Abstract
Although we have used an intravenous continuous glucose monitor for blood glucose management, a previous study reported that a subcutaneous continuous glucose monitor was also reliable for use in critically ill patients. The aim of this study was to compare the subcutaneous and intravenous continuous glucose monitors. This was an observational trial (UMIN-CTR, ID:000013338). We included patients who were admitted to our intensive care units (ICU) after hepato-biliary pancreatic surgery. Continuous blood glucose measurement was performed from the beginning of the operation to ICU discharge using the intravenous continuous monitor STG-55 (Nikkiso, Tokyo, Japan) and the subcutaneous continuous monitor iPro2 (Medtronic Japan, Tokyo, Japan). The STG-55 measured the glucose level in real time, and the iPro2 measured this every 5 min. We compared glucose levels obtained using the two devices every 5 min using a Bland–Altman plot and a regression analyses. A total of 3592 comparative samples in 15 cases were analyzed. The mean glucose level measured using the STG-55 was 139 ± 21 mg/dl, and that measured using the iPro2 was 144 ± 31 mg/dl. A linear regression line had the equation of the form y = 0.225x + 106. The coefficient of determination was 0.11, and the F-test significance level was set as p < 0.01. The mean of the differences was −5.2 mg/dl, with a 95 % agreement limit of −67 to + 57 mg/dL. The percent error was 44 %. In conclusion, the current study suggests that subcutaneous and intravenous continuous glucose monitoring was not highly correlated during either surgery or ICU stay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood glucose management is important for surgical patient in operating room and intensive care units (ICUs). Recent evidences suggest that perioperative hyperglycemia significantly contributes to the development of infection and it is associated with poor surgical outcomes after cardiac surgery [1, 2]. In the ICU, hyperglycemia is a risk factor of hospital mortality [3]. On the other hand, an association also exists between even mild or moderate hypoglycemia and mortality in critically ill patients [4]. Therefore, it is important to avoid not only hyperglycemia, but also hypoglycemia in perioperative glycemic control. We used intravenous continuous glucose monitoring with closed-loop glucose control to avoid hypoglycemia and reduce the nurses’ workloads [5, 6]; however, this technique was problematic because of the interruption of blood glucose measurement and automatic glucose control caused by blood removal failure [6].
On the other hand, a previous study reported that a subcutaneous continuous glucose monitor was reliable for use in critically ill patients [7]. We felt a subcutaneous continuous glucose monitor would be more useful compared to an intravenous continuous glucose monitor because blood removal failure would be less likely to occur. Although some studies have evaluated the accuracy of a subcutaneous continuous glucose monitor compared to standard devices such as a blood gas analyzer or other subcutaneous device, no study has as yet compared a subcutaneous continuous device with an intravenous continuous device [7–10]. Therefore, we conducted a prospective observational study to evaluate the accuracy of a subcutaneous continuous glucose monitor compared to an intravenous continuous glucose monitor in an operating room and in an ICU.
Materials and methods
This was a prospective observational trial registered in the University Hospital Medical Information Network Clinical Trial Registry System (UMIN-CTR, ID:000013338). Our hospital’s ethics committee approved the study, and informed consent was obtained from all patients. We included patients who had undergone scheduled hepato-biliary pancreatic surgery.
After general anesthesia induction, a 20-G intravenous catheter was inserted into a peripheral vein and was connected to the intravenous continuous glucose monitor STG-55 (Nikkiso, Tokyo, Japan). The subcutaneous continuous glucose monitor iPro2 (Medtronic Japan, Tokyo, Japan) was inserted into the subcutaneous tissue of the ipsilateral upper arm. Continuous blood glucose measurement was performed from the beginning of the operation to ICU discharge. The STG-55 measured the glucose level in real time.
The STG-55 continuously monitored blood glucose levels via a blood-sampling technique using a dual-lumen catheter and a glucose sensor electrode with a glucose oxidase membrane [6, 11]. Before starting the procedure, two-point internal calibration was performed using a standard solution (glucose concentration, 0 mg/dL) and a standard glucose solution (200 mg/dL). During blood glucose monitoring, internal calibration was automatically performed at 4-h intervals using the standard solution. The STG-22, the previous generation of the model, is a highly accurate and reliable system [11, 12]. In a previous study, the correlation between blood glucose levels, measured with the STG-22 and a blood gas analyzer (ABL800FLEX, Radiometer Medical Aps, Denmark), was evaluated by calculating the Pearson’s correlation coefficient, which was estimated at 0.96 [12].
The iPro2 measured glucose levels every 5 min using the glucose oxidase method. This device stored the sensor signal information internally, and it was retrospectively calibrated after the device was removed from the patient [13]. Retrospective calibration allowed the calibration algorithm to use information both before and after the time point of interest to obtain an optimal calibration for each reference point [13]. In this study, we defined 1 and 4 h after the start of the operation, at ICU admission, and at 7 a.m. on postoperative day 1 as reference points. Reference glucose data were based on arterial blood values and were obtained using the ABL800FLEX. The uploading of these data to web-based software provided a summary of the glucose measurements. Scheme of methodology in measurements of glucose is shown in Fig. 1.
We excluded the data during the automatic calibration of the STG-55, which occurred every 4 h. In addition, we calculated points at which blood glucose measurement was interrupted by a blood removal failure of the STG-55. Then, we excluded these points from analysis.
Data are presented as mean ± standard deviation. We compared the glucose levels obtained using the two devices every 5 min using a Bland–Altman plot and regression analyses. The Bland–Altman analysis was used to compare the bias (the mean of the differences) and limits of agreement (bias ±2 standard deviations of bias) between blood glucose measured by STG-55 and iPro2. The percentage errors were also calculated. We defined the STG-55 as the standard device in this study. In addition, we tested whether our measurements met the International Organization for Standardization (ISO) criteria; that is, for glucose values measured less than 100 mg/dl by the STG-55, the iPro2 values were within ±15 mg/dl, and for glucose valued measured more than 100 mg/dl by the STG-55, the iPro2 values were within ±15 % [14].
Results
A total of 19 patients were enrolled in this study. The STG-55 broke down in three cases, and the iPro2 did not export data in one case; thus, 15 cases were analyzed. Three patients received 1–5 μg/kg/min of dopamine for hypotension (Table 1).
Operation room
We collected 685 comparative samples. Of these, 34 were excluded because the STG-55 could not measure blood glucose owing to calibration. In addition, 46 samples were excluded because the STG-55 could not measure blood glucose owing to blood removal failure (6.7 % of all samples). The mean glucose level measured using the STG-55 was 125 ± 29 mg/dl, and that measured using the iPro2 was 138 ± 26 mg/dl. A scatter diagram of the blood glucose levels measured with the STG-55 and iPro-2 is shown in Fig. 2a. A linear regression line had the equation of the form y = 0.814x + 12.5, where x is the STG-55 blood glucose level and y is the iPro-2 blood glucose level; the coefficient of determination R 2 was 0.516 (<100 mg/dl, 0.050; 100–150 mg/dl, 0.268; >150 mg/dl, 0.324). Then, we constructed a Bland–Altman plot, as shown in Fig. 3a. The x-axis represents arithmetical averages (STG-55 blood glucose level + iPro2 blood glucose level)/2) across the range of 82.1–266.4. The y-axis represents differences (STG-55 blood glucose level—iPro2 blood glucose level). In this case, the differences vary from −90.7 to 65.4. The three lines represent the 95 % upper limit of agreement, the arithmetical average of the differences and the 95 % lower limit of agreement from the top. The SD of the differences was 21.0. We computed the upper and lower limit agreement as −13.1 (the arithmetical average of the differences) plus and minus 2 times 21.00(SD), which yielded 28.9 and −55.1. We found that 4.95 % (30 of 605) of the data points are outliers, exceed the upper limit of agreement, or fall in the lower limit of agreement. For the limits of agreement, the differences were evaluated for a Normal distribution in two ways: (1) Kolmogorov–Smirnov test (p value = 0.071 > 0.05) and (2) histogram (Fig. 4a). The x-axis contains the differences in increment of 5 mg/dl. The y-axis represents relative frequencies. The percentage error is 30.5 % (2 times 21.0 (SD of the differences) divided by 145 (the arithmetical average of STG-55 blood glucose levels) multiplied by 100). Thirty-six percent were within ±15 mg/dl of the intravenous values at glucose concentrations below 100 mg/dl, and 59 % were within ±15 % of the intravenous values at glucose concentrations at or above 100 mg/dl.
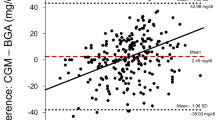
The x-axis is for arithmetical averages ((STG-55 value + iPro2 value)/2), and the y-axis for differences (STG value—iPro2 value). The a, b and c are during operation, during ICU and overall, respectively. For every figure, three straight lines has the equations: from the top, y = arithmetical average of the differences + 2 × (standard deviation of the differences), y = arithmetical average of the differences, y = arithmetical average of the differences—2 × (standard deviation of the differences)
ICU room
We collected 3145 comparative samples. Of these, 158 were excluded because of removal failure (4.5 % of all samples). The mean glucose level measured using the STG-55 was 142 ± 18 mg/dl, and that measured using the iPro2 was 145 ± 31 mg/dl. A scatter diagram of the blood glucose levels measured with the STG-55 and iPro-2 is shown in Fig. 2b. A linear regression line had the equation of the form y = 0.125x + 123, where x is the STG-55 blood glucose level and y is the iPro-2 blood glucose level, the coefficient of determination R 2 was 0.051 (<100 mg/dl, 0.017; 100–150 mg/dl, 0.067; >150 mg/dl, 0.019). Then, we constructed a Bland–Altman plot, as shown in Fig. 3b. The x-axis represents arithmetical averages across the range of 85.9–266. In this case, the differences vary from −157 to 129. The SD of the differences was 32.4. We computed the upper and lower limit agreement as −3.56 plus and minus 2 times 32.4 (SD of the differences), which yielded 61.3 and −68.4. 5.35 % (160 of 2987) of the data points are outliers, exceed the upper limit of agreement or fall the lower limit of agreement. For the limits of agreement, the differences were evaluated for a Normal distribution in two ways: (1) Kolmogorov–Smirnov test (p value <0.01) and (2) histogram (Fig. 4b). The x-axis contains the differences in increment of 5 mg/dl. The y-axis represents relative frequencies. The percentage error is 44.7 % [2 times 32.4 (SD of the differences) divided by 138 (the arithmetical average of STG-55 blood glucose levels) multiplied by 100]. Twenty-four percent of the patients were within ±15 mg/dl of the intravenous values at glucose concentrations below 100 mg/dl, and 61 % were within ±15 % of the intravenous values at glucose concentrations at or above 100 mg/dl.
Overall
The mean glucose level measured using the STG-55 was 139 ± 21 mg/dl, and that measured using the iPro2 was 144 ± 31 mg/dl. A scatter diagram of the blood glucose levels measured with STG-55 and iPro-2 is shown in Fig. 2c. A linear regression line had the equation of the form y = 0.225x + 106, where x is the STG-55 blood glucose level and y is the iPro-2 blood glucose level; the coefficient of determination R 2 was 0.109 (<100 mg/dl, 0.041; 100–150 mg/dl, 0.097; >150 mg/dl, 0.036). Then, we constructed a Bland–Altman plot as shown in Fig. 3c. The x-axis represents arithmetical averages across the range of 82.1–266. In this case, the differences vary from −157 to 129. The SD of the differences was 31.0. We computed the upper and lower limit agreement as −5.17 plus and minus 2 times 31.01(SD of the differences), which yields 56.9 and −67.2. Furthermore, 8.29 % (298 of 3592) of the data points are outliers, exceed the upper limit of agreement, or fall in the lower limit of agreement. For the limits of agreement, the differences were evaluated for a Normal distribution in two ways: (1) Kolmogorov–Smirnov test (p value <0.01) and (2) histogram (Fig. 4c). The percentage error is 43.1 % (2 times 31.0 (SD of the differences) divided by 144 (the arithmetical average of STG-55 blood glucose levels) multiplied by 100). Thirty-three percent of the patients were within ±15 mg/dl of the intravenous values at glucose concentrations below 100 mg/dL, and 61 % of the patients were within ±15 % of the intravenous values at glucose concentrations at or above 100 mg/dL.
Discussion
Several guidelines recommended arterial or venous whole-body sampling for blood glucose management in ICU patients [15, 16]. Although capillary glucose measurement is accurate in normotensive patients, several factors such as the presence of edema, a shock state, or the use of vasoconstrictors affect peripheral perfusion in ICU patients [17]. Thus, alternated peripheral perfusion might affect the accuracy of capillary glucose measurements [17]. In fact, previous reports described low accuracy of capillary glucose measurement with patients who received vasopressors, had edema, and were in a shock state [17–19]. On the other hand, one recent study showed that subcutaneous continuous glucose monitoring to guide insulin treatment in critically ill patients is as safe and effective as intermittent point-of-care measurements [20]. In our study, pH value and body temperature were within normal ranges. In addition, 80 % of the patients did not use vasopressors. Therefore, we thought that there were few factors that could affect subcutaneous measurement. The Pearson correlation coefficient was 0.92 in the previous study comparing subcutaneous continuous monitoring with a blood gas analyzer [7].
In another study that concluded the accuracy of subcutaneous continuous monitoring was relatively low in critically ill patients, the coefficient of determination was 0.65 and 75.8 % within ±20 % of the standard device at glucose concentrations at or above 75 mg/dl [9]. In our study, the coefficient of determination was 0.11 and 61 % within ±15 % of the intravenous values at glucose concentrations at or above 100 mg/dl. Our study defined the STG-55 as a standard device, unlike other studies. Therefore, the numbers of comparison points in our study differed greatly from those of other studies: 239 ± 45 vs. 11–12 per patient [7, 9]. This study included patients who received hepatectomy. In hepatectomy, the temporary clamp of the hepatic artery and the portal vein, namely the Pringle maneuver, is used to reduce intraoperative bleeding. Previous studies have shown a rapid, profound transition in glucose concentrations during hepatic resection using the Pringle maneuver [21, 22]. In fact, a rapid alternation of blood glucose occurred in this study. Interstitial fluid glucose generally correlated with blood glucose, with a lag time of between 0 and 45 min and an average lag of 8–10 min and a gradient between interstitial and plasma glucose concentrations varying between 20 and 110 % [23]. That is, if this situation were to greatly change, the differences would be caused by a time lag at almost all points. After ICU admission, patients received peripheral parental nutrition, and insulin administration was begun via the STG-55. The same situation was created by developing a fluid that included 7.5 % of glucose and insulin. Although the rapid changes in blood glucose levels caused by the Pringle maneuver was observed for a short period, the changes due to parenteral nutrition and insulin therapy continued during the ICU stay. In addition, both the volume of the plasma and the interstitial fluid affected interstitial fluid glucose concentration [23]. Therefore, altered fluid balance between the plasma and the interstitial space, such as edema, might have a harmful effect on the measurement results especially in patients in the ICU. We believe these factors were responsible for the lower correlation observed in the ICU compared to that in the operation room.
Although sensors were inserted under the abdominal skin in most other trials, we inserted it into the subcutaneous tissue of the upper arm in this study because the patients received abdominal surgery [9]. Previous studies have described how better accuracy was observed in subcutaneous continuous glucose sensors placed in the shoulder compared with those placed in the upper leg during the postoperative period [10]. Although subcutaneous sensors were inserted into the ipsilateral side in which the venous catheter was inserted to minimize influence, we could not deny the possibility that an upper arm and an abdominal difference affected the results.
We selected hepatectomy as one of the inclusion criteria because we thought it was important for continuous glucose monitoring devices to detect rapid alterations of glucose in ICU patients to prevent hypoglycemia. This study showed that the iPro2 did not follow glucose alterations in patients who received major surgery. However, we thought the results of this study did not deny the overall accuracy of subcutaneous continuous glucose monitor. Therefore, further prospective studies are required to prepare patients for subcutaneous continuous glucose monitoring.
Both subcutaneous and intravenous continuous glucose monitoring reduced the workloads of ICU nurses [5, 20]. Therefore, it is important for ICU staff, as well as patients, to improve the accuracy and usefulness of these devices. In this study, the STG-55 could not measure glucose levels in 5.1 % of all the samples. This value did not change for over a decade because in a previous study, we reported blood sampling was interrupted in 5.2 % of the cases [6]. In addition, the STG-55 might not be suitable for an emergency situation because it involves a preparation time of 60 min. On the other hand, the iPro2 involves a simple preparation and was conducted at all points in this study. However, the iPro2 was limited in terms of its utility as a continuous blood glucose measurement device for operation rooms and intensive care units because it could not display the real time blood glucose levels owing to the need for retrospective calibration. We thought blood glucose measurement during surgery and hemodynamic or fluid balance instability might be suitable for the intravenous method and measurements after stabilization might be suitable for the subcutaneous method.
Some studies have already reported a comparison between the subcutaneous continuous glucose device and standard devices [20]. On the other hand, we reported that intravenous continuous monitoring had acceptable accuracy compared to the blood gas analyzer [11, 12]. In addition, the Ministry of Health, Labor, and Welfare approved automatic blood glucose control using the STG-55 because this was a highly accurate device. One of the aims of this study was to evaluate whether subcutaneous continuous glucose monitoring could detect alterations of glucose at many points within one patient. We did not compare two continuous devices with a blood gas analyzer because of a reduction in the amount of blood sampling. Based on these aspects, we defined the STG-55 as a “standard device.”
In addition, there are no clearly defined metrics for reporting what is sufficient in terms of accuracy and reliability between the continuous method and standard devices [24]. The ISO 15197 criteria recommended that at glucose concentrations <100 mg/dl, 95 % of the test results are required to be within ±15 mg/dl, and at higher glucose concentrations, 95 % of the test results are required to be within ±15 % [14]. However, these criteria did not apply to the continuous glucose monitoring systems [14]. Therefore, we are not certain that our evaluation method was absolutely right.
Conclusion
The current study suggests that subcutaneous and intravenous continuous glucose monitoring were not highly correlated during either surgery or ICU stay. In addition, the iPro2 might not exactly reflect a rapid change. However, in the near future, more improvement will be needed for both devices because blood removal failure was observed in 5.1 % of all the samples with the STG-55.
References
Hanazaki K, Maeda H, Okabayashi T. Tight perioperative glycemic control using an artificial endocrine pancreas. Surg Today. 2010;40:1–7.
Sebranek JJ, Lugli AK, Coursin DB. Glycaemic control in the perioperative period. Br J Anaesth. 2013;111(Suppl 1):i18–34.
Badawi O, Waite MD, Fuhrman SA, Zuckerman IH. Association between intensive care unit-acquired dysglycemia and in-hospital mortality. Crit Care Med. 2012;40:3180–8.
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85:217–24.
Mibu K, Yatabe T, Hanazaki K. Blood glucose control using an artificial pancreas reduces the workload of ICU nurses. J Artif Organ. 2012;15:71–6.
Yatabe T, Yamazaki R, Kitagawa H, Okabayashi T, Yamashita K, Hanazaki K, Yokoyama M. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive care unit patients. Crit Care Med. 2011;39:575–8.
Brunner R, Kitzberger R, Miehsler W, Herkner H, Madl C, Holzinger U. Accuracy and reliability of a subcutaneous continuous glucose-monitoring system in critically ill patients. Crit Care Med. 2011;39:659–64.
Sechterberger MK, van der Voort PH, Strasma PJ, DeVries JH. Accuracy of intra-arterial and subcutaneous continuous glucose monitoring in postoperative cardiac surgery patients in the ICU. J Diabetes Sci Technol. 2015;9:663–7.
van Hooijdonk RT, Leopold JH, Winters T, Binnekade JM, Juffermans NP, Horn J, Fischer JC, van Dongen-Lases EC, Schultz MJ. Point accuracy and reliability of an interstitial continuous glucose-monitoring device in critically ill patients: a prospective study. Crit Care. 2015;19:34.
Vriesendorp TM, DeVries JH, Holleman F, Dzoljic M, Hoekstra JB. The use of two continuous glucose sensors during and after surgery. Diabetes Technol Ther. 2005;7:315–22.
Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. The accuracy of a continuous blood glucose monitor during surgery. Anesth Analg. 2008;106:160–3.
Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. Accuracy and reliability of continuous blood glucose monitor in post-surgical patients. Acta Anaesthesiol Scand. 2009;53:66–71.
Signal M, Thomas F, Shaw GM, Chase JG. Complexity of continuous glucose monitoring data in critically ill patients: continuous glucose monitoring devices, sensor locations, and detrended fluctuation analysis methods. J Diabetes Sci Technol. 2013;7:1492–506.
International Standards Organization. In vitro diagnostic test systems-requirements for blood-glucose monitoring systems for self-testing in managing Diabetes mellitus. Reference number ISO 15197:2013. Geneva: International Organization for Standardization; 2013.
Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C, Freire AX, Geehan D, Kohl B, Nasraway SA, Rigby M, Sands K, Schallom L, Taylor B, Umpierrez G, Mazuski J, Schunemann H. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40:3251–76.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637.
Ellis MF, Benjamin K, Cornell M, Decker K, Farrell D, McGugan L, Porter GP, Shearin H, Zhao Y, Granger BB. Suitability of capillary blood glucose analysis in patients receiving vasopressors. Am J Crit Care. 2013;22:423–9.
Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33:2778–85.
DuBose JJ, Inaba K, Branco BC, Barmparas G, Lam L, Teixeira PG, Belzberg H, Demetriades D. Discrepancies between capillary glucose measurements and traditional laboratory assessments in both shock and non-shock states after trauma. J Surg Res. 2012;178:820–6.
Boom DT, Sechterberger MK, Rijkenberg S, Kreder S, Bosman RJ, Wester JP, van Stijn I, DeVries JH, van der Voort PH. Insulin treatment guided by subcutaneous continuous glucose monitoring compared to frequent point-of-care measurement in critically ill patients: a randomized controlled trial. Crit Care. 2014;18:453.
Maeda H, Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Hanazaki K. Hyperglycemia during hepatic resection: continuous monitoring of blood glucose concentration. Am J Surg. 2010;199:8–13.
Yatabe T, Kitagawa H, Kawano T, Munekage M, Okabayashi T, Yamashita K, Hanazaki K, Yokoyama M. Continuous monitoring of glucose levels in the hepatic vein and systemic circulation during the Pringle maneuver in beagles. J Artif Organ. 2011;14:232–7.
Cengiz E, Tamborlane WV. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;11(Suppl 1):S11–6.
Wernerman J, Desaive T, Finfer S, Foubert L, Furnary A, Holzinger U, Hovorka R, Joseph J, Kosiborod M, Krinsley J, Mesotten D, Nasraway S, Rooyackers O, Schultz MJ, Van Herpe T, Vigersky RA, Preiser JC. Continuous glucose control in the ICU: report of a 2013 round table meeting. Crit Care. 2014;18:226.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Munekage, M., Yatabe, T., Sakaguchi, M. et al. Comparison of subcutaneous and intravenous continuous glucose monitoring accuracy in an operating room and an intensive care unit. J Artif Organs 19, 159–166 (2016). https://doi.org/10.1007/s10047-015-0877-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-015-0877-2