Abstract
Important advances from both therapeutic and clinical assessment have recently been reported in psoriatic arthritis (PsA). Moreover, the constant challenge to provide a more comprehensive assessment of this heterogeneous disease results in a variety of clinical instruments that help the clinician for a global evaluation of both disease activity and responsiveness. The current European League Against Rheumatism (EULAR) recommendations on the use of imaging suggest the use of ultrasound (US) in chronic arthritis to increase the diagnostic accuracy and improvement of its management as compared to clinical examination alone. Although US findings are not firmly established in daily clinical practice, it demonstrated several positive aspects such as good sensitivity and specificity, acceptable reliability, and adequate sensitivity to change, especially in the peripheral PsA. Additionally, recent works introduced the role of US in the assessment of skin and nails opening interesting area of research. The aim of this paper is to describe the potential role of US in the assessment of PsA and to discuss the current evidence supporting its application in daily clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease that may develop in 5–40 % of individuals with psoriasis [1]. Patients affected may have a heterogeneous clinical presentation, including articular and dermatological features and diverse disease courses and outcomes.
In recent years, important advances from both therapeutic and clinical assessment have been reported in PsA. The introduction of biological treatments has dramatically improved the disease control and, consequently, the functional capacity and quality of life of psoriatic patients [2, 3]. Moreover, the constant challenge to provide a more comprehensive assessment of the disease results in a variety of clinical instruments such as Disease Activity index for Psoriatic Arthritis (DAPSA), PsA Response Criteria (PsARC), Composite Psoriatic Disease Activity Index (CPDAI), and Psoriatic Arthritis Disease Activity Score (PASDAS) that help the clinician for a global evaluation of both disease activity and responsiveness [4–7].
Imaging findings play a valuable role in PsA. The current European League Against Rheumatism (EULAR) recommendations on the use of imaging techniques suggest the use of ultrasound (US) in chronic arthritis in order to increase the diagnostic accuracy and improvement of its management as compared to clinical examination alone [8, 9].
Although US findings are not firmly “evidence-based” established in daily clinical practice, it demonstrated several positive aspects such as good sensitivity and specificity, acceptable reliability, and adequate sensitivity to change, especially in the peripheral PsA [10–17]. US can facilitate the early detection and careful characterization of the inflammatory process in the different stages of the disease (including aggressive findings at soft tissues and bone level) and the evaluation of subclinical inflammatory changes in patients with PsA and psoriasis [18–21].
Recently, interesting reports introduced the utility of US in the assessment of skin and nails which open a new interesting area of research, providing a more holistic assessment of psoriatic disease spectrum [22, 23].
The aim of this paper is to describe the potential role of US in helping the physician for the assessment of PsA and to discuss the current evidence supporting its application in daily clinical practice.
Methods
Literature review criteria and search strategy
All US relevant literature in the field of PsA published in the last 25 years was reviewed. The search for original articles concerning humans, published between June 1990 and May 2015, and referring to the use of US in the evaluation of PsA and/or psoriasis was carried out in the PubMed and EMBASE databases. A systematic search was performed using the following search terms in all possible combinations: “ultrasound, sonography, ultrasonography, psoriatic arthritis, psoriatic disease, psoriasis, joint, tendon, enthesis, skin, nail, synovitis, tenosynovitis, enthesitis, psoriatic skin, and onychopathy”. In addition, the reference lists of all retrieved articles were manually reviewed. In case of missing data, study authors were contacted by e-mail to retrieve original data.
Inclusion and exclusion criteria
We excluded from this review the following types of publications: articles not published in English, case reports, letters to the editor that were purely commentary, and/or non-human studies. Search results were screened to avoid duplicates. Manuscripts were reviewed and data extracted and categorized into those assessing the following anatomical targets: joint, enthesis, tendons, skin, and nail. Titles, abstracts, and full reports of articles identified were systematically screen by one author with regard to inclusion and exclusion criteria. Extracted data addressed aspects of study design, US acquisition technique, methodology and reproducibility, and study findings. The references of published review articles were further screened for additional manuscripts that met our inclusion and exclusion criteria.
The references of published review articles were screened for additional manuscripts that met our inclusion and exclusion criteria.
Results
Since the initial published report of US in PsA, an important body of evidence in this topic has been published. Approximately 675 publications were identified in PubMed and EMBASE databases between June 1990 and May 2015. The results of the search strategy are illustrated in Fig. 1.
Joint involvement (synovitis and bone erosions)
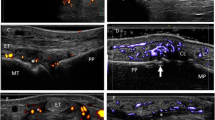
Synovitis is generally the main pathological finding in chronic arthritis including PsA (Fig. 2a). It is associated with long-term erosive radiological progression and poor outcome (Fig. 2b). Recently, Gladman et al. [24] described as the proportion of PsA patients with more than five joints involved is increased in the last years from 19 to 41 %.
a Metacarpophalangeal in longitudinal scan showing a proliferative synovitis with presence of intra-articular power Doppler. b Metacarpophalangeal joint in longitudinal scan showing a large bone erosion at metacarpal neck level (arrow). c Tenosynovitis of the flexor finger tendons of the hand in transverse scan. Note the power Doppler signal surrounding the tendons (t). d Achilles tendon in longitudinal scan. Note the presence of intra-tendinous power Doppler indicating enthesitis, and the erosion (arrow) at calcaneal bone level (c). e Epidermal layer at psoriatic lesion appears thickened and inhomogeneous (arrowheads). Beneath, it is evident a focal hypoechoic thickening of the dermis (d) with evident power Doppler signal revealing increase of blood perfusion in the dermis. Note the hypoechoic band in the upper dermis in correspondence of the psoriatic plaque (asterisk). f Power Doppler US revealing marked signal indicative of an increase of blood flow at the nail bed level. m = metacarpal bone, pp = proximal phalanx, dp = proximal phalanx, mp = middle phalanx
Backhaus et al. [25] in a pilot study compared different imaging tools in the assessment of joint inflammation. They reported a statistically significant advantage of US, MRI, and scintigraphy in the evaluation of synovitis as compared to X-ray examination.
Another study by Backhaus et al. [26] demonstrated the high value of MRI and US, surpassing conventional X-ray of detecting synovial hypertrophy. This study evaluated a group of 49 patients with rheumatoid arthritis (RA) and PsA assessing the metacarpophalangeal joints (MCPj) as well as proximal and distal interphalangeal joints. The authors revealed inflammatory lesions of synovial membrane in 55 % of the patients undergoing US and in 42 % undergoing MRI.
Wiell et al. [12] in a single-center study in PsA patients evaluated the inflammatory lesions in the small joints of the hand using US, MRI, and X-ray and subsequently compared the effectiveness of these imaging techniques to physical examination. Imaging studies were found more sensitive compared to physical examination. In addition, the number of detected erosions pointed to a higher effectiveness of US and MRI comparing to X-ray examination. Erosions were found in 18 % of joints on US examination, in 23 % of joints on MRI, and in 12 % of joints on X-rays. MRI was slightly more sensitive than US in synovitis. They also reported a superior sensitivity than X-ray for the detection of erosions. Additionally, US demonstrated a good inter-observer agreement for synovitis and bone erosions [27].
Weiner et al. [28] carried out a similar study comparing the potential of US, MRI, bone scintigraphy, and X-ray in the detection of synovitis and bone erosions at hand and foot joints. This study included X-ray as gold standard for erosions, whereas MRI was used for synovitis. Both US and MRI demonstrated to have higher sensitivity in the detection of overall joint pathology than radiography. Interestingly, similar findings were found between patients with painful and swollen joints and clinically unaffected joints.
Sankowski et al. [14] conducted recently a single-center, longitudinal study in PsA patients aimed to measure the efficacy of X-ray, US, and MRI in the assessment of erosions at wrist and MCPj joints level. In line with previous studies, US and MRI showed to be similar diagnostic value and surpass conventional X-ray in detection of periosteal reactions. US and MRI revealed even small, asymptomatic lesions (small erosions, slight thickening of the tendon attachments).
Lin et al. [29] performed a cross-sectional study aimed to compare the US findings among 44 patients with PsA, 39 with RA, and 20 healthy controls. Among RA patients, 67.2 % showed joint effusion, 63.9 % synovial thickening, and 48.3 % bone erosions, while no tenosynovitis, soft tissue inflammation, or enthesitis were found. However, among the patients with PsA, 60.9 % showed joint effusion, 55.2 % synovial thickening, 57.7 % bone erosion, 57.7 % tenosynovitis, 35.7 % soft tissue inflammation, and 31.7 % enthesitis.
Recently, Ficjan et al. [30] in a prospective study developed an US composite score for the assessment of inflammatory and structural lesions in PsA. Eighty patients underwent B-mode and power Doppler (PD) findings including 68 joints (evaluating synovia, perisynovial tissue, tendons, and bone) and 14 entheses. Two scores bilateral and unilateral called PsASon22 and PsASon13, respectively, were created. Both composite scores revealed a moderate to high sensitivity (bilateral composite score 43 to 100 %, unilateral 36 to 100 %) to detect inflammatory and structural lesions revealing sufficient convergent construct validity, sensitivity to change, reliability, and feasibility.
Tendon involvement (tenosynovitis and enthesitis)
Dactylitis is a common feature of PsA and takes part of classification criteria for the diagnosis of PsA (CASPAR) [31] (Fig. 2c). It also demonstrated to be a predictor of radiological damage due to its frequent association with synovitis [16, 32].
The role of US in tendon involvement is widely standardized in RA [33]. However, in PsA dactylitis, there is still lack of evidence regarding its feasibility, reliability, or responsiveness [17].
Olivieri et al. [34] conducted a comparative study assessing the potential role of US and MRI in determining “sausage-like” aspect of finger (tenosynovitis and arthritis) in 12 patients with dactylitis and their corresponding normal contralateral fingers. MRI revealed a significant increase in the volar bone-to-skin distance in dactylitic fingers with respect to that of the normal contralateral fingers. It was due to distension of the flexor synovial sheaths by fluid collection. Of the 36 joints of the 12 dactylitic fingers, only 1 showed capsule distension. Using MRI as the imaging gold standard, US showed a 100 % sensitivity and specificity for flexor tenosynovitis but lacked sensitivity for joint involvement because it failed to reveal joint capsule distension in the only joint involved. Similarly, physical examination showed a 100 % sensitivity and specificity for flexor sheath involvement.
Fournié et al. [35] conducted a single-center study comparing US findings in 25 fingers with RA and 25 PsA. Tenosynovitis was seen in both groups without significant statistical differences. Of interest is that extrasynovial changes (soft tissue inflammation or thickening and enthesitis) were found in 84 % of fingers with PsA with respect to none in the fingers with RA. The extrasynovial changes reflected enthesitis or soft tissue inflammation, with the main patterns being capsular enthesophyte, juxtaarticular periosteal reaction, enthesopathy at the site of deep flexor tendon insertion on the distal phalanx, and subcutaneous soft tissue thickening of the finger pad or entire finger.
Husic et al. [36] published the results of a prospective study aimed to investigate the association between PsA-specific clinical composite scores and US-verified pathology as well as comparison of clinical and US definitions of remission. DAPSA and CPDAI were the clinical variables, whereas minimal disease activity (MDA) and CPDAI = 0 or DAPSA ≤ 3.3 or Boolean’s remission definition and physician-judged remission were adopted as criteria of remission. The authors created minimal US disease activity which is MUDA defined as PD score = 0 and PD score ≤ 1 including joints, peritendinous tissue, tendons, and entheses. Interestingly, DAPSA but not CPDAI correlated with B-mode and PD synovitis, and US signs of enthesitis, dactylitis, tenosynovitis, and perisynovitis were not linked with clinical composites. Clinical remission or MDA was observed in 15.7 to 47.1 % of PsA patients, whereas US remission and MUDA were present in 4.3 and 20 % of patients. The results of this study induced to consider that PsA-specific composite scores partially reflect US findings.
To date, there is a wide body of evidence supporting the validity of US in the assessment of entheses [37–40] (Fig. 2d). Moreover, several scoring systems for the assessment of entheseal involvement have also been developed. However, most of them were not developed specifically in PsA patients [30, 41].
Frediani et al. [42], in a single-center study including 40 patients affected by PsA and 40 patients affected by RA, compared the prevalence of quadricipital enthesitis in PsA and RA patients and clinical or echostructural differences in this lesions between the two diseases. The study documented that enthesitis is more frequent in PsA patients. Knee inflammation was found in PsA patients with enthesitis regardless of the concomitant presence of joint effusion; none of the RA patients suffered from enthesitis alone. No significant correlation between the presence of peripatellar psoriatic lesions and enthesitis was found.
Similar results were found by Delle Sedie et al. in a cross-sectional study of 83 patients with PsA, who have demonstrated a prevalence of knee enthesitis of 39.7 % [43]. Scarpa et al. [44] studied 47 patients with early PsA. All patients underwent clinical evaluation, bone scintigraphy, and US assessment. US revealed signs of enthesitis on each site, which was positive to the bone scintigraphy.
US demonstrated also the ability to detect pre-clinical enthesitis. Preliminary studies showed the presence of enthesitis in asymptomatic patients with psoriasis [18, 19, 45].
Gisondi et al. [19] performed a cross-sectional study assessing sonographically the lower limb entheses of 30 asymptomatic patients with psoriasis and 30 healthy controls. The entheseal abnormalities were significantly higher in patients with psoriasis with respect to healthy controls. In particular, the thickness of all tendons examined was significantly higher in cases than in controls as well as the number of enthesophytes in all sites examined. Interestingly, the presence of enthesopathy was directly correlated with age, body mass index, and waist circumference and not with the duration and severity of psoriasis.
Following this line of research, we conducted a single-center study assessing the lower limb entheses, by B-mode and PD, of 45 patients with psoriasis and 45 healthy controls. US signs of enthesopathy were significantly higher in the group of psoriasis (32.9 %) than in healthy controls (8.4 %). PD was also more frequent in psoriasis group (0.9 %) than in healthy controls (none) [18]. With regards the distribution of entheseal abnormalities, the most affected site was the Achilles enthesis (9.5 %), followed by distal patellar enthesis (9.1 %), proximal patellar enthesis (6.9 %), quadriceps enthesis (6.4 %), and plantar aponeurosis enthesis (0.9 %). Finally, good reproducibility of US findings was reported in the study.
Tinazzi et al. [46] performed a longitudinal study in order to investigate the predictive value of US in diagnosing PsA in patients with psoriasis. The study included psoriatic patients without any clinical signs of arthritis. After 2 years of follow-up, about 23 % of patients (7 of 28 patients) with psoriasis developed PsA according the CASPAR criteria. The pattern of joint involvement was polyarticular in two patients and oligoarticular in five patients.
Bandinelli et al. [47] performed a cross-sectional study aimed to determine the prevalence of subclinical lower limb enthesitis in patients with early PsA using as comparator the clinical examination. The authors reported at least one entheseal abnormality in all PsA patients including the positivity of PD in 40.2 %. Moreover, US demonstrated higher sensitivity with respect the clinical examination in the detection of enthesopathy (100 versus 29.3 %).
Eder et al. [48] conducted a US study determining the utility of MAdrid Sonographic Enthesitis Index (MASEI) in classifying patients as having PsA and comparing entheseal abnormalities between patients with PsA, psoriasis alone, and healthy controls. The MASEI score was higher in patients with PsA than in those with psoriasis, and both those groups were higher than healthy control. MASEI damage was higher in patients with PsA compared to both patients with psoriasis and healthy controls, whereas no differences were found in the inflammatory MASEI. In discrepancy with the other studies, no significant difference in MASEI scores was found across the three groups in patients with a body mass index > 30. The sensitivity of the MASEI score to correctly classify patients as having PsA was 30 %, and the specificity was 95 % when compared to healthy control and 89 % when compared to psoriasis.
Recently, Acquacalda et al.[45] in a multicenter study determined the prevalence of US enthesitis in patients with psoriasis with or without musculoskeletal symptoms showing their evolution under 6 months of systemic treatments. US abnormalities were found in 97.1 % of the total population. A total of 27.9 % enthesitis were observed in all the population, 25.9 % in psoriasis, and 31.7 % in PsA. Neither group displayed PD signal.
Bandinelli et al. [20] demonstrated that hand and wrist US findings were independent of clinical early PsA. A total of 1,120 fingers and 224 wrists of 112 early PsA patients were assessed looking for active synovitis, erosions, and tenosynovitis. Active synovitis was more frequent at wrists (22.3 %), followed by tenosynovitis (2.6 %), while erosions were rare (0.8 %).
Skin and nail involvement
The recent availability of US equipment with PD frequency higher than 10 MHz enables a very sensitive visualization of the blood flow also at dermal level.
Recently, the main US changes of psoriatic plaque that include structural changes of both epidermis and dermis associated with blood flow increase within the dermis detected by PD technique were described. The thickening of both epidermis and dermis with respect to the surrounding normal skin and the hypoechoic band under the psoriatic area showing vascularization resulted in the most common US findings [22] (Fig. 2e).
At the nail level, the US changes are characterized by loss of the hyperechoic definition of the nail plates, thickening, and the fusion of both plates (with loss of the intermediate anechoic layer). It is important taking into account that a minimal quantity of blood flow can be detected in normal conditions within the nail bed [9] (Fig. 2f).
Preliminary results demonstrating the potential role of PD in the assessment of psoriatic plaque and onychopathy in patients with psoriasis and/or PsA have been recently published [49]. We demonstrated the PD criterion validity and responsiveness to change by a positive correlation with the clinical and histologic findings at psoriatic plaque level in patients receiving tumor necrosis factor (TNF)-α antagonist therapy [23, 50].
El Miedany et al. [51] performed a study to identify the US predictors of arthritis in 126 patients with psoriasis. They were evaluated both clinically and by US at 0, 6, and 12 months for synovitis/joint damage, enthesitis, and onychopathy. Joint changes were observed in 47 % of patients. Baseline synovial score/PD score ≥ 2 was associated with increased risk of structural progression: odds ratio (OR) = 1.98 versus 2.61 versus 2.66 (P < 0.001) for the clinical versus US and PD evaluation, respectively. An increased probability for structural progression in the presence of enthesitis was also observed (OR = 2.79 and 3.50) for our findings, whereas OR was 2.46 for clinical examination. Onychopathy was associated with structural joint damage (OR = 2.30). In multivariate logistic regression analysis, persistence of synovitis/enthesitis at 6 months of therapy was predictive of subsequent structural progression. Baseline grayscale score of ≥2, PD score of ≥2, presence of enthesitis, and onychopathy, all at baseline as well as persistent synovitis and enthesitis at 6 months, are predictors of progressive early PsA.
Sandobal et al. [52] investigated the US findings at fingernail level in patients with PsA and psoriasis compared with RA and healthy controls. All patients and control subjects showed US abnormalities. Those with PsA and psoriasis showed a higher number of compromised nails. When classifying those abnormalities, patients with PsA revealed loosening of the borders of the ventral plate, whereas patients with psoriasis showed focal hyperechoic involvement of the ventral plate without involvement of the dorsal plate. Patients of the control group could not be classified, although 31 of 55 showed thinning of the ventral plate without hyperechoic deposits. Patients with PsA showed significantly an increase of PD in distal interphalangeal joints and nail beds.
Treatment monitoring
Fiocco et al. [53] demonstrated the potential role of US in evaluating the response to therapy of persistent knee joint synovitis by a 12-month longitudinal study in pre- and post-arthroscopic synovectomy in RA and PsA patients. In another 12-month open label single-center study, the same author [54] followed up 27 knees in a small cohort of 20 patients (12 with RA and 8 with PsA) receiving TNF-α antagonist therapy. US showed a reduction of synovial membrane thickness (grayscale) and intra-articular blood flow (PD technique) over time.
Aydin et al. [55] in a study that included 43 patients with spondyloarthritis found a significant decrease of blood flow at the Achilles enthesis level after 2 months of TNF-α antagonist therapy.
Naredo et al. [56] in a large study involving 35 centers demonstrated that US including PD assessment can be used as a valuable tool to assess enthesis response to therapy in spondyloarthritis. This is the first study that separately evaluated response to therapy of different US abnormalities at multiple entheseal sites demonstrating a highly significant improvement of both grayscale abnormalities (hypoechogenicity and/or thickening) and PD signal. A significant improvement of adjacent bursitis has also been observed. Conversely, entheseal cortical abnormalities (bone erosion and/or enthesophytes) and calcifications did not improve throughout the follow-up period, in spite of therapy.
Recently, our group [23] demonstrated the sensitivity to change of PD assessments of psoriatic plaque over an 8-week period in a cohort of psoriasis and PsA patients receiving TNF-α antagonist therapy.
To date, the few data available in literature testing the sensitivity to change of PD in PsA have depicted its potential assessing only a single domain (i.e., joint, enthesis, tendon, or skin).
We have recently proposed [57] a preliminary PD composite score for the global assessment of blood flow changes after treatment in PsA patients. The PD composite score was called “Five Targets Power Doppler for Psoriatic Disease (5TPD)” that includes the assessment of joint, tendon with synovial sheath, enthesis, skin, and nail together. PD for each target was graded from 0 to 3 on the basis of the semiquantitative scoring systems previously suggested. The maximum total score of 5TPD is 15 as a result of the sum of all the five target PD scores. Our preliminary results showed a significant improvement of global 5TPD scores from baseline to 8 weeks after anti TNF-α treatment. Moreover, the inter and intra-observer κ values varied from good to excellent at baseline and follow-up, and the time spent on baseline US examinations was mean 10.5 ± 2.0 min SD and no more than 7 min for follow-up assessment.
More recently, Schäfer et al. [58] validated the Sonography of Large Joints in Rheumatology (SOLAR) score for the assessment of large joints of PsA and ankylosing spondylitis (AS) patients (which has previously been validated for RA). The score includes the assessment of synovitis and vascularization at shoulder, elbow, hip, and knee joint level using semiquantitative scorings (grade 0–3). A longitudinal assessment at baseline and 3 and 6 months after initiation of local or systemic therapy (DMARDs/biologics) was performed. Eighty-three of 126 patients enrroled were followed up for 6 months. All US scores demonstrated a marked improvement. The GSUS and the PDUS scores for all joint areas, except the PD score of the hip, exhibited a significant improvement (p < 0.05), while the grayscale findings of the knee showed even a highly significant (p < 0.001) change.
Differential diagnosis
There is a lack of evidence supporting the role of US in the differential diagnosis in PsA. Only one study conducted by our group raised the potential role of US in the differential diagnosis between RA and PsA distinguishing different pattern of inflammation at metacarpophalangeal (MCP) joint level [59]. We studied 80 RA patients and 20 PsA for detecting US findings indicative of the presence of joint cavity widening (JCW), due to synovial fluid and/or synovial hypertrophy, peritendinous extensor tendon inflammation (PTI pattern), and intra-articular or peritendinous PD signal.
In 83 MCP joints of PsA patients were found different degress of JCW. The 18 % of MCP joints showed synovial fluid whereas 82 % synovial hypertrophy. In 86.7 % MCP joints, intra-articular PD was detected. No PTI pattern was found. In PsA patients, a total of 82 MCP joints were assessed. The 65.8 % of MCP joints showed PTI pattern. In 92.5 % MCP joints, extra-articular PD signal was detected, whereas 34.1 % of MCP joints showed different degrees of JCW. Of note, the PTI pattern was frequently observed in patients with short disease duration (mean = 13.2 months; SD = 3.67).
Conclusions
Since the introduction of US in the field of rheumatology, the attention was concentrated to produce data supporting its utility in different types of chronic arthritis. This effort is now well compensated since the current literature offers data showing the key role that US plays in helping of early diagnosis, disease activity, and sensitivity to change [10–21].
In the last years, there have been open interesting areas of research aimed to develop new applications of US beyond the musculoskeletal area. Thanks to the increased competency of the rheumatologist, the standardization of the technique, and the availability of high-resolution transducers, it has been possible to provide a multi-target organ assessment of PsA including skin, nail, and vessels [10, 57].
PsA offers a multi-target, specific window for US. Its wide heterogeneity offers different possible applications for the US. Although there remains a long way to drive, the current published evidence reaffirms the insensitivity of standard clinical assessment and supports a range of feasible and effective uses for US in PsA, particularly in quantifying the inflammatory process, in defining the classification of the disease, and in treating more globally the psoriatic disease.
The joint and tendon inflammations are consolidated findings in PsA. US demonstrated to be a valid, sensitive, and reliable in the assessment of synovitis, tenosynovitis, and enthesitis including anatomical damage (bone and/or tendon erosions). Although the property of US in detecting subclinical joint or tendon involvement in patients with psoriasis seems to be a promise in terms of prognostic value, it still requires accumulating more evidence by longitudinal multicenter studies. However, it includes an interesting future area of research that can integrate dermatologists and rheumatologists in order to facilitate an early diagnosis in patients with psoriasis.
Other new potential area for US is represented by psoriasis of skin and nail. The studies in these topics are still preliminary but contain solid basis to think that US can follow the scientific process towards a validation, especially in the assessment of the activity of inflammatory process and in the responsiveness. Brief reports depicting the concurrent validity and treatment monitoring efficacy of US are already provided [22, 23]. Prospective studies assessing the strength of correlation between US results, clinical scoring systems, and histologic findings are needed in order to define the value of US findings as an outcome measure.
Despite a great deal of work much remains to be done, this overview showed how US might possess the necessary attributes to facilitate best clinical practice in the management of patients with PsA.
Applications of this imaging technology continue to be developed, and further opportunities are likely to arise, informed by ongoing research strategies, especially to test its concurrent validity, reliability, and responsiveness in multicenter studies.
References
Weger W (2010) Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents. Br J Pharmacol 160:810–820
Thorlund K, Druyts E, Aviña-Zubieta JA, Mills EJ (2012) Anti-tumor necrosis factor (TNF) drugs for the treatment of psoriatic arthritis: an indirect comparison meta-analysis. Biologics 6:417–427
Ash Z, Gaujoux-Viala C, Gossec L, Hensor EM, FitzGerald O, Winthrop K, van der Heijde D, Emery P, Smolen JS, Marzo-Ortega H (2012) A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 71:319–326
Nell-Duxneuner VP, Stamm TA, Machold KP, Pflugbeil S, Aletaha D, Smolen JS (2010) Evaluation of the appropriateness of composite disease activity measures for assessment of psoriatic arthritis. Ann Rheum Dis 69:546–549
Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS (2010) Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 69:1441–1447
Mumtaz A, Gallagher P, Kirby B, Waxman R, Coates LC, Veale JD, Helliwell P, FitzGerald O (2011) Development of a preliminary composite disease activity index in psoriatic arthritis. Ann Rheum Dis 70:272–277
Helliwell PS, Fitz Gerald O, Fransen J, Gladman DD, Kreuger GG, Callis-Duffin K, McHugh N, Mease PJ, Strand V, Waxman R, Azevedo VF, Beltran Ostos A, Carneiro S, Cauli A, Espinoza LR, Flynn JA, Hassan N, Healy P, Kerzberg EM, Lee YJ, Lubrano E, Marchesoni A, Marzo-Ortega H, Porru G, Moreta EG, Nash P, Raffayova H, Ranza R, Raychaudhuri SP, Roussou E, Scarpa R, Song YW, Soriano ER, Tak PP, Ujfalussy I, de Vlam K, Walsh JA (2013) The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 72:986–991
Colebatch AN, Edwards CJ, Ostergaard M, Van der Heijde D, Balint PV, D’Agostino MA, Forslind K, Grassi W, Haavardsholm EA, Haugeberg G, Jurik AG, Landewe RB, Naredo EO, Connor PJ, Ostendorf B, Potocki K, Schmidt WA, Smolen JS, Sokolovic S, Watt I, Conaghan PG (2013) EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis 72:804–814
Mandl P, Navarro-Compán V, Terslev L, Aegerter P, van der Heijde D, D’Agostino MA, Baraliakos X, Pedersen SJ, Jurik AG, Naredo E, Schueller-Weidekamm C, Weber U, Wick MC, Bakker PA, Filippucci E, Conaghan PG, Rudwaleit M, Schett G, Sieper J, Tarp S, Marzo-Ortega H, Østergaard M (2015) EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 74:1327–1339
Gutierrez M, Filippucci E, De Angelis R, Filosa G, Kane D, Grassi W (2010) A sonographic spectrum of psoriatic arthritis: “the five targets”. Clin Rheumatol 29:133–142
De Simone C, Caldarola G, D’Agostino M, Carbone A, Guerriero C, Bonomo L, Amerio P, Magarelli N (2011) Usefulness of ultrasound imaging in detecting psoriatic arthritis of fingers and toes in patients with psoriasis. Clin Dev Immunol 2011:390726
Wiell C, Szkudlarek M, Hasselquist M, Møller JM, Vestergaard A, Nørregaard J, Terslev L, Østergaard M (2007) Ultrasonography, magnetic resonance imaging, radiography, and clinical assessment of inflammatory and destructive changes in fingers and toes of patients with psoriatic arthritis. Arthritis Res Ther 9:R119
Coates LC, Hodgson R, Conaghan PG, Freeston JE (2012) MRI and ultrasonography for diagnosis and monitoring of psoriatic arthritis. Best Pract Res Clin Rheumatol 26:805–822
Sankowski AJ, Lebkowska UM, Cwikła J, Walecka I, Walecki J (2013) The comparison of efficacy of different imaging techniques (conventional radiography, ultrasonography, magnetic resonance) in assessment of wrist joints and metacarpophalangeal joints in patients with psoriatic arthritis. Pol J Radiol 78:18–29
Kane D, Stafford L, Bresnihan B, FitzGerald O (2003) A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology 42:1460–1468
Kane D, Greaney T, Bresnihan B, Gibney R, FitzGerald O (1999) Ultrasonography in the diagnosis and management of psoriatic dactylitis. J Rheumatol 26:1746–1751
Bakewell CJ, Olivieri I, Aydin SZ, Dejaco C, Ikeda K, Gutierrez M, Terslev L, Thiele R, D’Agostino MA, Kaeley GS (2013) Ultrasound and magnetic resonance imaging in the evaluation of psoriatic dactylitis: status and perspectives. J Rheumatol 40:1951–1957
Gutierrez M, Filippucci E, De Angelis R, Salaffi F, Filosa G, Ruta S, Bertolazzi C, Grassi W (2011) Subclinical entheseal involvement in patients with psoriasis: an ultrasound study. Semin Arthritis Rheum 40:407–412
Gisondi P, Tinazzi I, El-Dalati G, Gallo M, Biasi D, Barbara LM, Girolomoni G (2008) Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case–control study. Ann Rheum Dis 67:26–30
Bandinelli F, Denaro V, Prignano F, Collaku L, Ciancio G, Matucci-Cerinic M (2015) Ultrasonographic wrist and hand abnormalities in early psoriatic arthritis patients: correlation with clinical, dermatological, serological and genetic indices. Clin Exp Rheumatol 33:330–335
Pistone G, La Vecchia M, Pistone A, Bongiorno MR (2014) Achilles tendon ultrasonography may detect early features of psoriatic arthropathy in patients with cutaneous psoriasis. Br J Dermatol 171:1220–1222
Gutierrez M, Wortsman X, Filippucci E, De Angelis R, Filosa G, Grassi W (2009) High-frequency sonography in the evaluation of psoriasis: nail and skin involvement. J Ultrasound Med 28:1569–1574
Gutierrez M, De Angelis R, Bernardini ML, Filippucci E, Goteri G, Brandozzi G, Lemme G, Campanati A, Grassi W, Offidani A (2011) Clinical, power Doppler sonography and histological assessment of the psoriatic plaque: short-term monitoring in patients treated with etanercept. Br J Dermatol 164:33–37
Gladman DD, Stafford-Brady F, Chang CH, Lewandowski K, Russell ML (1990) Longitudinal study of clinical and radiological progression in psoriatic arthritis. J Rheumatol 17:809–812
Backhaus M, Kamradt T, Sandrock D, Loreck D, Fritz J, Wolf KJ, Raber H, Hamm B, Burmester GR, Bollow M (1999) Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum 42:1232–1245
Backhaus M, Burmester GR, Sandrock D, Loreck D, Hess D, Scholz A, Blind S, Hamm B, Bollow M (2002) Prospective two year follow up study comparing novel and conventional imaging procedures in patients with arthritic finger joints. Ann Rheum Dis 61:895–904
Spadaro A, Lubrano E (2012) Psoriatic arthritis: imaging techniques. Reumatismo 64:99–106
Weiner SM, Jurenz S, Uhl M, Lange-Nolde A, Warnatz K, Peter HH, Walker UA (2008) Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis: a comparison with radiography, MRI and scintigraphy. Clin Rheumatol 27:983–989
Lin Z, Wang Y, Mei Y, Zhao Y, Zhang Z (2015) High-frequency ultrasound in the evaluation of psoriatic arthritis: a clinical study. Am J Med Sci 350:42–46
Ficjan A, Husic R, Gretler J, Lackner A, Graninger WB, Gutierrez M, Duftner C, Hermann J, Dejaco C (2014) Ultrasound composite scores for the assessment of inflammatory and structural pathologies in psoriatic arthritis (PsASon-Score). Arthritis Res Ther 16:476. doi:10.1186/s13075-014-0476-2
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 54:2665–2673
Olivieri I, Padula A, Scarano E, Scarpa R (2007) Dactylitis or “sausage-shaped” digit. J Rheumatol 34:1217–1222
Naredo E, D’Agostino MA, Wakefield RJ, Möller I, Balint PV, Filippucci E, Iagnocco A, Karim Z, Terslev L, Bong DA, Garrido J, Martínez-Hernández D, Bruyn GA, OMERACT Ultrasound Task Force* (2013) Reliability of a consensus-based ultrasound score for tenosynovitis in rheumatoid arthritis. Ann Rheum Dis 72:1328–1334
Olivieri I, Barozzi L, Favaro L, Pierro A, de Matteis M, Borghi C, Padula A, Ferri S, Pavlica P (1996) Dactylitis in patients with seronegative spondylarthropathy. Assessment by ultrasonography and magnetic resonance imaging. Arthritis Rheum 39:1524–1528
Fournié B, Margarit-Coll N, Champetier de Ribes TL, Zabraniecki L, Jouan A, Vincent V, Chiavassa H, Sans N, Railhac JJ (2006) Extrasynovial ultrasound abnormalities in the psoriatic finger. Prospective comparative power Doppler study versus rheumatoid arthritis. Joint Bone Spine 73:527–531
Husic R, Gretler J, Felber A, Graninger WB, Duftner C, Hermann J, Dejaco C (2014) Disparity between ultrasound and clinical findings in psoriatic arthritis. Ann Rheum Dis 73:1529–1536
Filippucci E, Aydin SZ, Karadag O, Salaffi F, Gutierrez M, Direskeneli H, Grassi W (2009) Reliability of high-resolution ultrasonography in the assessment of Achilles tendon enthesopathy in seronegative spondyloarthropathies. Ann Rheum Dis 68:1850–1855
De Miguel E, Muñoz-Fernández S, Castillo C, Cobo-Ibáñez T, Martín-Mola E (2011) Diagnostic accuracy of enthesis ultrasound in the diagnosis of early spondyloarthritis. Ann Rheum Dis 70:434–439
Wang CH, Feng Y, Ren Z, Yang X, Jia JF, Rong MY, Li XY, Wu ZB (2015) Performance of ultrasound to monitor Achilles enthesitis in patients with ankylosing spondylitis during TNF-a antagonist therapy. Clin Rheumatol 34:1073–1078
Grassi W, Filippucci E, Farina A, Cervini C (2000) Sonographic imaging of tendons. Arthritis Rheum 43:969–976
Alcalde M, Acebes JC, Cruz M, González-Hombrado L, Herrero-Beaumont G, Sánchez-Pernaute O (2007) A sonographic enthesitic index of lower limbs is a valuable tool in the assessment of ankylosing spondylitis. Ann Rheum Dis 66:1015–1019
Frediani B, Falsetti P, Storri L, Allegri A, Bisogno S, Baldi F, Marcolongo R (2002) Ultrasound and clinical evaluation of quadricipital tendon enthesitis in patients with psoriatic arthritis and rheumatoid arthritis. Clin Rheumatol 21:294–298
Delle Sedie A, Riente L, Filippucci E, Scirè CA, Iagnocco A, Gutierrez M, Valesini G, Montecucco C, Grassi W, Bombardieri S (2010) Sonographic assessment of the knee in patients with psoriatic arthritis. Clin Exp Rheumatol 28:147–152
Scarpa R, Cuocolo A, Peluso R, Atteno M, Gisonni P, Iervolino S, Di Minno MN, Nicolai E, Salvatore M, del Puente A (2008) Early psoriatic arthritis: the clinical spectrum. J Rheumatol 35:137–1341
Acquacalda E, Albert C, Montaudie H, Fontas E, Danre A, Roux CH, Breuil V, Lacour JP, Passeron T, Ziegler LE (2015) Ultrasound study of entheses in psoriasis patients with or without musculoskeletal symptoms: a prospective study. Joint Bone Spine 82:S1297–S1319
Tinazzi I, McGonagle D, Biasi D, Confente S, Caimmi C, Girolomoni G, Gisondi P (2011) Preliminary evidence that subclinical enthesopathy may predict psoriatic arthritis in patients with psoriasis. J Rheumatol 38:2691–2692
Bandinelli F, Prignano F, Bonciani D, Bartoli F, Collaku L, Candelieri A, Lotti T, Matucci-Cerinic M (2013) Ultrasound detects occult entheseal involvement in early psoriatic arthritis independently of clinical features and psoriasis severity. Clin Exp Rheumatol 31:219–224
Eder L, Jayakar J, Thavaneswaran A, Haddad A, Chandran V, Salonen D, Rosen CF, Gladman DD (2014) Is the Madrid Sonographic Enthesitis Index useful for differentiating psoriatic arthritis from psoriasis alone and healthy controls? J Rheumatol 41:466–472
Rodgers M, Epstein D, Bojke L, Yang H, Craig D, Fonseca T, Myers L, Bruce I, Chalmers R, Bujkiewicz S, Lai M, Cooper N, Abrams K, Spiegelhalter D, Sutton A, Sculpher M, Woolacott N (2011) Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess 15:1–329
Atteno M, Peluso R, Costa R (2010) Comparison of effectiveness and safety of infliximab, etanercept, and adalimumab in psoriatic arthritis patients who experienced an inadequate response to previous disease-modifying antirheumatic drugs. Clin Rheumatol 29:399–403
El Miedany Y, El Gaafary M, Youssef S, Ahmed I, Nasr A (2015) Tailored approach to early psoriatic arthritis patients: clinical and ultrasonographic predictors for structural joint damage. Clin Rheumatol 34:307–313
Sandobal C, Carbó E, Iribas J, Roverano S, Paira S (2014) Ultrasound nail imaging on patients with psoriasis and psoriatic arthritis compared with rheumatoid arthritis and control subjects. J Clin Rheumatol 20:21–24
Fiocco U, Cozzi L, Rubaltelli L, Rigon C, De Candia A, Tregnaghi A, Gallo C, Favaro MA, Chieco-Bianchi F, Baldovin M, Todesco S (1996) Long-term sonographic follow-up of rheumatoid and psoriatic proliferative knee joint synovitis. Br J Rheumatol 35:155–163
Fiocco U, Ferro F, Vezzù M, Cozzi L, Checchetto C, Sfriso P, Botsios C, Ciprian L, Armellin G, Nardacchione R, Piccoli A, Todesco S, Rubaltelli L (2005) Rheumatoid and psoriatic knee synovitis: clinical, grey scale, and power Doppler ultrasound assessment of the response to etanercept. Ann Rheum Dis 64:899–905
Aydin SZ, Karadag O, Filippucci E, Atagunduz P, Akdogan A, Kalyoncu U, Grassi W, Direskeneli H (2010) Monitoring Achilles enthesitis in ankylosing spondylitis during TNF-alpha antagonist therapy: an ultrasound study. Rheumatology (Oxford) 49:578–582
Naredo E, Batlle-Gualda E, García-Vivar ML, García-Aparicio AM, Fernández-Sueiro JL, Fernández-Prada M, Giner E, Rodriguez-Gomez M, Pina MF, Medina-Luezas JA, Toyos FJ, Campos C, Gutiérrez-Polo R, Ferrer MA, Martínez O, Díaz-Torne C, Gonzalez T, Campos S, Queiro R, Castaño-Sánchez M, Aznar JJ, Bustabad S, Paez-Camino M, Tuneu R, Ruiz T, Mateo L, Pujol M, Ponce A, Ros I, Gallegos A, Moreno J, Gumbau D, Sianes M, Poveda-Elices MJ, Romero-Gómez M, Raya E, Ultrasound Group of the Spanish Society of Rheumatology (2010) Power Doppler ultrasonography assessment of entheses in spondyloarthropathies: response to therapy of entheseal abnormalities. J Rheumatol 37:2110–2117
Gutierrez M, Di Geso L, Salaffi F, Bertolazzi C, Tardella M, Filosa G, Filippucci E, Grassi W (2012) Development of a preliminary US power Doppler composite score for monitoring treatment in PsA. Rheumatology (Oxford) 51:1261–1268
Schäfer VS, Fleck M, Kellner H, Strunk J, Sattler H, Schmidt WA, Ehrenstein B, Backhaus M, Hartung W (2013) Evaluation of the novel ultrasound score for large joints in psoriatic arthritis and ankylosing spondylitis: six month experience in daily clinical practice. BMC Musculoskelet Disord 19(14):358
Gutierrez M, Filippucci E, Salaffi F, Di Geso L, Grassi W (2011) Differential diagnosis between rheumatoid arthritis and psoriatic arthritis: the value of ultrasound findings at metacarpophalangeal joints level. Ann Rheum Dis 70:1111–1114
Disclosures
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Gutierrez, M., Draghessi, A., Bertolazzi, C. et al. Ultrasound in psoriatic arthritis. Can it facilitate a best routine practice in the diagnosis and management of psoriatic arthritis?. Clin Rheumatol 34, 1847–1855 (2015). https://doi.org/10.1007/s10067-015-3053-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3053-4