive fashion as per protocol. The analytical design was cross-sectional. All patients were classified as per the standard classification criteria [22]. Patients were to have a disease duration ≥6 months and a maximum articular pain in the preceding 24 h of 40 mm or more on a visual analogue scale (VAS) while performing some activity. Patients should have been on supervised standard rheumatology care for at least 6 months.
A subcohort of 57 patients from the current sample who were receiving standard of care treatment with supervised methotrexate was strictly followed at monthly intervals for 16 weeks; this was a control arm for the main diet intervention study (data to be published elsewhere). This cohort provided data for sensitivity to change/effect size analysis.
All patients were clinically examined by rheumatologists. Observations were recorded in standard case record forms. Standard recommendations [6] were followed to assess physician centric (including joint counts and physician global assessment) and patient centric measures (recorded by TK and a nurse and included pain VAS and functional/quality of life instruments). An Internet-based application was used to calculate disease activity score (DAS) 28. All patients completed the following evaluations/measurements in a face to face interview in the local Marathi language prior to rheumatology examination (i) pain VAS [11]: The pain VAS was a horizontal line, 0–10-cm scale and anchored at ‘0’ for ‘No pain at all’ and at ‘10’ for ‘Pain as bad as it could be’. Patients were instructed to mark a vertical mark on this line to indicate the severity of active pain (not on rest) in the preceding 24 h. (ii) Global assessments of disease activity: A Likert scale of five-category response was used—asymptomatic, mild, moderate, severe, and very severe with an ascending score ranging from 1 to 5 (asymptomatic to severe). (iii) General health: Patients were requested to score their overall health as per their belief on a horizontal 100-mm line (VAS), anchored at 0 for ‘extremely poor health’ and 100 for ‘perfect health’. (iv) RAPS [19]: As a preliminary step, it was translated by an independent professional into a local Marathi language and discussed for comprehension amongst paramedics and doctors and with few patients (RA). The translation was contextual rather than ad verbatim. The primary structure of the questionnaire and scoring system was maintained. Subsequently, another professional back-translated the local version into English language to ensure good similarity. In consultation with the translators, the authors fine-tuned the final draft and administered it to ten patients of RA selected at random on a single day from the CRD outpatient. Overall, more than 90 % questions were answered by each patient. The draft questionnaire was understood by the patients and took about 8–10 min to complete. It was adopted as the final version in the current study. RAPS contained 24 questions classified into four subclass domains. The domains were physiologic (P), affective (A), sensory–discriminative (S) and cognitive (C). Pain items were scored using a seven-point Likert scale ranging from “never” (0 score) to “always” (6 score); maximum score for each question was 6, and the range of total score was 0–144. We used an independent pain VAS. The original version of RAPS which was translated in the current report is attached as Appendix
(v) Modified Stanford health assessment questionnaire (HAQ): a validated Indian version [23] catering to the customs and lifestyle of the Indian (Asian) community was used. The HAQ disability score (HAQ) was an ordinal scale with 24 items dealing with daily functioning during the past week. These covered eight component areas: dressing and grooming, arising, eating, walking, hygiene, reach, grip and outdoor activities. Each response was scored on a four-point category scale of ability: without any difficulty, with some difficulty, with much difficulty and unable to do and did not differ from the Stanford HAQ [12].
(vi) Medical outcome study short form 36 (SF 36) (with permission) [15, 24]: A translated version for local use was obtained from the vendor. The SF 36 had 36 questions that measure the following eight dimensions: physical functioning, physical role limitations, bodily pain, social functioning, general mental health, social role limitations, vitality and general health perceptions. There is an overlap between dimensions. We used a 1-week recall period. Two summary scores are calculated, one for the physical component summary (PCS) and one for the mental component summary (MCS) scores: some dimensions overlap. The predominant scales in ‘physical health score’ (SF 36 PCS) are physical function, physical role, bodily pain and general health. The predominant scales in ‘mental health score’ (SF 36 MCS) are vitality, social role, emotional role and mental health. However, all scales in the questionnaire contribute to the two principle scores. We used a vendor-provided automated software program for scoring (NBS). In NBS, each scale was scored to have the same average (50) and the same standard deviation (10), meaning each point equals one tenth of a standard deviation. For all scales and summary measures, individual respondent scores below 45 and group mean scores below 47 can be interpreted as being below the average range for the general population; higher score mean better health [24].
The categories used for physician global assessment was similar to patient global assessment [6].
We have used some data recorded at baseline (close to current study time point) of the main diet study to calculate the body mass index (BMI) and average daily calories consumption (energy) for correlation analysis [21].
Laboratory investigations were carried out as per routine rheumatology practice and were the following: haemoglobin (normal range, male 13–16 g/dl and female 12–14 g/dl), erythrocyte sedimentation rate (Westergren, reported as millimeters fall at the end of the first hour), C-reactive protein (CRP, nephelometry; cutoff value 5 mg/dl) and rheumatoid factor (RF, nephelometry; cutoff value 40 IU/ml).
Statistical analysis
This was a convenience sample with an essential cross-sectional design. A subcohort was followed to evaluate the sensitivity to change. We were guided by patient logistics and feasibility. Our target was to enrol as many patients as possible over a 4-month period but not less than 150 patients. SPSS version 16 (2007) software was used. Standard statistical tests were chosen to evaluate the internal and external validity of RAPS and included test–retest, Cronbach’s alpha, interclass correlation, correlation matrix (Pearson coefficient (r), linear multiple regression model, factor analysis (principle component analysis with orthogonal rotation) and sensitivity to change [25]. The strength of correlation was guided by standard recommendation [25] but empirically classified into nil, fair (0.1–0.25), moderate (0.25–50), strong (0.5–0.8) and very strong to perfect (0.8–1.0). Significant p (0.05, two-tailed) was determined using ANOVA. The standardized response mean (SRM) effect size was based on mean change over 16 weeks divided by the standard deviation [25].
Observations and results
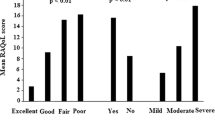
One hundred seventy-two patients (92.4 % women) were enrolled in the study with a mean age and disease duration of 48.18 and 8.83 years, respectively. Table 1 describes the baseline demographics and other characteristics of the study cohort. Overall, the disease was active and moderately severe (Table 1). The HAQ score (mean 5.09, 95 % confidence interval 4.6, 5.5) indicated a mild functional disability. All patients were on supervised treatment (mostly methotrexate) for at least 16 weeks prior to enrolment.
General validity
The instrument had good face and content validity as assessed independently by current authors, patients (pilot evaluation, see above) and an in-house team of rheumatologist (NK), research coordinator (MS) and a biostatistician (SS). A consensus approach was used for the current final version.
Construct validity
The Cronbach’s coefficient alpha (internal consistency) was 0.91. The coefficient alpha of the subscales (Table 2) was 0.80 to 0.83 for the domains of physiologic, sensory discrimination and cognitive. It was 0.28 for the affective domain and thereby suggestive of a poor construct validity. The alpha (adjusted) for each of the questions pertaining to the affective domain was also low (data not shown) as compared to the other subclass domains.
Test–retest
Patients were provided with a fresh blank questionnaire after they collected the prescription (post rheumatology evaluation) and asked to complete the same within the next 24 h to evaluate test–retest agreement. The overall correlation (Table 2) between the two RAPS readings was strong (0.78, 95 % confidence interval 0.63–0.86) and meant that the patients understood the questions and could reproduce reasonably consistent answers over a short period. The reliability was further endorsed by the interclass correlation coefficients for each of the domains in the two interval readings (Table 2). The correlation was best and almost perfect for the ‘physiologic domain’ and least but still strong for the ‘affective domain’.
Concurrent criterion validity
We ran correlations for the subscale domains (Table 3). The correlation was strong and significant between each of the subscales (range 0.6–0.8). The correlation between each of the subscales and RAPS (total score) was moderate and statistically significant (range 0.3–0.4). The correlation was moderate (p < 0.05) between SF 36 (both for physical and mental scores) and each of the subscales except for weak correlation between physiologic domain and SF 36 mental score. And this suggests that each of the domains contributes towards generic measure of quality of life. As per a priori classification, the strength of association between each of the domains and pain VAS was ‘fair’, but not statistically significant, and was much less than expected. Several other meaningful correlations classified into the categories of fair or moderate (as per the classification described in ‘Patients and methods’) were observed—affective scale and HAQ (0.3, p < 0.05), sensory scale and pain VAS (0.2), cognitive scale and HAQ (0.2) and patient global (0.23, p < 0.05). Several significant correlations (p < 0.05) were observed between RAPS and several variables (Table 4), in particular JCSW, HAQ, GHA, MStiff, SF 36 (P), SF 36 (M) and CRP. The correlation between pain VAS and each of the variables for JCPT, physician and patient global assessments and some surrogate markers of general health (BMI, blood haemoglobin and daily calorie/energy consumption) was nil to weak and not significant.
Regression
RAPS was the dependent variable, and variables with correlation ≥0.1 were included as independent variables (Table 1) in a multiple linear regression model. The model achieved goodness of fit (p < 0.001, ANOVA), and the combined variables could explain 25 % of the variation (adjusted R square). Several variables were found to be significant predictors of RAPS, namely age, duration of disease and each of the SF 36 subscales (physical and mental).
Factor analysis (principle component with orthogonal rotation)
The loading of variables into each of the six components (descending disorder) is shown in Table 5. Each of the six components explained 10–12 % variance in the model and when combined explained 63.58 % variance (cumulative). RAPS was observed to load along with SF 36 (for physical and mental subscales).
Sensitivity to change
The SRM for RAPS was 0.6 and superior to several other variables—pain VAS (0.6), JCPT (0.4), JCSW (0.1), physician global assessment (0.6), patient global assessment (0.2), HAQ (0.2), SF 36 PSC (0.04), SF 36 MSC (0.2), DAS 28 (0.6), ESR (0.4) and CRP (0.2).
Discussion
In this cross-sectional design study, we demonstrate the validity and relevance of a translated version of RAPS in patients suffering from painful RA in a distinct Indian (Asian) ethnic cohort. Our community shares several traditions and customs and lifestyle with neighbouring Asian communities. The face and content validity was established by expert consensus. The Indian version was reliable and internally consistent for the total questionnaire (Cronbach’s coefficient alpha 0.91) and the subclass domains. The construct and concurrent criterion validity was shown by correlation matrix (Tables 2, 3 and 4). The short-term reproducibility was good (Table 2). The Indian RAPS showed significant correlation with HAQ, SF 36 and CRP. SF 36 scores were significant predictors and loaded with RAPS in a factorial analysis (Table 4). Overall, the current RAPS data endorsed the core elements of truth and feasibility as described in the OMERACT filter [26].
Though RAPS was developed more than a decade ago, the instrument is relatively new to clinical use. It has not been validated after its initial development [27]. The WHO ICF components are body function, body structure, impairment and activity limitation. No cutoff points or critical score points have been established, and no data on sensitivity to change has been reported. Therefore, the current study vindicates the usefulness of RAPS. Further, it showed a moderate sensitivity (0.6) to change over 16 weeks in patients on standard of rheumatology care in a community setting and this was comparable to DAS 28.
Pain VAS is a too simple measure and often not a satisfying experience for the patient in our clinic setting. Conceptually, the evaluation of chronic pain using VAS does not seem to be an attractive proposition although it is the most popular method in global use [1, 11]. Methods to measure pain usually focus on intensity and perception and are critical to the overall management of chronic musculoskeletal pain and disability [2, 9, 11]. Several factors influence the pain experience [2, 28–32] including personality traits like memory, ethnicity, social, culture and traditions, age, religion and socioeconomic environment. There may be difference in perception and impact of pain between urban and rural areas. This was elegantly demonstrated in an earlier study carried out in The Netherlands which also highlighted the fact that more than pain, people suffer because of a frustration of being unable to do things they used to do and dependency on other [30]. Pain is a complex construct and not easy to measure [9, 10]. Pain needs a comprehensive measure. Suitable translation of pain questionnaires into local language to suit the needs of community is important to capture the real-life scenario but difficult as shown by several studies [30]. Our experience with the HAQ from a patient’s perspective has been very encouraging [23, 29].
RAPS uses a graphic scale to address the different domains of pain. In a recent study in RA patients, the graphic rating scale (numerical VAS) and a verbal rating scale (Likert) demonstrated a somewhat different pattern of correlations with other measures of disease activity, suggesting that these should not be used interchangeably to measure pain in RA patients [29]. This supports the contention that RAPS is not a substitute for pain VAS but complimentary towards a more holistic approach.
Sim and Waterfield described pain experience as having sensory, affective, evaluative, cognitive and behavioural dimensions with sensory, emotional and physiological outcomes [33]. And these pain components were used in a clinical setting to develop and validate RAPS [19]. But to the best of our knowledge (and after Internet search), there has been a delay in accepting RAPS for wider clinical use in rheumatology.
Pain is a personal experience [1]. Apart from that, there are ethnocultural differences in pain reports between countries, although the mechanisms underlying these differences remain unclear [29, 30]. It is indeed a challenge to record it in a meaningful descriptive and analytical fashion [7, 9, 10]. Translations are cumbersome, and pain is subject to the several nuances of the language. We used essentially a contextual approach. Patients, paramedics and doctors were involved. We did not discard any question or add a new item to the original RAPS version [19]. The Indian version was completed in a face to face interview rather than self-reported. Despite cajoling and encouragement, our patients resent self-reporting and prefer face to face interview [23, 32]. This can be a deterrent in our high patient volume setting because of time and manpower constraints. However, we have reported the overarching usefulness of HAQ in community rheumatology care [23, 32] and believe that patient centric questionnaires indeed compensate for the limited time that rheumatologists spend with the patients in examination rooms. In our experience, instruments like HAQ provide reassurance to the patient that pain and its impact are being taken care of by the rheumatologist. RAPS should be considered a value addition.
The validity statistics of RAPS in the current report favour its clinical application. Indian RAPS and its subclass domains were best correlated (though modest) with HAQ and SF 36 (Table 3); correlations with other efficacy variables were nil-fair. Age, disease durations and SF 36 were the only significant predictors. The correlation with pain VAS was unexpectedly only fair. All this would also mean that RAPS was not redundant, and its value as an important clinical measure probably extended beyond several conventional efficacy and disease severity measures in the current study. The SRM of the current RAPS (over 16-week supervised rheumatology care) was comparable to DAS 28, pain VAS and physician global assessment (disease) and superior to other efficacy measures (see ‘Observations and results’). Thus, it would be appropriate to conclude that the current RAPS was certainly a comprehensive and a clinically useful patient measure. RAPS seems to measure unique aspects of pain which are not captured by the simplistic pain VAS.
On comparison, there were some important differences between the clinometric properties of the Indian version and the original RAPS (Anderson) [19]. It is prudent to add that though both were validated in rheumatology settings; there were several differences in the methodology—actual investigation site, investigators and patient population. The current study was single centre. Also, the evaluation in our setting was more comprehensive and elaborate and a subcohort of patients was followed. The Pearson correlation (p < 0.05) between RAPS and tender joint count (0.52) and pain VAS (0.67) in the initial validation [19] was much higher than that reported in the current report (Table 3). The current Indian version had a significant association with quality of life and functional measure of SF 36 and HAQ (Tables 3 and 4). It is prudent to add that the correlation of pain has been best with HAQ and poor to fair with tender and swollen joint counts in several studies [1]. Interestingly, similar to that reported by Anderson [19], the metrics of the ‘affective subscale’ (Table 2) in the current study was a matter of concern and cast a doubt on the construct validity (Table 2). However, we found a significant correlation between the affective subscale and the HAQ and SF 36 score (Table 3) and the test–retest correlation was good (Table 2). We conclude that the affective subscale is important to the core significance of RAPS and should be retained. Some questions (affective) may need a change.
In a recent review of different pain measurements in RA including the RAPS, the authors were rather critical: ‘RAPS were found to be more laborious to evaluate pain status since pain in only one of the several core measures that are suggested to be monitored in clinical routine investigation of inflammatory arthritis. With regard to standardization, all pain measurement tools provided standardized instruction for calculation of test scores. Nevertheless, especially those instruments covering multiple facets of pain were found to be lacking additional information on how to interpret the resulting individual total score between minimum and maximum score, or to provide certain information on the meaning of each pain domain with respect to the corresponding score.’ [34] This will also require studies to ascertain minimum clinically important difference for RAPS.
The current study was limited in its cross-sectional design though we did follow a subcohort to determine standardized response mean. All patients in the current study had moderately severe painful RA; thus, it may not be appropriate to extrapolate the current observations to milder lesser painful cases of RA (floor effect). Also, the current study data was not sufficient to describe the ‘discrimination’ properties (OMERACT filter) [26]. RAPS has multiple pain domains, and each needs to be validated independent of each other. Though the original RAPS [19] has aptly addressed all these concerns, there may be a shift in construct validity after translation. To an extent, we did carry out construct and concurrent validity for the subscales (Tables 2 and 3) but are limited in the data to support convergent and divergent validity. Cronbach’s alpha assumes unidimensionality, but we have used it to endorse the construct validity of the multidimensional RAPS questionnaire (Table 2) and this is appropriately supported by the correlation results with other important measures in RA (Table 4).
In conclusion the Indian (Asian) translated version of RAPS was a valid and reliable measure of pain in patients suffering from painful active RA. It deserves greater recognition and application by the rheumatology fraternity.
References
Sokka T (2003) Assessment of pain in rheumatic diseases. Clin Exp Rheumatol 23:S77–S84
Buckelew SP, Parker JC (1989) Coping with arthritis pain: a review of the literature. Arthritis Care Res 2:136–145
Minnock P, FitzGerald O, Bresnihan B (2003) Women with established rheumatoid arthritis perceive pain as the predominant impairment of health status. Rheumatology 42:995–1000
Skevington S (1986) Psychological aspects of pain in rheumatoid arthritis: a review. Soc Sci Med 23:567–575
Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C et al (2006) Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis. Ann Intern Med 144:867–876
Felson DT, Anderson JJ, Boers M, Bombardier C, Chemoff M, Fried B et al (1993) American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum 36:729–740
Carlsson AM (1983) Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 16:87–101
Huskisson EC (1974) Measurement of pain. Lancet 2:1127–1131
Huskisson EC (1982) Measurement of pain. J Rheumatol 9:768–769
Turk D, Melzack R (1992) Handbook of pain assessment. The Guilford Press, New York
Hawker GA, Mian S, Kendzerska T, French M (2011) Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 63(Suppl 11):S240–S252
Fries JF, Spitz P, Kraines RG, Holman HR (1980) Measurement of patient outcome in arthritis. Arthritis Rheum 23:137–145
Meenan RF, Gertman PM, Mason JH (1980) Measuring health status in arthritis: the arthritis impact measurement scales. Arthritis Rheum 23:146–152
Ware JE, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36): conceptual framework and item selection. Med Care 30:473–483
Tugwell P, Idzerda L, Wells GA (2007) Generic quality-of-life assessment in rheumatoid arthritis. Am J Manag Car 13:S224–S236
Callahan LF, Brooks RH, Summey JA, Pincsu T (1987) Quantitative pain assessment for routine care of rheumatoid arthritis patients, using a pain scale based on activities of daily living and a visual analog pain scale. Arthritis Rheum 30:630–636
Melzack R (1975) The McGill Pain Questionnaire: major properties and scoring methods. Pain 1:277–299
Melzack R (1987) The Short-Form McGill Pain Questionnaire. Pain 30:191–197
Anderson LD (2001) Development of an instrument to measure pain in rheumatoid arthritis. Rheumatoid Arthritis Pain Scale (RAPS). Arthritis Care Res 45:317–323
Centre for Rheumatic Diseases, Pune [Internet. Accessed March 15, 2015.] Available from: www.rheumatologyindia.org.
Kianifard T, Saluja M, Chopra A (2014) Is dietary potassium important in rheumatoid arthritis? Preliminary observations. Ann Rheum Dis 73(Suppl_2):1169–1170
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Chopra A, Saluja M (2012) Validation and usefulness of an Indian version (CRD Pune) health assessment questionnaire: drug trials, community practice and COPCORD Bhigwan population study (1994-2012). Indian J Rheumatol 7:74–82
Ware JE, Kosinski M, Dewey JE (2000) How to score version two of the SF-36 health survey. Quality Metric, Lincoln
Portney LG, Watkin MP (2000) Foundations of clinical research. Applications to practice, 2nd edn. Prentice Hall Health, New Jersey, pp 79–110
Boers M, Brooks P, Strand CV, Tugwell P (1998) The OMERACT filter for outcome measures in rheumatology. J Rheumatol 25:198–199
Burckhardt CS, Jones KD (2003) Adult measures of pain. The McGill Pain Questionnaire (MPQ), Rheumatoid Arthritis Pain Scale (RAPS), Short-Form McGill Pain Questionnaire (SF-MPQ), Verbal Descriptive Scale (VDS), Visual Analog Scale (VAS), and West Haven-Yale Multidisciplinary Pain Inventory (WHYMPI). Arthritis Rheum (Arthritis Care Res) 49(5S):S96–S104
Melzack R, Dennis S (1979) Pain mechanisms: theoretical approaches. In: Beers RF, Basset EG (eds) Mechanisms of pain and analgesic compounds. Raven, New York, pp 185–187
ten Klooster PM, Vlaar APJ, Taal E, Gheith RE, Rasker JJ, El-Garf AK et al (2006) The validity and reliability of the graphic rating scale and verbal rating scale for measuring pain across cultures: a study in Egyptian and Dutch women with rheumatoid arthritis. Clin J Pain 22:827–830
Vlaar APJ, ten Klooster PM, Taal E, Gheith RE, El-Garf AK, Rasker JJ et al (2007) A cross-cultural study of pain intensity in Egyptian and Dutch women with rheumatoid arthritis. J Pain 8(9):730–736
Cornelissen PGJ, Rasker JJ, Valkenburg H (1988) The arthritis sufferer and the community: a comparison of arthritis sufferers in rural and urban areas. Ann Rheum Dis 47:150–156
Chopra A (2009) Community rheumatology in India. Indian J Rheumatol 4:119–126
Sim J, Waterfield J (1997) Validity, reliability and responsiveness in the assessment of pain. Physiother Theory Pract 13:23–38
Englbrecht M, Tarner IH, van der Heijde DM, Manger B, Bombardier C, Müller-Ladner U (2012) Measuring pain and efficacy of pain treatment in inflammatory arthritis: a systematic literature review. J Rheumatol Suppl 90:3–10
Acknowledgments
We thank the management of ARCF-CRD for this in-house PhD research project and all patients who participated in the study and its validation process. Dr S Sarmukkadam, a biostatistician, provided excellent statistical guidance and analysis. Several colleagues, namely Dr Nachiket Kulkarni, Ms Manjit Saluja and Dr N Nahar, from CRD Pune assisted in screening and enrolling patients, rheumatology examination and co-ordination of the project. Dr Anuradha Venugopalan, in charge at the lab and an immunologist, provided an invaluable assistance in carrying out the study laboratory tests.
Authors’ contributions
All the authors were involved in drafting the manuscript and approved the final version. Dr Arvind Chopra had full access to the data and takes full responsibility for the veracity of the data in the current study. Toktam Kianifard, Taghi Kianyfard and Arvind Chopra are responsible for the study concept and design. Toktam Kianifard and Arvind Chopra are responsible for the acquisition of data. Toktam Kianifard and Arvind Chopra are responsible for the analysis and interpretation of data.
Financial sponsorship/grant
None.
Compliance with Ethical Standards
ᅟ
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rheumatoid Arthritis Pain Scale [19].
For each item, choose one number from 0 (never) to 6 (always) to describe how you have felt in the last week. 0 1 2 3 4 5 6
1. I would describe my pain as gnawing. (S)
2. I would describe my pain as aching . (S)
3. I would describe the word exhausting to describe my pain (A)
4. I would describe my pain as annoying. (A)
5. I’m in constant pain. (S)
6. I would describe my pain as rhythmic. (S)
7. I have swelling of at least one joint. (P)
8. I have morning stiffness of one hour or more. (P)
9. I have pain on motion of at least one joint. (P)
10. I cannot perform all the everyday tasks I normally would because of pain. (C)
11. Pain interfere with my sleep. (C)
12. I cannot decrease my pain by using methods other than taking extra medication. (C)
13. I would describe my pain as burning. (S)
14. I find that I guard my joints to reduce pain. (A)
15. I brace myself because of pain. (A)
16. My pain is throbbing in nature. (S)
17. I would describe my pain as sharp. (S)
18. I would say my pain is severe. (S)
19. I feel stiffness in my joints after rest. (P)
20. My joints feel hot. (P)
21. I feel anxious because of pain. (C)
22. I would describe my pain as tingling. (S)
23. I feel my pain is uncontrollable. (C)
24. I feel helpless to control my pain. (C)
Each question is classified into the following subclass domains: physiologic (P), affective (A), sensory discriminative (S) and cognitive (C)
Rights and permissions
About this article
Cite this article
Kianifard, T., Kianyfard, T. & Chopra, A. Validation and relevance of Rheumatoid Arthritis Pain Scale (RAPS) in Indian (Asian) patients suffering from rheumatoid arthritis. Clin Rheumatol 35, 63–71 (2016). https://doi.org/10.1007/s10067-015-3071-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3071-2