Abstract
Introduction
Hypoglycemia is a frequent and feared complication of insulin therapy on the intensive care unit (ICU). Sedated patients in particular are at risk for hypoglycemia due to the absence of clinical symptoms. Furthermore, recent studies point to a correlation between the variability of blood glucose and mortality. Therefore, continuous glucose monitoring has the potential to influence outcome due to a better control of blood glucose in critically ill patients.
Materials and methods
We evaluated the efficacy, accuracy and safety of a new commercially available subcutaneous continuous glucose monitoring system (sCGM; Sentrino®, Medtronic) in a pilot study in critically ill adult patients. sCGM data were recorded for up to 72 h and values were compared with blood glucose values measured by cassette-based blood gas analyzer (BGA).
Results
A total of 14 patients (eight male, six female), with a mean age of 62.1 ± 9.8 years, referred to the ICU after major abdominal surgery were studied. The average simplified acute physiology score (SAPS II) was 35 ± 9. Three patients had known type II diabetes. The average runtime of sensors was 44.1 ± 22.1 h. In comparison to BGA, measurement of blood glucose by sCGM revealed an accuracy of 1.5 mg/dl, and a precision of + 34.2 mg/dl to − 31.2 mg/dl. Linn’s concordance correlation coefficient yielded 0.74 with a 95 % confidence interval of 0.68–0.78. No hypoglycemic events, defined as a blood glucose level below 70 mg/dl, occurred during treatment.
Conclusions
sCGM monitoring via a subcutaneous sensor demonstrated high accuracy and considerable variability compared to blood gas samples, even in critically ill patients.
Zusammenfassung
Hintergrund
Die Hypoglykämie stellt eine häufige und gefürchtete Komplikation der Insulintherapie auf der Intensivstation dar. Insbesondere bei sedierten Patienten besteht aufgrund fehlender klinischer Symptome das Risiko einer Hypoglykämie. Außerdem weisen aktuelle Studien auf eine Korrelation zwischen der Variabilität von Blutzucker und Mortalität hin. Daher hat das kontinuierliche Glukosemonitoring das Potenzial, den Verlauf durch eine bessere Blutzuckereinstellung bei kritisch kranken Patienten zu beeinflussen.
Material und Methoden
Untersucht wurden die Wirksamkeit, Genauigkeit und Sicherheit eines neuen, kommerziell verfügbaren, subkutanen kontinuierlichen Glukosemonitoringsystems (sCGM; Sentrino®, Fa. Medtronic) in einer Pilotstudie an kritisch kranken erwachsenen Patienten. Die sCGM-Daten wurden für bis zu 72 h aufgezeichnet und die Werte mit den Blutzuckerwerten verglichen, die mittels eines kassettenbasierten Blutgasanalysators (BGA) gemessen wurden.
Ergebnisse
Es wurden insgesamt 14 Patienten (8 m, 6 w) mit einem Durchschnittsalter von 62,1 ± 9,8 Jahren untersucht, die nach einer großen Bauchoperation auf die Intensivstation verlegt wurden. Der durchschnittliche SAPS II („simplified acute physiology score“) betrug 35 ± 9. Bei 3 Patienten war ein Typ-2-Diabetes bekannt. Die durchschnittliche Nutzungsdauer der Sensoren lag bei 44,1 ± 22,1 h. Im Vergleich zur BGA ergab die Messung des Blutzuckers mit dem sCGM eine Genauigkeit von 1,5 mg/dl und eine Variabilität von + 34,2 mg/dl bis − 31,2 mg/dl. Der Konkordanz-Korrelationskoeffizient nach Linn ergab 0,74 bei einem 95 %-Konfidenzintervall von 0,68–0,78. Es traten keine Hypoglykämien, definiert als Blutzuckerspiegel unter 70 mg/dl, während der Behandlung auf.
Schlussfolgerungen
Das sCGM-Monitoring über einen subkutanen Sensor wies eine hohe Genauigkeit und eine beträchtliche Variabilität im Vergleich zu den Blutgasanalysen auf, selbst bei kritisch kranken Patienten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent large multicenter studies evaluating the use of tight glycemic control in critically ill patients, showed 7 %–25 % of episodes of hypoglycemia to have a negative impact on patient outcome [1, 2, 3]. Although the desired target glucose corridor has been shifted [2, 3], there is still strong focus on glucose control, underlined by the fact that there is a significant association between both hyperglycemia and hypoglycemia and mortality [4, 5, 6, 7]. Moreover, it has been shown that glucose variability due to oxidative stress as well as neuronal and mitochondrial damage is associated with increased mortality in critically ill patients [8, 9, 10]. The issue of optimal glucose control becomes more complex as there are confounders that influence the therapy, such as the type and accuracy of the device used to measure blood glucose [11] and the level of experience of the care-giving team [12].
Continuous monitoring of blood glucose with the ability to immediately identify changes may lead to improved glycemic control. Such continuous measurements would surely give caregivers the opportunity to react on blood glucose variations immediately. However, there is no evidence to date that this would have any influence on clinical outcome.
In this feasibility study we tested a new commercially available subcutaneous sensor (Sentrino®, Medtronic) for subcutaneous continuous glucose monitoring (sCGM). We retrospectively compared the blood glucose values obtained by the Sentrino® system against blood glucose values obtained by blood gas analysis, a measurement with recognized high accuracy [13] in 14 critically ill patients on a surgical intensive care unit (ICU).
Methods
Study design and patients
The study was approved by the ethics committee of the Hamburg Chamber of Physicians. Because of the retrospective nature of the study, the need for informed consent was waived by the ethics committee. In all, 14 patients on a surgical ICU following major abdominal surgery were included in the study and received continuous blood glucose monitoring via a subcutaneously inserted sensor for a maximum duration of 72 h. All patients included were over 18 years old and had an expected stay on the ICU > 72 h.
Blood glucose monitoring
The Sentrino® sCGM System (Medtronic) combines a monitor with a minimally invasive, subcutaneous sensor, which is inserted subcutaneously with a needle sensor into the upper thigh. The sensor has novel drug interference rejection technology that ensures minimal interference with a wide array of pharmaceuticals used in the critical care unit. The monitor continuously determines blood glucose values and provides these continuously, either numerically or as a graph over time with the option to set levels of alarms for hypo- and hyperglycemia.
Following the recommendations of the manufacturer, sensors were only used for 72 h. All sensors could be placed appropriately. We used a target blood glucose corridor between 100 and 180 mg/dl. Values below 70 mg/dl were defined as a hypoglycemic event. Measurements of blood glucose with blood gas analysis were performed during the ICU stay every hour within the first 6 h after placement, and then every 2 h. All blood glucose measurements via blood gas analysis were performed with a cassette-based blood gas analyzer (Radiometer Copenhagen ABL 90 FLEX, Radiometer Copenhagen, Denmark). The blood samples were collected in heparinized blood gas syringes (PICO50, Radiometer Copenhagen, Denmark), and measured at 37 °C. The blood gas analyzer was regularly maintained and equilibrated according to national laws (Guidelines of the German Federal Board of Medicine) and to the recommendations of the manufacturer.
Statistical analysis
Statistical analysis was performed by using a linear correlation, a Bland-Altman plot and Linn’s concordance correlation coefficient. All statistical comparisons were calculated using IBM SPSS (Version 20, IBM Deutschland GmbH, Ehningen, Germany). Data were tested for normality distribution with the Shapiro-Wilk test. Mean ± standard deviation are shown; for abnormal data, distributed median and first and third quartile are added in brackets; n = number of patients.
Results
A total of 14 patients (six female and eight male) were included in the study. On average, they were 62.1 ± 9.8 years old, weighed 78.1 ± 23.5 kg (71.5, I.Q: 58.0, III.Q: 100.8) and were 172.4 ± 23.5 cm tall. They had an average SAPS II score of 35.9 ± 9.1 and a TISS score of 15.6 ± 4.2. Three Patients had type II diabetes; six patients were receiving enteral, five parenteral and three combined enteral and parenteral nutrition. The majority (11) of patients were on mechanical ventilation during the observation period and 13 of these received catecholamine therapy. The average runtime of the sensors was 44.1 ± 22.1 h (47.5, I.Q: 19.0, III.Q: 71.3). In two cases the monitor displayed that the sensor had low activity, and in three cases the sensors were dislocated accidently during routine care by the nursing staff. No complications, such as bleeding or infection at the insertion site, could be detected.
Fig. 1 illustrates the linear regression between the values of the sensor and the values of blood glucose measurement via blood gas analyzer, which yielded a positive correlation (R = 0.74).
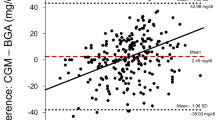
As illustrated in Fig. 2, Bland-Altman analysis revealed good accuracy for the sensor with a shift of the mean blood glucose level of 1.5 mg/dl, but poor precision with a range of + 34.2 mg/dl and − 31.2 mg/dl. Linn’s concordance correlation coefficient yielded 0.74 with a 95 % confidence interval of 0.68–0.78, as displayed in Fig. 2.
All patients received insulin continuously intravenously by a computerized syringe pump system (Space®, B. Braun Melsungen, Melsungen, Germany) targeting a blood glucose level of between 100 and 180 mg/dl. No hypoglycemic events, defined as a blood glucose level below 70 mg/dl, occurred during the study period.
Discussion
In this observational study, we assessed the accuracy and precision of blood glucose measurement with a subcutaneous sensor recently released commercially for use in critically ill patients compared with conventional blood glucose determination using a blood gas analyzer. When compared with this clinical gold standard of blood analysis, sCGM showed acceptable accuracy, with a slight overestimation of the mean blood glucose level of 1.5 mg/dl. However, at a range from + 34.2 mg/dl to − 31.2 mg/dl, precision was slightly above the clinically acceptable limits recently suggested by Critchley for the comparison of monitoring techniques [14].
Since the initial study by van den Berghe et al. [15], the debate about glucose control in critically ill patients has been ongoing. Not only the discussion about the best blood glucose corridor to treat critical ill patients [1, 2, 3, 15], but also the increasing evidence that hypo-, hyperglycemia and ultimately glucose variability can negatively influence outcomes in these patients [8, 9, 10, 17, 18] has led to a broader recognition of the importance of glycemic control under different circumstances of critical illness. Also, clear treatment recommendations have been implemented (Surviving Sepsis Campaign), thereby implicating the demand for optimal glucose monitoring on the ICU. Moreover, by nature, continuous monitoring of a clinical variable allows its closer regulation. Therefore, the ability to continuously measure blood glucose by sCGM can potentially help to ensure preemptive intervention in glycemic dysregulation and thus reduce hazardous hypo- and hyperglycemic events, caused both by the underlying disease and therapeutic intervention. In concordance with the above, we did not observe any hypoglycemic events in our patients with blood glucose levels below 70 mg/dl during the observation period.
Different sCGM products have been extensively studied in patients with diabetes outside the ICU [18, 19], as well as in pediatric patients [20]. Data in ICU patients, in particular in whom the technical requirements for the sensors are more complex due to critical status of the patient and the complex environment, are scarce. Two initial studies in ICU patients with the predecessor device (Minimed® CGM, Medtronic) of the device tested in our study (Sentrino® CGM, Medtronic) were published recently [21, 22]. Compared with those two patient populations, the severity of illness as assessed by SAPS and the complexity of treatment in terms of mechanical ventilation and catecholamine therapy were alike in our study. Regarding the reliability and robustness of this measurement technology, it is encouraging that our results are comparable with those of the two afore-mentioned studies. However, both studies failed to show significant improvement in terms of glycemic control or glycemic variability [21, 22].
In all 14 patients, placement of the needle sensor in the subcutaneous tissue of the upper thigh was successful and uneventful and did not lead to any complications, such as hemorrhage or infection. The sensor was dislocated in three patients during routine care by the nursing team, although the sensors were securely taped. This underlines a conceptional weakness in the tested device in terms of its use in the intensive care unit, which is clinically relevant and needs further development. In two patients, the monitor activated a user alarm indicating low activity (in case 1 after 3 h and in case 2 after 12 h of use), and measurement was therefore ceased. Without being able to analyze the reasons for this malfunction more deeply, it is apparent that the level of experience of the care-giving team with such a new device will also contribute to the runtime of the sensors.
This study has, of course, clear limitations. Firstly, it is a retrospective data analysis in a small cohort of patients. Furthermore, the time span of our comparative observation ranged from 3 to 72 h, with a mean time period of 44 h. Therefore, based on these data, we cannot make a judgement on the level of accuracy when using the sensor for longer periods of time. Also, vasoconstriction due to catecholamine therapy, low cardiac output and hypothermia may affect subcutaneous blood flow and therefore may have an influence on the accuracy of the subcutaneous sensor. Therefore, future prospective clinical trials focusing on patient outcomes are warranted.
In summary, continuous blood glucose monitoring via a subcutaneous sensor was feasible in a cohort of complex critically ill patients and showed acceptable accuracy compared with blood sample analysis. Therefore, this approach might contribute to closer and safer glycemic control in critically ill patients.
Conclusions
-
Continuous blood glucose monitoring with a subcutaneous sensor is feasible in a cohort of complex critically ill patients.
-
Blood glucose values obtained by the subcutaneous sensor correlated with blood glucose values obtained by blood gas analysis.
-
Compared with blood gas analysis, continuous blood glucose monitoring showed acceptable accuracy and considerable precision in our study.
-
Continuous blood glucose monitoring might contribute to closer and safer glycemic control in critically ill patients; however, further studies focussing on patient outcome are warranted.
References
Brunkhorst FM, Engel C, Bloos F et al (2008) German Competence Network Sepsis (SepNet): intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 358:125–139
Finfer S, Chittock DR, Su SY et al (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360:1283–1297
Preiser JC, Devos P, Ruiz-Santana S et al (2009) A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 35:1738–1748
Egi M, Bellomo R, Stachowski E et al (2010) Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 85:217–224
Jacka MJ, Torok-Both CJ, Bagshaw SM (2009) Blood glucose control among critically ill patients with brain injury. Can J Neurol Sci 36:436–442
Finfer S (2011) Hypoglycemia in critically ill adults—association yes, causation not proven. Crit Care 15:1012
Holzinger U (2013) Glucose control in the critically ill. Innovations and contemporary strategies. Med Klin Intensivmed Notfmed 108:422–428
Egi M, Bellomo R, Stachowski E et al (2006) Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 105:244–252
Bagshaw SM, Bellomo R, Jacka MJ et al (2009) ANZICS CORE Management Committee. The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit Care 13:R91
Hermanides J, Vriesendorp TM, Bosman RJ et al (2010) Glucose variability is associated with intensive care unit mortality. Crit Care Med 38:838–842
Van den Berghe G (2012) Intensive insulin therapy in the ICU—reconciling the evidence. Nat Rev Endocrinol 8:374–378
Schultz MJ, Harmsen RE, Korevaar JC et al (2012) Adoption and implementation of the original strict glycemic control guideline is feasible and safe in adult critically ill patients. Minerva Anestesiol 78:982–995
Inoue S, Egi M, Kotani J et al (2013) Accuracy of blood-glucose measurements using glucose meters and arterial blood gas analyzers in critically ill adult patients: systematic review. Crit Care 17:R48
Critchley LA, Lee A, Ho AM (2010) A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg 111:1180–1192
Van den Berghe G, Wouters P, Weekers F et al (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345:1359–1367
Umpierrez GE, Isaacs SD, Bazargan N et al (2002) Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 87:978–982
Doenst T, Wijeysundera D, Karkouti K et al (2005) Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 130:1144
Damiano ER, El-Khatib FH, Zheng H et al (2013) A comparative effectiveness analysis of three continuous glucose monitors. Diabetes Care 36:251–259
Kapitza C, Lodwig V, Obermaier K et al (2003) Continuous glucose monitoring: reliable measurements for up to 4 days with the SCGM1 system. Diabetes Technol Ther 5:609–614
Bridges BC, Preissig CM, Maher KO et al (2010) Continuous glucose monitors prove highly accurate in critically ill children. Crit Care 14:R176
Holzinger U, Warszawska J, Kitzberger R et al (2010) Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care 33:467–472
Brunner R, Adelsmayr G, Herkner H et al (2012) Glycemic variability and glucose complexity in critically ill patients: a retrospective analysis of continuous glucose monitoring data. Crit Care 16:R17
Acknowledgements
We would like to thank the nursing staff of the surgical ICU (1 F) Department of Intensive Care Medicine, Center of Anesthesiology and Intensive Care Medicine, Hamburg-Eppendorf University Medical Center, Hamburg, Germany for helping us to perform the study.
Compliance with ethical guidelines
Conflict of interest. M. A. Punke, C. Decker, K. Wodack, D. A. Reuter and S. Kluge declare that there are no conflicts of interest. We received sensors and monitors from Medtronic free of charge.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Punke, M., Decker, C., Wodack, K. et al. Continuous glucose monitoring on the ICU using a subcutaneous sensor . Med Klin Intensivmed Notfmed 110, 360–363 (2015). https://doi.org/10.1007/s00063-014-0453-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00063-014-0453-1