Abstract
The patients with respiratory failure need high tidal volume by mechanical ventilation, which lead to the ventilator-induced lung injury. We developed an extracorporeal lung and renal assist device (ELRAD), comprising acid infusion, membrane lung, continuous hemodiafiltration and alkaline infusion. To evaluate this system, we conducted in vivo studies using experimental swine which were connected to the new system. In vivo experiments consist of four protocols; baseline = hemodiafiltration only (no O2 gas flow to membrane lung); membrane lung = “Baseline” plus O2 gas flow to membrane lung; “Acid infusion” = “Membrane lung” plus continuous acid infusion; ELRAD = “Acid infusion” plus continuous alkaline infusion. We changed the ventilatory rate of the mechanical ventilation to maintain PCO2 at 50–55 mmHg during the four protocols. The results showed that there was statistically no significant difference in the levels of pH, HCO3−, and base excess when each study protocol was initiated. The amount of CO2 eliminated by the membrane lung significantly increased by 1.6 times in the acid infusion protocol and the ELRAD protocol compared to the conventional membrane lung protocol. Minute ventilation in the ELRAD protocol significantly decreased by 0.5 times compared with the hemodiafiltration only protocol (P < 0.0001), the membrane lung (P = 0.0006) and acid infusion protocol (P = 0.0017), respectively. In conclusion, a developed CO2 removal system efficiently removed CO2 at low blood flow and reduced minute ventilation, while maintaining acid–base balance within the normal range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with respiratory failure including acute respiratory distress syndrome often require a high minute volume of the mechanical ventilation to prevent not only hypoxia but also hypercapnia [1,2,3,4,5]. The high minute volume results in high driving pressure, which can potentially cause lung injury; recent guidelines recommend a low tidal volume of 4–8 ml/kg/predicted body weight [6, 7]. Because additional lung injury due to mechanical ventilation is obviously harmful to patients with respiratory failure, the development of alternative methods for CO2 removal which can avoid lung injury is a crucial research topic, and may contribute to improved clinical outcomes [8,9,10,11].
An extracorporeal carbon dioxide removal system (ECCO2R system), which is a system for CO2 removal from blood using extracorporeal circulation and an artificial membrane lung, was considered to be a practical method for clinical application [8, 12,13,14,15]. However, a conventional ECCO2R system requires high blood flow to achieve sufficient CO2 removal, which potentially limits the clinical application. To overcome this limitation, numerous CO2 removal approaches have been investigated [16, 17]. The initially reported techniques involved the addition of acid (e.g., lactic acid) to blood upstream to the ECCO2R system, which increased CO2 removal by changing bicarbonate ion to CO2 (H+ + HCO3−↔ H2CO3 ↔ CO2 + H2O) [18,19,20]. However, these trials resulted in a high level of accumulated molecules such as lactic acid. Since the blood lactate level is an important marker for critically ill patients, hyperlactatemia due to the infusion of lactic acid reduces the utility of blood lactate levels as a marker [21]. Furthermore, lactic acid causes vasodilation which may be harmful for critically ill patients [22]. More recent studies have reported on another CO2 removal system in which acid is added to the blood using an “electrolyte bath.” In these reports, the acid was generated by oxidation–reduction reaction instead of direct H+ addition [23, 24]. However, the system required a complex circuitry including several hemodialysis systems and multiple pumps, which would potentially result in the limitation of clinical use.
We have developed an ECCO2R system, which has a high efficiency of CO2 removal based on a simple and easy-to-setup to permit clinical application. The system comprises an acid infusion, membrane lung, continuous hemodiafiltration (CHDF) for pH adjustment and removal of accumulated molecules, and alkaline infusion. To evaluate this system, we conducted in vivo study using experimental swine, taking potential application for humans into consideration. Since this system has the ability to remove CO2 and provide renal support through continuous hemodiafiltration, we coined it as the Extracorporeal Lung and Renal Assist Device (ELRAD).
Materials and methods
Extracorporeal circulation system
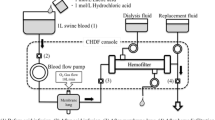
The ELRAD system was created by combining devices which are commonly used in clinical practice including vascular access catheter (12Fr, GentleCath®, Nippon Sherwood, Tokyo, Japan), bedside console and extracorporeal circuit for CHDF (TR-525®, TORAY Medical, Tokyo, Japan), hemofilter (FB-150Uβeco® [membrane surface 1.5 m2], NIPRO, Osaka, Japan), dialysate fluid (Sublood BSG® [HCO3− 35 mEq/L, Cl− 111.5 mEq/L], Fuso Pharmaceutical Industries, Osaka, Japan), replacement fluid (Bicarbon® [HCO3− 25 mEq/L, Cl− 113 mEq/L], AY Pharmaceuticals, Tokyo, Japan) and a membrane lung (Capiox RX05 Baby RX Oxygenator® [membrane surface 0.5 m2], TERUMO, Ann Arbor, MI, USA). The membrane lung was interposed between the blood pump and hemofilter (Fig. 1).
Hydrochloric acid (Wako Pure Chemical Industries, Osaka, Japan) was titrated with distilled water to produce a 0.5 mol/L acid solution [25], and trometamol (concentration 0.3 mol/L) (THAM®, Otsuka, Tokushima, Japan) was used as an alkaline solution for adjustment of the bicarbonate ion. The acid infusion port was set upstream to the membrane lung, and the alkaline infusion port was set downstream to the hemofilter. Unfractionated heparin (Heparin Sodium, Mochida Pharmaceutical Co., Ltd. Tokyo, Japan.) was used as an anticoagulant for extracorporeal circulation.
Swine model
Six healthy female crossbred pigs (age, 3–4 months; body weight, 38 ± 2 kg) were studied at Fujita Health University, Japan. Propofol and rocuronium bromide were used through an ear vein as their respiration was synchronized for mechanical ventilation after intubation [see Appendix (Supplemental Digital content)].
Mechanical ventilator and ELRAD setting
Oral endotracheal intubation and pressure-controlled mechanical ventilation (EvitaXL®, Dräger Medical, Lübeck, Germany) were performed under anesthesia. The inspired oxygen fraction was fixed at 0.4, the positive end expiratory pressure (PEEP) at 5 cm H2O, and tidal volume at 8 mL/kg, respectively.
The double lumen vascular access catheter, which was inserted into the right jugular vein, was connected to the ELRAD circuit. The blood flow in the ELRAD circuit was 200 mL/min, O2 gas flow of the membrane lung was 10 L/min, dialysate flow was 1000 mL/h, filtration flow 1500 mL/h and replacement flow 500 mL/h. The acid and alkaline infusion rate was 480 mL/h.
Experimental protocols
The in vivo experiment consisted of four protocols; each protocol took 1 h. To align the initial condition of each protocol, an interval period of 1 h was utilized between protocols (Fig. 2).
- Baseline:
hemodiafiltration only (no O2 gas flow to membrane lung)
- Membrane lung:
“Baseline” plus O2 gas flow to membrane lung
- Acid infusion:
“Membrane lung” plus continuous acid infusion
- ELRAD:
“Acid infusion” plus continuous alkaline infusion
To evaluate the efficiency of CO2 removal using the extracorporeal system for the four protocols, we changed the ventilatory rate of the mechanical ventilation to maintain PCO2 at 50–55 mmHg during the each of the protocols. The ventilator setting in the interval period was the same as during the preparation period and replacement flow was set to 20 mL/h in the acid infusion and ELRAD protocol.
Sample collection and measurement
Blood was collected from the artery line at the end of each protocol and interval, and immediately measured using the blood gas analyzer (iSTAT®, Abbott Point of Care Inc., Abbott Park, Illinois, USA). Blood cell counts (white, red blood cells, and platelet) and blood chemistry were determined using blood collected before the implementation of the membrane lung protocol and after the ELRAD protocol (Bio Majesty JCA-BM6050®; JEOL Ltd., Tokyo, Japan). Previously reported normal ranges for swine blood data were used as Ref. [26].
The amount of CO2 eliminated by the membrane lung (VCO2ML) was measured using a CO2 analyzer (CGP-31®; TOA-DKK Co. Ltd., Tokyo, Japan) which was connected to the gas-out port of membrane lung. Vital signs (blood pressure, heart rate, and body temperature) and SpO2 were continuously recorded in all experiments.
Tidal volume, ventilatory rate, minute ventilation (tidal volume × ventilatory rate), peak pressure and plateau pressure were recorded at the point of the blood gas analysis and the beginning and end of each protocol.
This study was approved by the Institutional Animal Care and Use Committee of Fujita Health University. All experiments were performed with strict adherence to the Guidelines for proper conduct of animal experiments proposed by Science Council of Japan.
Statistical analysis
The primary outcome variables were VCO2ML and minute ventilation to maintain PCO2 at 50–55 mmHg. The secondary outcome variables were measurement parameters of the blood cell counts and blood chemistry. The variables were compared using a paired t test based on an initial baseline and the preceding baseline of each protocol, and Tukey’s multiple comparisons test for mechanical ventilator variables and VCO2ML. Any differences were considered to be statistically significant using a two-tailed value of P < 0.05. Data were presented as mean values with standard errors. Analyses were performed using R version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-roject.org/) and PRISM version 7 (GraphPad Software, Inc., La Jolla, California, USA).
Results
All the experiments were successfully conducted as planned in the six healthy swine. The levels of PCO2 were tightly maintained within a target range of 50–55 mmHg and did not significantly differ throughout the entire period of the experiment (P = 0.22) (Table 1). There was no statistically significant difference in the levels of pH, HCO3− and the base excess between baseline, interval I and II (pH, P = 0.44; HCO3−, P = 0.31; base excess, P = 0.30).
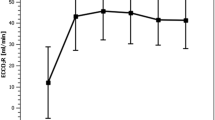
In the analysis of VCO2ML which is a primary outcome variable, this parameter significantly increased by 1.6 times in the acid infusion protocol and ELRAD protocol compared to the membrane lung protocol (acid infusion, P < 0.0001; ELRAD, P = 0.0003) (Fig. 3). There was no statistically significant difference between the acid infusion protocol and the ELRAD protocol (P = 0.55).
In the analysis of minute ventilation which is co-primary outcome variable, this parameter significantly decreased in the ELRAD protocol by 0.5 times compared to the baseline protocol (P < 0.001), and also significantly decreased compared to the membrane lung and acid infusion protocol (membrane lung, P = 0.0006; acid infusion, P = 0.0017) (Table 2).
In the secondary analysis of the study on the blood tests, there was a decrease in the sodium level for the blood gas analysis (Table 1), the white blood cells, and levels of total protein, albumin, ALT, and magnesium (Table 3). An increase in the AST levels was observed at the end of the experiment compared to the initial baseline protocol, while all these values were within the normal range for the experimental swine (Table 3).
Discussion
The ELRAD, which comprised an acid infusion system, a membrane lung, hemodiafiltration and an alkaline infusion, significantly increased CO2 removal compared to the control condition of the membrane lung. The ELRAD significantly reduced the minute ventilation by half compared to the baseline protocol.
The investigation of efficient CO2 elimination by regional acidification has been reported [19, 20]. A previous study on the addition of 0.5 mEq/L lactic acid upstream of the membrane lung was shown to remove 70% more CO2 compared to the baseline condition without acid infusion [18]. In accord with this result, our ELRAD system using hydrochloric acid as an acid solution could remove 60% more blood CO2 content compared to the conventional method using a membrane lung.
In another previous study, regional blood acidification using lactic acid in an ECCO2R system increased VCO2ML, but failed to reduce minute ventilation [27]. In accord with this result, the acid infusion protocol of our experiment was not able to reduce the minute ventilation. Investigators have speculated that the decreased pH [28, 29] or caloric intake effect of lactic acid which decreases glycolysis [30, 31], might contribute to the failure. Since the hydrochloric acid chosen in the current study has no effect on the caloric intake, the unchanged minute ventilation in the acid infusion protocol may be associated with a decreased pH. In the ELRAD protocol, we used trometamol to correct the acid state due to loss of CO2 buffer, other than the sodium bicarbonate which potentially generates CO2 [32, 33]. Trometamol, which has low toxicity and short half-life (1.2 h), is used as an alkaline agent as a pure proton acceptor in metabolic acidosis according to the following formula; (CH2OH)3C-NH2 + HA ↔ (CH2OH)3C-NH3+ + A− (HA: acid) [34]. A pharmaceutical product of trometamol approved for intravenous administration was chosen in the study. In addition, it has been reported that trometamol might preserve myocardial contractility and hemodynamic stability in patients with acute respiratory distress syndrome. It may also attenuate ventilator-induced lung injury by decreasing intracellular CO2 content that impairs the pH-dependent protecting system against cellular injury and plasma membrane injury [35, 36].
Recently, another ECCO2R system using a new acidification method called “respiratory electrodialysis” was reported [23, 24]. In respiratory electrodialysis, electrons are used for blood acidification instead of the addition of H+ ions. These electrons are generated by oxidation–reduction reactions with a 14 Fr double lumen catheter, three hemodialysis systems, and four blood pumps, and the system was shown to reduce the minute ventilation from 6.66 ± 1.2 to 3.36 ± 0.8 L per minute in experiments using swine of similar size. In the current study, ELRAD had a similar efficiency of CO2 removal (minute ventilation reduced from 5.53 ± 0.33 to 2.87 ± 0.21 L per minute) compared to the respiratory electrodialysis system. Since the ELRAD system is constructed by incorporating the membrane lung into the circuit of a conventional continuous hemodiafiltration, it is more simple and easy-to-setup. ECCO2R system connected with continuous renal replacement therapy console without regional acidification and alkaline infusion was also reported [37]. An existing lung and renal replacement system reduced tidal volume from 6 to 4 ml/kg at blood flow rate of 420 mL/min but increased PaCO2 from 43 to 53 mmHg and decreased pH from 7.39 to 7.32. Compared to this existing lung and renal replacement system, our system had higher CO2 removal efficiency and halved minute ventilation at lower blood flow rate (200 mL/min) within normal range of PaCO2 and pH, which highlighted the clinical advantage of ELRAD.
The ELRAD system does not only play the role of respiratory assist, but also plays the role of renal assist, which may help to mitigate against several undesired outcomes that were highlighted in previous studies. First, respiratory electrodialysis and several ECCO2R studies using lactic acid have shown a significant increase of the creatinine level [20, 23], while the creatinine level remained within the normal range in the current ELRAD study. Second, acid infusion can possibly increase the levels of accumulated molecules. In a previous study on acid infusion using lactic acid, the blood level of lactate increased from 4.2 at the baseline time point to 15.1 mmol/L at the 15 min time point. However, in our study, there was no significant increase of the blood chlorine ion. Thus, regional acidification by hydrochloric acid with hemodiafiltration can maintain normal levels of blood chlorine ions.
In previous studies based on lactic acid infusion, there was no hemolysis observed when the pH of the blood reached 6.9–7.0 [18, 20]. In accord with these results, no signs of hemolysis were observed in the laboratory test of the blood cell counts and blood chemistry, in the present study. Since inhalation anesthetic had an effect on the CO2 exchange at the swine lung, we chose intravenous anesthetic for this study [38]. This resulted in the requirement of a large amount of fluid due to the vasodilation effect of anesthetic. A positive fluid balance may contribute to a slight decrease in the levels of hemoglobin, total protein, and albumin.
The present experimental study has several limitations. First, the ELRAD system requires a high administration rate of the acid solution. In addition, the safety use of acid infusion to human blood is not established. However, in a previous study using up to 1 mol/L of hydrochloric acid for the treatment of metabolic alkalosis, no hemolysis was observed [25, 39,40,41]. We decided to use 0.5 mol/L hydrochloric acid as a safe concentration and an administration rate of 480 ml/h, in reference to another blood acidification study which reported 5 mEq/min infusion of 0.5 mol/L lactic acid to 500 mL/min blood flow and obtained more than 130 mmHg CO2. A safety mechanism which checks the pH upstream to the reinfusion will be considered for clinical application. Second, we did not evaluate the oxygenation of the ELRAD system, while the oxygen saturation was kept in the range of 99–100% during all experiments. The oxygenation ability of the membrane lung we used at a blood flow rate of 200 mL/min could only maintain 15 mL/min oxygen [42], which is far less than the 3.5 mL/kg/min oxygen consumption of humans [43]. The ELRAD system is inadequate for oxygenation, therefore, we did not evaluate the oxygenation capability. Finally, we fixed the dialysate flow rate of 1000 mL/h and filtration flow rate of 500 mL/h in all protocol to evaluate whether hydrochloric acid infusion would alter serum levels of chlorine ion or not. We maintained the fluid balance by adjusting replacement flow rate from 500 to 20 mL/h in the acid infusion protocol, while we did not add further adjustment of the hemodiafiltration settings in ELRAD protocol in which the fluid balance increased. This positive fluid balance may contribute to temporarily decreased hemoglobin level in the ELRAD protocol. In further study, the fluid balance should be controlled by adjustment of dialysate flow.
Conclusions
We developed an efficient CO2 removal system, ELRAD using acid and alkaline infusion, with a membrane lung incorporated into a CHDF console at low blood flow. This novel system removed 1.6 times the volume of CO2 compared to a conventional method and reduced minute ventilation to about a half compared to the control model while maintaining the acid–base equilibrium within normal range. Further studies are needed prior to the clinical application of this easy setup system comprising of materials that are typically found in a clinical setting.
Abbreviations
- CHDF:
-
Continuous hemodiafiltration
- ECCO2R system:
-
Extracorporeal carbon dioxide removal system
- ELRAD:
-
Extracorporeal lung and renal assist device
- PEEP:
-
Positive end expiratory pressure
- VCO2ML:
-
The amount of CO2 eliminated by the membrane lung
References
Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903.
Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med. 2006;32:24–33.
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36.
Biehl M, Kashiouris MG, Gajic O. Ventilator-induced lung injury: minimizing its impact in patients with or at risk for ARDS. Respir Care. 2013;58:927–37.
Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710.
Zampieri FG, Mazza B. Mechanical ventilation in sepsis: a reappraisal. Shock (Augusta, Ga). 2017;47:41–6.
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–63.
Cove ME, MacLaren G, Federspiel WJ, Kellum JA. Bench to bedside review: extracorporeal carbon dioxide removal, past present and future. Crit Care (London, England). 2012;16:232.
Mani RK, Schmidt W, Lund LW, Herth FJ. Respiratory dialysis for avoidance of intubation in acute exacerbation of COPD. ASAIO J. 2013;59:675–8.
Cressoni M, Zanella A, Epp M, Corti I, Patroniti N, Kolobow T, Pesenti A. Decreasing pulmonary ventilation through bicarbonate ultrafiltration: an experimental study. Crit Care Med. 2009;37:2612–8.
Pesenti A, Patroniti N, Fumagalli R. Carbon dioxide dialysis will save the lung. Crit Care Med. 2010;38:S549–54.
Kreyer S, Scaravilli V, Linden K, Belenkiy SM, Necsoiu C, Li Y, Putensen C, Chung KK, Batchinsky AI, Cancio LC. Early utilization of extracorporeal CO2 removal for treatment of acute respiratory distress syndrome due to smoke inhalation and burns in sheep. Shock. 2016;45:65–72.
Sklar MC, Beloncle F, Katsios CM, Brochard L, Friedrich JO. Extracorporeal carbon dioxide removal in patients with chronic obstructive pulmonary disease: a systematic review. Intensive Care Med. 2015;41:1752–62.
Del Sorbo L, Pisani L, Filippini C, Fanelli V, Fasano L, Terragni P, Dell’Amore A, Urbino R, Mascia L, Evangelista A, et al. Extracorporeal Co2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med. 2015;43:120–7.
Wearden PD, Federspiel WJ, Morley SW, Rosenberg M, Bieniek PD, Lund LW, Ochs BD. Respiratory dialysis with an active-mixing extracorporeal carbon dioxide removal system in a chronic sheep study. Intensive Care Med. 2012;38:1705–11.
Batchinsky AI, Jordan BS, Regn D, Necsoiu C, Federspiel WJ, Morris MJ, Cancio LC. Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med. 2011;39:1382–7.
Livigni S, Maio M, Ferretti E, Longobardo A, Potenza R, Rivalta L, Selvaggi P, Vergano M, Bertolini G. Efficacy and safety of a low-flow veno-venous carbon dioxide removal device: results of an experimental study in adult sheep. Crit care (London, England). 2006;10:R151.
Zanella A, Patroniti N, Isgro S, Albertini M, Costanzi M, Pirrone F, Scaravilli V, Vergnano B, Pesenti A. Blood acidification enhances carbon dioxide removal of membrane lung: an experimental study. Intensive Care Med. 2009;35:1484–7.
Zanella A, Mangili P, Giani M, Redaelli S, Scaravilli V, Castagna L, Sosio S, Pirrone F, Albertini M, Patroniti N, et al. Extracorporeal carbon dioxide removal through ventilation of acidified dialysate: an experimental study. J Heart Lung Transpl. 2014;33:536–41.
Zanella A, Mangili P, Redaelli S, Scaravilli V, Giani M, Ferlicca D, Scaccabarozzi D, Pirrone F, Albertini M, Patroniti N, et al. Regional blood acidification enhances extracorporeal carbon dioxide removal: a 48-hour animal study. Anesthesiology. 2014;120:416–24.
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M. Sepsis definitions task F: developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:775–87.
De La Lande IS, Whelan RF. The role of lactic acid in the vasodilator action of adrenaline in the human limb. J Physiol. 1962;162:151–4.
Zanella A, Castagna L, Salerno D, Scaravilli V, Abd El Aziz El Sayed Deab S, Magni F, Giani M, Mazzola S, Albertini M, Patroniti N et al. Respiratory electrodialysis. A novel, highly efficient extracorporeal CO2 removal technique. Am J Respir Crit Care Med. 2015;192:719–726.
Zanella A, Castagna L, Abd El Aziz El Sayed Deab S, Scaravilli V, Ferlicca D, Magni F, Giani M, Salerno D, Casati M, Pesenti A. Extracorporeal CO2 removal by respiratory electrodialysis: an in vitro study. ASAIO J. 2016;62:143–149.
Kwun KB, Boucherit T, Wong J, Richards Y, Bryan-Brown CW. Treatment of metabolic alkalosis with intravenous infusion of concentrated hydrochloric acid. Am J Surg. 1983;146:328–30.
Swindle MM, Smith AC. Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques. 3rd ed. Boca Raton: CRC Press; 2015. p. 523–36.
Scaravilli V, Kreyer S, Belenkiy S, Linden K, Zanella A, Li Y, Dubick MA, Cancio LC, Pesenti A, Batchinsky AI. Extracorporeal carbon dioxide removal enhanced by lactic acid infusion in spontaneously breathing conscious sheep. Anesthesiology. 2016;124:674–82.
Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit care (London, England). 2006;10:R79.
Chang YC, Huang KT, Chen YM, Wang CC, Wang YH, Tseng CC, Lin MC, Fang WF. Ventilator dependence risk score for the prediction of prolonged mechanical ventilation in patients who survive sepsis/septic shock with respiratory failure. Sci Rep. 2018;8:5650.
Zanella A, Giani M, Redaelli S, Mangili P, Scaravilli V, Ormas V, Costanzi M, Albertini M, Bellani G, Patroniti N, et al. Infusion of 2.5 meq/min of Lactic Acid minimally increases CO2 production compared to an isocaloric glucose infusion in healthy anesthetized, mechanically ventilated pigs. Crit care (London, England). 2013;17:R268.
Choi CS, Kim YB, Lee FN, Zabolotny JM, Kahn BB, Youn JH. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am J Physiol Endocrinol Metab. 2002;283:E233–40.
Hoste EA, Colpaert K, Vanholder RC, Lameire NH, De Waele JJ, Blot SI, Colardyn FA. Sodium bicarbonate versus THAM in ICU patients with mild metabolic acidosis. J Nephrol. 2005;18:303–7.
Kallet RH, Jasmer RM, Luce JM, Lin LH, Marks JD. The treatment of acidosis in acute lung injury with tris-hydroxymethyl aminomethane (THAM). Am J Respir Crit Care Med. 2000;161:1149–53.
Nahas GG, Sutin KM, Fermon C, Streat S, Wiklund L, Wahlander S, Yellin P, Brasch H, Kanchuger M, Capan L, et al. Guidelines for the treatment of acidaemia with THAM. Drugs. 1998;55:191–224.
Weber T, Tschernich H, Sitzwohl C, Ullrich R, Germann P, Zimpfer M, Sladen RN, Huemer G. Tromethamine buffer modifies the depressant effect of permissive hypercapnia on myocardial contractility in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;162:1361–5.
Caples SM, Rasmussen DL, Lee WY, Wolfert MZ, Hubmayr RD. Impact of buffering hypercapnic acidosis on cell wounding in ventilator-injured rat lungs. Am J Physiol Lung Cell Mol Physiol. 2009;296:L140–4.
Schmidt M, Jaber S, Zogheib E, et al. Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care. 2018;22:122.
Tranquilli WJ. Techniques of inhalation anesthesia in ruminants and swine. Vet Clin North Am Food Anim Pract. 1986;2:593–619.
Knutsen OH. New method for administration of hydrochloric acid in metabolic alkalosis. Lancet (London, England). 1983;1:953–6.
Brimioulle S, Vincent JL, Dufaye P, Berre J, Degaute JP, Kahn RJ. Hydrochloric acid infusion for treatment of metabolic alkalosis: effects on acid-base balance and oxygenation. Crit Care Med. 1985;13:738–42.
Nasimi A, Cardona J, Berthier M, Oriot D. Hydrochloric acid infusion for treatment of severe metabolic alkalosis in a neonate. Clin Pediatr. 1996;35:271–2.
Terumo cardiovascular systems corporation. http://www.terumo-cvs.com/doc/863551_CAPIOX-FX-Advance-Brochure_DEC2015_FINAL.pdf.
Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–65.
Funding
Yoshimi Memorial T.M.P. Grant.
Author information
Authors and Affiliations
Contributions
NT and TN contributed to the study’s conceptualization and design, the acquisition of data, analysis, and interpretation of data, statistical analysis, and drafting and critical revision of the manuscript for intellectual content. TS, YK, and KM contributed to the acquisition of data, interpretation of data, and critical revision of the manuscript for intellectual content. ON and SO contributed to the study’s conceptualization and design and critical revision of the manuscript for intellectual content. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. This work was supported by JSPS KAKENHI Grant Number 17K17042 and Yoshimi Memorial TMP Grant from the Japanese Society for Artificial Organs. The funder had no role in the study design, experiments, collection, analysis, interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takahashi, N., Nakada, Ta., Sakai, T. et al. A CO2 removal system using extracorporeal lung and renal assist device with an acid and alkaline infusion. J Artif Organs 23, 54–61 (2020). https://doi.org/10.1007/s10047-019-01136-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-019-01136-0