Abstract
A computed tomography (CT)-based navigation system is one of the support tools to place implant with appropriate alignment and position in total hip arthroplasty (THA). To determine whether the higher performance of the navigation would further improve the accuracy of implant placement in the clinical setting, we retrospectively compared the navigation accuracy of two different versions of a navigation system. The newer version of the navigation system had an upgraded optical sensor with superior positional accuracy. Navigation accuracy, defined as differences between postoperative measurements on CT images and intraoperative records on the navigation system, of 49 THAs performed with the newer version of the navigation system was compared with that of 49 THAs performed with the older version. With the newer version, the mean absolute accuracy (95% limits of agreement) of implant alignment was 1.2° (± 3.3°) for cup inclination, 1.0° (± 2.4°) for cup anteversion, 2.0° (± 4.9°) for stem anteversion, and 1.1° (± 2.4°) for stem valgus angle. The accuracy of the implant position was 1.5 mm (± 3.1 mm), 1.3 mm (± 3.0 mm), and 1.5 mm (± 3.1 mm) for cup x-, y-, and z-axes, respectively, 1.6 mm (± 3.2 mm), 1.4 mm (± 2.9 mm), and 1.5 mm (± 2.7 mm) for stem x-, y-, and z-axes, respectively, and 2.4 mm (± 4.5 mm) for leg length discrepancy. The values for the newer version were significantly more accurate with less variation compared to those of the older version. With upgraded navigation performance, more accurate implant placement was demonstrated in the clinical setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In total hip arthroplasty (THA), implant malalignment is one of the major technical problems leading to clinical complications due to edge loading [1, 2] or implant impingement [3,4,5,6]. Clinical complications include accelerated wear [1], dislocation [3, 4], implant breakage [2], and implant loosening [5, 6]. Postoperative leg length discrepancy (LLD) is another complication of THA. Excessive LLD may cause back pain, gait disorders, or limping, which leads to patient dissatisfaction [7]. Therefore, implant placement with appropriate alignment and position is essential to make the artificial joint function well without these problems.
Computed tomography (CT)-based navigation systems can help to minimize these technical errors. Use of this navigation system involves the following steps: (1) preoperative planning of target alignment and positioning using a computer model created from preoperative CT images; (2) registration of the computer model and actual bone landmarks; and (3) tracking and measurement of the target bone and surgical tools or implants during the operation. Therefore, to best support surgeons, the tracked and measured implant alignment and position information should be shown as accurately as possible. This accuracy is based on several factors, including the quality of the optical sensor and other hardware, the quality of software, technical errors, and system measurement error [8,9,10]. Accordingly, hardware improvement has been attempted to provide a more accurate system. However, no reports have shown that improved performance has provided a more accurate display for implant alignment and positioning in the clinical setting.
To confirm whether a better performance of the CT-based navigation system improves the accuracy of implant placement in the clinical setting, we retrospectively compared the accuracy of THA implant placement between an older version of the navigation system and a newer version with upgraded performance.
Materials and methods
Navigation systems
Two different versions of the CT-based navigation system were used for THA. The older version (Stryker Navigation Cart System, Stryker Leibinger GmbH & Co. KG, Freiberg, Germany) had an optical sensor (Stryker FlashPoint 5000 Senor Array Camera, Stryker Leibinger GmbH & Co. KG, Freiberg, Germany), and the newer version (Stryker Navigation System2 Cart, Stryker Leibinger GmbH & Co. KG, Freiberg, Germany) had a higher quality optical sensor (Stryker FlashPoint 6000 Senor Array Camera, Stryker Leibinger GmbH & Co. KG, Freiberg, Germany). The FlashPoint 6000 showed superior positional accuracy with superior spatial reasoning capacity, a wider optical sensor sweet spot, and a digital communication line (Table 1).
Subjects
This retrospective study was approved by the institutional review board of our hospital. In our institution, a CT-based navigation system has been used to perform THA since October 2007. The older version of the navigation system was replaced with the newer version in January 2012. To investigate the accuracy of both navigation systems, subjects who underwent primary cementless THA with the same brand of prosthesis were selected according to the following criteria: (1) both cup and stem implantation were carried out under the guidance of the CT-based navigation system without any intraoperative failure of the navigation system; (2) the same brand of cementless acetabular cup (Trident PSL, Stryker Orthopaedics, Mahwah, NJ) and cementless femoral stem (Accolade TMZF, Stryker Orthopaedics, Mahwah, NJ) were implanted; and (3) there were no intraoperative or postoperative complications related to implant fixation, such as early implant migration or periprosthetic fracture. We selected the femoral stem to evaluate accuracy, because this stem was implanted with both versions of the navigation system and a sufficient period of time had elapsed to eliminate the influence of the initial learning curve for using the navigation system. 49 hips from 48 patients who underwent THA with the prostheses between July 2010 and December 2011 were included for the older version group. Consecutive 49 hips from 47 patients who underwent THA between January 2012 and June 2012 were included for the newer version group. All operations were performed by three surgeons: 1 senior surgeon (experienced surgeon) who was performing more than 100 THAs annually, and 2 resident surgeons (inexperienced surgeons) who were performing less than 30 THAs annually. Table 2 shows the demographic data and operator of both cohorts.
Preoperative planning
Preoperative CT images of the entire pelvis and bilateral femurs from each patient were taken using a helical CT scanner with 3-mm slice thickness. CT data were uploaded to the navigation planning workstation. Eight reference points for the pelvis (bilateral anterior superior iliac spines, bilateral pubic tubercles, the most distal point of bilateral ischia, mid pubic symphysis, and sacral mid-plane) and six reference points for each femur (center of femoral head, trochanteric fossa, the most posterior point of the greater trochanter, bilateral posterior condyles, and the center of the knee) were taken to create the pelvic and femoral coordinate systems. Three-dimensional (3D) surface models of the pelvis and bilateral femurs were then constructed using a semiautomatic segmentation procedure. The optimal alignment and position of the cup and stem were planned using three multiplanar reconstruction views and a 3D volume-rendering view. Cup alignment was adjusted based on stem anteversion [11].
Surgical procedure
All THAs were performed using either version of the navigation system through a mini-posterior approach. In both the groups, operation including registration was performed in the same manner consistently. A pelvic tracker was percutaneously fixed on the ilium before skin incision. Following dislocation of the hip joint, a femoral tracker was fixed on the greater trochanter. Registration of the femur was performed by surface-matched digitizing of at least 30 points on the femoral surface with a pointer, confirmed by touching characteristic points of the femur such as the greater trochanter, lesser trochanter, trochanteric fossa, and medial and lateral epicondyles. A verification point of the femur to check for intraoperative loosening of femoral tracker fixation was created on the greater trochanter. After femoral rasping was performed, orientation (anteversion and valgus angle) and position were checked.
Registration of the pelvis was performed by surface matching and was confirmed by touching characteristic points of the pelvis such as the anterior, posterior, and superior rim of the acetabulum, acetabular fossa, anterior superior iliac spine, and greater sciatic notch. A verification point for the pelvis was created on the posterosuperior portion of the acetabular rim. After reaming, the acetabular cup was implanted using the navigation guide, and final alignment (anteversion and inclination) and position of the cup were recorded. Fixation of the pelvic tracker was checked by touching its verification point.
Subsequently, the femoral stem was implanted, and the final alignment (anteversion and valgus angle) and position of the stem and LLD were recorded after changing the neck length of the femoral head to achieve optimal leg length. Fixation of the femoral tracker was checked by touching its verification point. Hips in which stability of either the pelvic or femoral trackers could not be verified were excluded.
Postoperative measurements
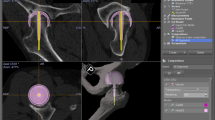
Postoperative CT images were routinely obtained within 2-week postoperation to detect the early complications. To adjust for the differences in the pelvic and femoral coordinate systems between the pre- and postoperative CT images, the semiautomatic volume registration technique was used with 3D image-processing software (Virtual Place-M; Medical Imaging Laboratory, Tokyo, Japan) [12, 13]. The reproducibility of this method has been reported as within 0.7° and 0.8 mm [14]. Then, the same-sized computer-aided design (CAD) model of the implanted cup or stem was superimposed on the postoperative images of the actually implanted prostheses. Thereafter, the postoperative alignment (anteversion and inclination) and position of the cup, the postoperative alignment (anteversion and valgus angle) and position of the stem, and LLD were recorded. Navigation accuracy, defined as the difference between postoperative measurements on CT images and intraoperative records on the navigation system, was evaluated in both groups. The radiographic definition of cup anteversion and inclination was used for the cup alignment [15]. To spatially evaluate the cup and stem position, three orthogonal axes [x-(transverse) axis, y-(sagittal) axis, and z-(longitudinal) axis] were defined in the pelvic and femoral coordinate systems. Cup position was evaluated based on the location of the point of the cup’s spherical center, and stem position was evaluated based on the location of the point where the stem neck axis and stem body axis intersected.
Statistical analysis
Power analysis suggested that 32 hips would be required in each group to achieve adequate power of 0.8 for detecting a minimum difference of 1° (α = 0.05, one-sided), which was assumed according to a previous report [16]. Statistical analyses were performed between the groups using the two-sided Student’s t test for age and body mass index (BMI) and the χ2 test for gender, diagnosis, and distribution of surgeons. To compare navigation accuracy, one-sided Student’s t tests for comparisons of absolute differences and F tests for comparisons of variation were performed. The 95% limits of agreement (± 1.96 × standard deviation) were used to show variation. To assess inter- and intra-observer error with this measurement method, 20 cases were randomly selected and the alignment and position of the cup and stem were measured twice at a 1-month interval by two of the authors. The Pearson’s correlation coefficients (r) were evaluated to determine inter- and intra-observer variability. Statistical significance was assigned for p values less than 0.05.
Results
There were no significant differences between the groups in age, sex, BMI, preoperative diagnosis, and distribution of surgeons (Table 2).
In all the alignment parameters, the navigation accuracy in the newer version group showed significantly smaller absolute differences and less variation than the older version group (Table 3). For cup inclination, cup anteversion, stem anteversion, and stem valgus angle, 95% limits of agreement were within ± 3.3°, ± 2.4°, ± 4.9°, and ± 2.4° in the newer version group and ± 4.8°, ± 3.9°, ± 7.4°, and ± 3.4° in the older version group, respectively. The improvement of the mean absolute difference was 0.7°, 0.6°, 1.2°, and 0.7° for cup inclination, cup anteversion, stem anteversion, and stem valgus angle, respectively.
In all positional parameters of implants and LLD, the navigation accuracy in the newer version group showed significantly smaller absolute differences and less variation than the older version group (Table 3). For cup x-, y-, and z-axes, stem x-, y-, and z-axes, and LLD, 95% limits of agreement were within ± 3.1, ± 3.0, ± 3.1, ± 3.2, ± 2.9, ± 2.7, and ± 4.5 mm in the newer version group and ± 5.5, ± 5.1, ± 5.7, ± 5.2, ± 5.2, ± 5.5, and ± 9.4 mm in the older version group, respectively. The improvement in the mean absolute difference was 0.7, 0.6, 0.7, 0.7, 0.9, 0.8, and 1.4 mm for cup x-, y-, z-axes, stem x-, y-, z-axes, and LLD, respectively.
Inter- and intra-observer variabilities for implant alignment were 0.4° ± 1.8° and 0.1° ± 0.8° for cup inclination, 0.1° ± 1.8° and 0.1° ± 1.1° for cup anteversion, 0.7° ± 1.5 and 0.2° ± 1.0° for stem anteversion, and 0.1° ± 0.5° and 0.1° ± 0.2° for stem valgus angle. The Pearson’s correlation coefficients for implant alignment were 0.90, 0.92, 0.78, 0.96, 0.97, 0.99, 0.96, and 0.94, respectively. Inter- and intra-observer variabilities for implant position were 0.1° ± 0.4 mm and 0.1° ± 0.3 mm for cup x-axis, 0.2 ± 0.3 mm and 0.2° ± 0.3 mm for cup y-axis, 0.4° ± 0.8 mm and 0.0° ± 0.6 mm for cup z-axis, 0.0° ± 0.6 mm and 0.2° ± 0.3 mm for stem x-axis, 0.3° ± 0.5 mm and 0.0° ± 0.3 mm for stem y-axis, and 0.5° ± 0.9 mm and 0.4° ± 1.0 mm for stem z-axis. All the Pearson’s correlation coefficients for implant position were 0.99.
Discussion
This study was the first to compare navigation accuracy between older and newer navigation system versions in the clinical setting. In the present study, the same implant, the same operation technique, and the same postoperative measurement method were used in two similar cohorts. Significantly superior navigation accuracy could then be demonstrated in the newer version group, including superior alignment, positioning, and LLD. Some previous reports have investigated accuracy of imageless navigation systems [17,18,19,20,21,22,23,24,25] and CT-based navigation systems [16, 25,26,27,28,29] (Table 4). The in the older version group was comparable to the reported accuracy of the CT-based navigation systems and was superior to the reported accuracy of the imageless navigation systems. The accuracy in the newer version group was further improved compared to those reported previously.
There are some conceivable causes for differences between the intraoperative record and postoperative measurement, including navigation error and postoperative measurement error. Postoperative measurement error may be caused by differences in evaluated coordinate systems between the intraoperative record and postoperative measurement [30] as well as measurement error from postoperative CT images by manual CAD templating. Therefore, we created postoperative coordinate systems using the volume registration technique to minimize the deviation. Moreover, inter- and intra-observer variability analysis for the manual CAD templating demonstrated high reliability. These indicated that postoperative measurement error could be minimized and the differences between the intraoperative record and postoperative measurement were mainly caused by navigation error.
In addition to the superior spatial reasoning capacity of the optical sensor, the wider sweet spot of the optical sensor can have reduced errors associated with operation at a boundary area of the sweet spot. Moreover, fast communication by digitalization can have reduced errors associated with time lag between operation of navigation tools and computer recognition. These improved navigation specifications might enable more accurate intraoperative measurement of implant alignment and position as well as more acceptable registration. Then, the improved navigation specifications lead to the superior accuracy in clinical setting.
It was reported that cup inclination less than 35° significantly reduced prosthetic range of motion [31] and cup inclination more than 45° accelerated wear [1]. Widmer et al. [32] suggested that acetabular cups should be oriented between 20° and 28° of anteversion to clear the required range of motion in the simulation. In clinical setting, Sugano et al. [33] suggested that the true safe zone to avoid dislocation or mechanical complication seemed to be 36°–45° of inclination and 10°–24° of anteversion. Accordingly, acceptable range of cup alignment is about 10° and navigation accuracy for cup alignment should preferably be within ± 5° to prevent complications. In the present study, although significant differences could be noticed in navigation accuracy for cup alignment, variations of navigation accuracy were within ± 5° in both groups, which indicated that the difference was negligible in terms of the impact on clinical results.
Stem orientation is also an important factor for achieving optimal range of motion and joint stability in THA [32]. Previously, however, there has been no report on the required accuracy for stem alignment. In consideration of our target angle [11], approximately 5° of cup anteversion change is necessary for each 10° increment of stem anteversion. Thus, the navigation accuracy for stem anteversion should be within ± 10° when the required accuracy for cup alignment was set to ± 5°. In the present study, although there were significant differences between the groups, variations of navigation accuracy for stem anteversion were within ± 10° in both groups, which indicated that the difference may also be negligible in consideration of clinical results.
In the current CT-based navigation system, acetabular reaming and cup placement could be performed by checking the alignment and position in real time on the monitor. The positional navigation accuracy was, therefore, important, because surgeons could be informed of correct position of the implant in bone and thereby perform the operation safely. In a simulation, perforation of the acetabular wall could be observed when an acetabular cup was displaced 5-mm backward from the optimal position (Fig. 1). In such a case, the actual cup position in the bone should also be verified to confirm that perforation did not occur. One anatomical report showed that the minimum thickness of the medial acetabular wall in Crowe-I was an average of 3.8 ± 2.1 mm [34], suggesting that the acetabular bone stock is relatively thin and high positional accuracy is required to accurately display the cup position. The newer version group demonstrated within ± 3.1 mm of variation in any direction, while the older version group demonstrated within ± 5.7 mm of variation in any direction. This result indicates that safer surgery can be performed with trust of surgeons when the newer version navigation system is used. Standardization of treatment outcome can be expected by eliminating an error the navigation system.
LLD following THA is a common reason for patient dissatisfaction [7]. Some reports indicate that an LLD greater than 10 mm will cause patient dissatisfaction following THA [35, 36]. In the present study, the newer version group showed 2.4 mm of mean absolute navigation accuracy with ± 4.6 mm of variation, while the older version group showed 3.8 mm of mean absolute navigation accuracy with ± 9.6 mm of variation. This result indicated that the newer version navigation system was more reliable for the control of leg length in consideration of the safety margin. The superior positional accuracy also contributed to the reliable control of leg length using the newer navigation system.
There were a few limitations of the present study. Because period of operation was different between the groups, it might have been affected by a learning curve of the navigation. To eliminate the influence of the learning curve, we targeted cases in which THA was performed after sufficient time elapsed from the introduction of navigation. Although we observed a significant difference in accuracy between the groups, the effect of the difference on clinical results was not evaluated. Additional studies are needed to show clinical advantages of the upgraded navigation system. These clinical data will encourage further improvement of the navigation system.
Conclusion
The upgraded CT-based navigation system demonstrated superior navigation accuracy in terms of implant alignment and implant position. This improved accuracy of implant position helps surgeons to more easily adjust leg length and check accurate implant position in host bone in real time on the navigation monitor, which can result in safer surgery.
References
Wan Z, Boutary M, Dorr LD. The influence of acetabular component position on wear in total hip arthroplasty. J Arthroplasty. 2008;23:51–6.
Tower SS. Rim cracking of the cross-linked longevity polyethylene acetabular liner after total hip arthroplasty. J Bone Joint Surg. 2007;89:2212–7.
Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11:89–99.
Bader R, Steinhauser E, Zimmermann S, Mittelmeier W, Scholz R, Busch R. Differences between the wear couples metal-on-polyethylene and ceramic-on-ceramic in the stability against dislocation of total hip replacement. J Mater Sci Mater Med. 2004;15:711–8.
Chen PY, Wu CT, Hou CH, Hou SM. Loosening of total hip arthroplasty with a prosthesis employing a skirted femoral head. J Formos Med Assoc. 2005;104:370–3.
Shon WY, Baldini T, Peterson MG, Wright TM, Salvati EA. Impingement in total hip arthroplasty. J Arthroplasty. 2005;20:427–35.
Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85:2310–7.
Sugano N, Sasama T, Sato Y, Nakajima Y, Nishii T, Yonenobu K, Tamura S, Ochi T. Accuracy evaluation of surface-based registration methods in a computer navigation system for hip surgery performed through a posterolateral approach. Comput Aided Surg. 2001;6:195–203.
Li Q, Zamorano L, Jiang Z, Gong JX, Pandya A, Perez R, Diaz F. Effect of optical digitizer selection on the application accuracy of a surgical localization system?a quantitative comparison between the OPTOTRAK and flashpoint tracking systems. Comput Aided Surg. 1999;4:314–21.
Schmerber S, Chassat MSF. Accuracy evaluation of a CAS system: laboratory protocol and results with 6D localizers, and clinical experiences in otorhinolaryngology. Comput Aided Surg. 2001;6:1–13.
Sugano N, Tsuda K, Miki H, Takao M, Suzuki N, Nakamura N. Dynamic measurements of hip movement in deep bending activities after total hip arthroplasty using a 4-dimensional motion analysis system. J Arthroplasty. 2012;27:1562–8.
Munch B, Ruegsegger P. 3-D repositioning and differential images of volumetric CT measurements. IEEE Trans Med Imaging. 1993;12:509 – 14.
Holden M, Hill DL, Denton ER, Jarosz JM, Cox TC, Rohlfing T, Goodey J, Hawkes DJ. Voxel similarity measures for 3-D serial MR brain image registration. IEEE Trans Med Imaging. 2000;19:94–102.
Watanabe Y, Masumoto J, Sasama T, Sato Y, Sugano N, Nishii T, Miki H, Yoshikawa H, Ochi T, Tamura S. Preprocessing method for rigid registration between pre- and postoperative CT images in total hip replacement. Med Imag Tech. 2003;21:358 – 68.
Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75:228 – 32.
Iwana D, Nakamura N, Miki H, Kitada M, Hananouchi T, Sugano N. Accuracy of angle and position of the cup using computed tomography-based navigation systems in total hip arthroplasty. Comput Aided Surg. 2013;18:187–94.
Ybinger T, Kumpan W, Hoffart HE, Muschalik B, Bullmann W, Zweymüller K. Accuracy of navigation-assisted acetabular component positioning studied by computed tomography measurements: methods and results. J Arthroplasty. 2007;22:812–7.
Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89:494–9.
Ryan JA, Jamali AA, Bargar WL. Accuracy of computer navigation for acetabular component placement in THA. Clin Orthop Relat Res. 2010;468:169 – 77.
Jenny JY, Boeri C, Dosch JC, Uscatu M, Ciobanu E. Navigated non-image-based positioning of the acetabulum during total hip replacement. Int Orthop. 2009;33:83 – 7.
Fukunishi S, Nishio S, Fujihara Y, Okahisa S, Takeda Y, Fukui T, Yoshiya S. Accuracy of combined anteversion in image-free navigated total hip arthroplasty: stem-first or cup-first technique? Int Orthop. 2016;40:9–13.
Takeda Y, Fukunishi S, Nishio S, Fujihara Y, Yoshiya S. Accuracy of component orientation and leg length adjustment in total hip arthroplasty using image-free navigation. Open Orthop J. 2017;11:1432–9.
Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26:596–605.
Sendtner E, Schuster T, Wörner M, Kalteis T, Grifka J, Renkawitz T. Accuracy of acetabular cup placement in computer-assisted, minimally-invasive THR in a lateral decubitus position. Int Orthop. 2011;35:809 – 15.
Kalteis T, Handel M, Bäthis H, Perlick L, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty: is it as accurate as CT-based navigation? J Bone Joint Surg Br. 2006;88:163–7.
Hananouchi T, Takao M, Nishii T, Miki H, Iwana D, Yoshikawa H, Sugano N. Comparison of navigation accuracy in THA between the mini-anterior and -posterior approaches. Int J Med Robot. 2009;5:20–5.
Hirasawa N, Matsubara M, Ishii K, Hagio S, Okuda N, Sekiya I, Muneta T. Effect of CT slice thickness on accuracy of implant positioning in navigated total hip arthroplasty. Comput Aided Surg. 2010;15:83–9.
Kitada M, Nakamura N, Iwana D, Kakimoto A, Nishii T, Sugano N. Evaluation of the accuracy of computed tomography—based navigation for femoral stem orientation and leg length discrepancy. J Arthroplasty. 2011;26:674–9.
Kajino Y, Kabata T, Maeda T, Iwai S, Kuroda K, Tsuchiya H. Does degree of the pelvic deformity affect the accuracy of computed tomography-based hip navigation? J Arthroplasty. 2012;27:1651–7.
Kyo T, Nakahara I, Kuroda Y, Miki H. Effects of coordinate-system construction methods on postoperative computed tomography evaluation of implant orientation after total hip arthroplasty. Comput Aided Surg. 2015;20:52–60.
Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14:509–13.
Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22:815–21.
Sugano N, Takao M, Sakai T, Nishii T, Miki H. Does CT-based navigation improve the long-term survival in ceramic-on-ceramic THA? Clin Orthop Relat Res. 2012;470:3054–9.
Liu RY, Wang KZ, Wang CS, Dang XQ, Tong ZQ. Evaluation of medial acetabular wall bone stock in patients with developmental dysplasia of the hip using a helical computed tomography multiplanar reconstruction technique. Acta Radiol. 2009;50:791–7.
Austin MS, Hozack WJ, Sharkey PF, Rothman RH. Stability and leg length equality in total hip arthroplasty. J Arthroplasty. 2003;18:88–90.
O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20:505–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nakahara, I., Kyo, T., Kuroda, Y. et al. Effect of improved navigation performance on the accuracy of implant placement in total hip arthroplasty with a CT-based navigation system. J Artif Organs 21, 340–347 (2018). https://doi.org/10.1007/s10047-018-1041-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-018-1041-6