Abstract
Karst aquifers are very easily contaminated because of the surficial features that commonly exist in karst terranes. Pollutant releases into sinkholes, sinking streams, and/or losing streams commonly result in concentrated solutes rapidly infiltrating and migrating through the subsurface to eventually discharge at downgradient springs unless intercepted by production wells, but slow percolation through soils also may result in serious contamination of karst aquifers. The unique features of karst terranes tend to cause significant problems in the interpretation of results obtained from water-quality grab samples of karst groundwater. To obtain more representative samples, event-driven sampling was proposed some decades ago, but event-driven sampling can be difficult and expensive to implement. In this paper, application of passive-sampling strategies is advocated as a means for effectively obtaining representative water-quality samples from karst aquifers. A passive-sampling methodology may be particularly useful for karst aquifers that may be found in complexly folded and faulted terranes. For example, a groundwater tracing investigation of a contaminated site in a karst terrane confirmed that several offsite springs and wells are connected to the contaminated site. Tracer recoveries suggested transport rates that were relatively slow for flow in a karstic aquifer (~0.02 m/s). Breakthrough curves were erratic and spiky. To obtain representative groundwater samples, a passive-sampling methodology is recommended.
Résumé
Les aquifères karstiques sont très facilement contaminés en raison des caractéristiques de surface qui prévalent en terrains karstiques. Les rejets de polluants dans les dolines, les cours d’eau qui s’infiltrent et/ou les pertes entraînent généralement une percolation rapide de solutés concentrés et leur migration à travers le sous-sol, pour finalement ressortir au niveau des sources situées à l’aval hydraulique, sauf s’ils sont interceptés au niveau de puits avec pompages. Cependant la percolation lente à travers les sols peut également entraîner une contamination importante des aquifères karstiques. Les caractéristiques uniques des terrains karstiques ont tendance à poser des problèmes significatifs quant à l’interprétation des résultats de qualité des eaux souterraines karstiques obtenus à partir d’échantillons ponctuels. Pour obtenir des échantillons plus représentatifs, un échantillonnage basé sur les événements a été proposé depuis quelques décennies, mais celui-ci peut s’avérer difficile et coûteux à mettre en œuvre. Dans cet article, l’application de stratégies d’échantillonnage passif est. préconisée comme un moyen d’obtenir efficacement des échantillons représentatifs de la qualité de l’eau des aquifères karstiques. Une méthode d’échantillonnage passif peut être particulièrement utile dans les aquifères karstiques des terrains plissés ou fracturés de manière complexe. Par exemple, une étude de traçage des eaux souterraines d’un site contaminé en terrain karstique a confirmé que plusieurs sources et puits hors site sont reliés au site contaminé. Le taux de récupération des traceurs suggère des vitesses de transport relativement lentes pour un aquifère karstique (~0.02 m/s). Les courbes de restitution sont erratiques et présentent des pics. Pour obtenir des échantillons d’eau souterraine représentatifs, une méthode d’échantillonnage passif est. recommandée.
Resumen
Los acuíferos kársticos se contaminan muy fácilmente debido a las características superficiales que existen comúnmente en los terrenos kársticos. Las emisiones de contaminantes en sumideros, cursos de hundimiento y/o cursos de pérdida suelen dar lugar a que los solutos concentrados se infiltren y migren rápidamente a través del subsuelo para finalmente descargarse en manantiales con pendiente decreciente, a menos que sean interceptados por los pozos de extracción, pero la lenta filtración a través de los suelos también puede dar lugar a una grave contaminación de los acuíferos kársticos. Las características singulares de estos terrenos kársticos tienden a causar problemas importantes en la interpretación de los resultados obtenidos de las muestras de calidad de agua de las aguas subterráneas kársticas. Para obtener muestras más representativas, hace algunos decenios se propuso el muestreo por eventos, pero su aplicación puede ser difícil y costosa. En el presente documento se propugna la aplicación de estrategias de muestreo pasivo como medio para obtener eficazmente muestras representativas de la calidad del agua de los acuíferos kársticos. Una metodología de muestreo pasivo puede ser particularmente útil para los acuíferos kársticos que pueden encontrarse en terrenos complejos plegados y con fallas. Por ejemplo, una investigación de un seguimiento de las aguas subterráneas de un sitio contaminado en un terreno kárstico confirmó que varios manantiales y pozos situados fuera del sitio están conectados con el sitio contaminado. Las recuperaciones del trazador sugirieron tasas de transporte relativamente lentas para el flujo en un acuífero kárstico (~0.02 m/s). Las curvas de avance eran erráticas y con picos. Para obtener muestras representativas de aguas subterráneas, se recomienda una metodología de muestreo pasivo.
摘要
因为岩溶地层普遍存在于地面的特征,岩溶含水层很容易被污染。污染物释放到下落水洞,伏流和/或排泄型的河流通常会导致浓缩的溶质迅速渗透并迁移穿过地下,最终在下游的泉水中排泄出来,除非被生产井拦截,但通过土壤的缓慢渗透也会导致岩溶含水层严重污染。喀斯特地层的独特特征使得从喀斯特地下水的水质抽样结果分析可解释出现的相关问题。为了获得更多有代表性的样本,几十年前提出了事件驱动的采样方法,但是事件驱动的采样方法可能既难于操作又昂贵。在本文中,提出应用无源采样策略作为有效地从岩溶含水层中获得代表性水质样品的一种方法。被动采样方法对于可能在复杂折叠和断层的地层中探知岩溶含水层特别有用。例如,对喀斯特地层污染场地的地下水追踪调查证实,几个非现场泉水和水井已与该污染场地相关。示踪剂回收率表明,岩溶含水层中的流动传输率相对较慢(~0.02 m/s)。穿透曲线是奇怪的而且有尖峰。为了获得代表性的地下水样品,建议采用无源采样方法。
Resumo
Os aquíferos cársticos são facilmente contaminados pelas suas características superficiais que comumente existem em terrenos cársticos. Liberações de poluentes em sumidouros, riachos afundando e/ou riachos perdidos geralmente resultam em solutos concentrados que se infiltram e migram rapidamente através da subsuperfície para, eventualmente, descarregar em nascentes de gradiente, a menos que seja interceptado por poços de produção, mas a percolação lenta através dos solos também pode resultar em contaminação séria dos aquíferos cársticos. As características únicas dos terrenos cársticos tendem a causar problemas significativos na interpretação dos resultados obtidos a partir de amostras de qualidade da água subterrâneas cársticas. Para obter amostras mais representativas, a amostragem baseada em eventos foi proposta algumas décadas atrás, mas a amostragem baseada em eventos pode ser difícil e cara de implementar. Neste artigo, define-se a aplicação de estratégias de amostragem passiva como um meio para obter efetivamente amostras representativas da qualidade da água de aquíferos cársticos. Uma metodologia de amostragem passiva pode ser particularmente útil para aquíferos cársticos que podem ser encontrados em terrenos complexamente dobrados e falhados. Por exemplo, uma investigação de traçadores de água subterrânea de um local contaminado em um terreno cárstico confirmou que várias nascentes e poços externos estão conectados ao local contaminado. As recuperações de traçadores sugeriram taxas de transporte que eram relativamente lentas para o fluxo em um aquífero cárstico (~ 0.02 m/s). As curvas de identificação foram erráticas e com picos. Para obter amostras representativas de águas subterrâneas, recomenda-se uma metodologia de amostragem passiva.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Karst aquifers are generally regarded as some of the most difficult aquifer types to be effectively investigated for groundwater contamination (Kresic 2013). The difficulties arise from the nature of how karst aquifers develop, specifically the dissolution of soluble rocks in which the aquifer is formed. As is well documented in numerous sources (e.g., Ford and Williams 2007; Palmer 2007; White 1988), the dissolution of soluble rocks results in discrete input points that direct surface water in a relatively unrestricted manner into the subsurface. Dreybrodt (2004, pp. 295–300) provides a very brief overview of the dissolution of soluble rocks that emphasizes the complexities involved.
Below the soil zone, bedrock fissures, bedding-plane partings, and vadose shafts within the vadose zone continue to direct and concentrate the inflowing underground water to select phreatic solution conduits. Of significance is the epikarstic zone, typically the first 3–9 m of bedrock beneath the soil zone, which has been documented to be able to store and rapidly transmit pollutants (Field 1990, 1992–1993). The total conduit system typically develops in a rammiform manner that directs the flowing groundwater to one or more resurgences at a base level during low-flow periods. During moderate- and high-flow periods one or more high-level overflow springs may become activated for a relatively short period until the piezometric level recedes to base-level conditions.
Karst aquifers become easily contaminated by leakage from landfills, surface impoundments (municipal and hazardous-waste lagoons), underground storage tanks, inadvertent chemical spills, accidental or deliberate releases into sinkholes (dolines), and overloading of pasture lands and spray fields with manures to name just a few causes (e.g., Kresic 2013, pp. 558–562). Pollutant releases into sinkholes, sinking streams, and/or losing streams commonly result in concentrated solutes rapidly infiltrating and migrating through the subsurface to eventually discharge at downgradient springs unless intercepted by production wells. Diffuse percolation through less defined surface openings is also common as well, which tends to be ignored because of the apparent extreme situation of concentrated contaminant inflow via typical karst-surface features. Besides, pollutant releases from lined lagoons and landfills, both rapid-concentrated flow and slow-dispersed flow both frequently occur in karst terranes.
Further complicating investigations and evaluation of contamination of karst aquifers are typical geological structures—for example, folded, fractured, and faulted terranes may or may not influence the release, transport, and discharge of pollutants. Changing stratigraphic layers, overturned beds, and degree of rock purity may also affect water transit and fate-and-transport of pollutants (see, for example, Benson and Yuhr 2016, p. 275). The purpose of this paper is to illustrate the inappropriateness of applying typical grab sampling and general unworkability of event-driven sampling of karst springs and wells and to suggest an improved sampling methodology based on passive diffusion of contaminants to and onto or into sampling devices.
A literature search resulted in some papers describing the applications of passive sampling devices (PSDs) in karst terranes—for example, Bidwell et al. (2010) were interested in the impact of organic wastewater compounds on cave ecosystems. To obtain a sense of the impact Bidwell et al. (2010) deployed PSDs in six caves and two surface-water sites within the Ozark Plateau of northeastern Oklahoma and northwestern Arkansas. A total of 83 chemicals were detected, 55 of which were detected in the caves.
Fox et al. (2010) emphasized the difficulties associated with obtaining representative time-weighted average water-quality samples from karst aquifers for of months when analyte concentrations are low and precipitation events are of short duration. To overcome these difficulties Fox et al. (2010) deployed PSDs in caves and at downstream resurgences to effectively detect organic chemicals.
Schwarz et al. (2011) were interested in the transport route of polycyclic aromatic hydrocarbons (PAHs) in a south German karst system. PSDs were installed in three vadose caves to collect time-integrated samples of seepage water and were installed at Blautopf Spring to collect groundwater samples from the caves’ outlet.
Metcalfe et al. (2011) were interested in the water quality of the coastal karst aquifer system along the Caribbean coast of the Yucatan Peninsula, Mexico, that consists of a very highly permeable limestone. PSD deployments occurred in three caves that are parts of extensive conduit networks, and one cenote and one cave that are both connected to a different conduit network that discharges to an embayment. The PSDs effectively detected several dissolved organic chemicals of concern, albeit at low concentrations which is one of the benefits of deploying PSDs.
Demougeot-Renard et al. (2017) successfully deployed some PSDs at Betteraz Spring and various piezometers in northeast Porrentruy in the Canton of Jura, Switzerland to detect perchloroethylene and trichloroethylene. Significantly, not all PSDs tested by Demougeot-Renard et al. were determined to be appropriate for the aquifer system being evaluated. The important factor of determining the most appropriate PSDs to deploy for the given environmental conditions appears to be as significant as determining the correct PSDs to deploy for the contaminants of interest.
Levy et al. (2017) applied PSDs to an alpine karst system in the German Alps for long-term detection of PCDDs, PCDFs, PCBs, PAHs, and organochlorine pesticides. Deployment of the PSDs was at two permanently frozen sites in a tunnel belonging to the gallery systems of the massive rock Zugspitze where adequate percolation waters were available. Very low concentrations of the contaminants of concern were detected confirming the value of applying such devices at difficult sites to access.
Brief review of problem of conventional grab sampling
Grab sampling is the process of collecting an aliquot of water at a spring or from a monitoring well. The sample is then labeled, preserved, transported in a cooler to a laboratory, and analyzed. Under the simplest of conditions (e.g., continuous contaminant discharge at constant concentrations without any losses during transport and analysis), grab samples are assumed to be representative under changing environmental conditions because the period of sample acquisition (seconds) may reasonably be considered instantaneous with respect to contaminant variation in streams (or conduits), which might be expected to vary significantly over minutes to hours to days depending on the nature of the contamination. High resolution monitoring demonstrates the sort of timescales on which one might expect to see significant changes in water quality. The term, representative, as used in this context is intended to imply that the sample provides an accurate picture of the concentration at that specific moment in time (i.e., reflects the concentration actually in the water). Unfortunately, grab samples only provide a snapshot of the water quality at the time of sampling, can be difficult to implement in some instances, and be expensive, especially if numerous samples must be collected.
In regard to grab samples collected at springs downgradient of a contaminated site being just a snapshot in time, it is likely that the contaminants originating from the site will not be detected in the grab samples except as a result of extremely improbable circumstances (e.g., sample collection at a point in time when contaminants are discharging at the spring at peak concentrations). Past tracer studies have shown that contaminant discharges at sufficiently high concentrations are not continuous. Rather, contaminant discharges at offsite springs can exhibit very low concentrations and be quite erratic (Fig. 1) and thus not truly representative of contaminant discharges (see for example, Quinlan and Alexander Jr 1987; Malet et al. 2017). Representative is defined in this context as 95% confidence that a mean calculated from a finite set of samples gathered over a specific period of time is equivalent to a mean calculated from a very large sample set collected over the same period of time and including both periodic samples and samples collected at more frequent intervals during high-flow events (Currens 1999).
Contaminant concentration variation over time, illustrating the problems with grab sampling in which no consideration regarding any specific precipitation events is considered: a relatively infrequent sampling and b more frequent sampling, more peak values, and greater range of concentration values below the mean concentration
Mean concentration, as depicted in Fig. 1, was calculated using equation 1 of Schleppi et al. (2006), but their equations 2 and 3 could just as well have been used. Other possible methods for calculating mean concentration also exist (Preston et al. 1992).
Automatic water samplers can be programmed to collect water samples at sufficiently high sampling rates as to collect representative water samples from springs shown to be connected to the contaminated site, but such an approach would likely be prohibitively expensive. Even setting a sampling frequency as infrequent as daily for an entire year could both be extremely expensive and insufficiently frequent at the same time (Quinlan and Alexander Jr 1987; Felton and Taraba 1995; Currens 1999; Blatnik et al. 2020).
Typical groundwater grab sampling of springs and wells, whether haphazard timewise or on a systematized schedule was long ago questioned as being capable of presenting representative groundwater quality information when implemented in karst terranes because of the nature of flow in karst aquifers (Quinlan and Alexander Jr 1987; Vrba 1988; Coxon and Thorn 1989; Kačaroğlu 1999) and is still a concern (e.g., MPCA 2005, p. 7). Groundwater flow in karst aquifers is generally characterized by relatively fast flow constrained in solution conduits that is convergent throughout much of its upper reaches but tends to become distributary very near its lowest reaches. High-level overflow to springs that function during periods of high precipitation but are often dry during periods of low precipitation is also common. Monitoring wells installed in karst aquifers generally do not produce representative samples because of the convergent flow of subsurface water and pollutants into solution conduits (Quinlan and Ewers 1985; Field 1992–1993).
Brief review of event-driven sampling for pollutants
Event-driven sampling is a common sampling methodology applied to surface-water streams (e.g., Rabiet et al. 2010; Schleppi et al. 2006; Johnes 2007; Horowitz 2008). In its simplest sense, water samples are collected with specific regard to precipitation events.
As a solution to the problem of conventional groundwater grab sampling for pollutants in karst terranes, Quinlan and Alexander (1987) proposed application of an event-driven sampling methodology in which groundwater samples would be collected starting at the beginning of a precipitation event and continuing through the rising limb of the hydrograph at a very high frequency (e.g., every hour) until the hydrograph peak is reached. After the hydrograph peak is reached, the sampling frequency could be substantially reduced (e.g., every four to six hours). All the collected water samples would then be analyzed in the laboratory for analytes of concern unless the precipitation event resulted in too little actual precipitation in which case, all the samples would then be discarded. The event-driven sampling methodology was shown in Quinlan and Alexander (1987) to result in representative samples at the test site, but the problem of knowing when a precipitation event was going to start, how much precipitation should be deemed adequate, and associated costs in terms of personnel on standby and actual sample collection and the numerous laboratory analyses are all problematic. Although not greatly prevalent, some examples of event-driven sampling in karst terranes do exist (Currens 1999; Lerch et al. 2001; Kilroy and Coxon 2004; Trček 2007).
Event-driven sampling, as originally proposed by Quinlan and Alexander (1987), was based on research conducted at Mammoth Cave National Park that was developed within the context of the flushing of solution conduits during storm events. As with grab sampling, the methodology described by Quinlan and Alexander can be prohibitively expensive in nearly all instances. In-situ monitoring can help in the automatization and optimization of sampling with automatic samplers but may not be very useful if contaminants breakthrough is difficult to link to specific precipitation events. Personnel would need to be on continuous standby and ready to deploy sampling equipment quickly and be available to monitor the equipment for the duration of any precipitation event throughout the year. Also, the number of samples that might be analyzed in the laboratory could run into the tens or even hundreds of thousands of dollars over a year.
For slow-flowing water in some solution conduits (e.g., those that tend to occur in the Appalachian Mountains), the sampling protocol for karst springs suggested by Quinlan and Alexander (1987) would also likely result in nondetection of the expected contaminants. Nondetection of contaminants is a serious limitation in developing realistic exposure and risk assessments. This problem is exacerbated during periods following large storm events, depending on how readily the contaminants, temporarily detained in subsurface solution conduits, are flushed and entrained along the conduit. These peak contaminant concentrations, because of the long travel times, are not readily correlatable to any storm event. This latter problem thus renders the suggested event-driven sampling protocol described by Quinlan and Alexander (1987) as inadequate and inappropriate and requires a very different approach.
In such an instance, passive sampling of surface waters and groundwater appears to be the most appropriate method for consideration. Although still not commonly employed for environmental water sampling, passive sampling appears to be a viable method for sample collection at surface-water and groundwater-discharge locations at and around contaminated sites that have been shown by tracing studies to relate to the site. The concept of passive sampling is beginning to receive serious consideration by the US Environmental Protection Agency (EPA; e.g., Burgess et al. 2016) because of the possibility of obtaining representative samples under difficult circumstances.
Passive sampling
Passive sampling and/or extraction of analytes involve measurement of the concentration of an analyte as a weighted average over the sampling and/or extraction time. This is accomplished by integrating the concentration of the analyte over the whole exposure time, which makes such a method less susceptible to accidental, extreme variations of pollutant concentrations. In this way, a suitable means of obtaining information over a long-term period of pollutant levels in a given environmental compartment is obtained (Namieśnik et al. 2005). Passive sampling may be defined, in the broadest sense, as any method that is based on the free flow of contaminant molecules from the sampled media to a receiving phase in a PSD, as a result of differences between the chemical potentials of the analyte in the two media (Górecki and Namieśnik 2000; Vrana et al. 2005). Passive-sampling methods are generally classified as either adsorptive or absorptive. Adsorptive methods take advantage of the physical or chemical retention by surfaces and rely on parameters that involve surface binding and/or surface area. Absorptive methods involve not only surface phenomena but also analyte permeation into the interceding material. This latter approach provides the possibility for compound discrimination because of the membrane’s physicochemical characteristics (Kot et al. 2000). In addition, some studies have shown that the results obtained using some PSDs are significantly correlatable, from a statistical perspective, with the concentrations obtained using an event-driven sampling routine even when sampling rates were not corrected by flow (e.g., Fernández et al. 2014).
Currently, there are three generic forms of PSDs
-
1.
Thief (grab, equilibrium) PSDs.
-
2.
Diffusion (equilibrium) PSDs.
-
3.
Time integrating (kinetic, nonequilibrium) PSDs
All may be deployed down a well to the desired depth within the screened interval or open borehole to obtain a discrete sample without using pumping or a purging technique (ITRC 2006, pp. 3 and 10). Most can be stacked to obtain samples at multiple depths. Some PSDs can also be used to measure contaminants in groundwater as groundwater discharges to a surface-water body (e.g., at resurgences). Several PSDs were originally designed for surface-water sampling but may be modified for deployment in monitoring wells of appropriate diameter.
Equilibrium versus nonequilibrium sampling
The main advantage of PSDs is that they continue functioning until equilibrium is achieved. Once equilibrium conditions are achieved, additional enrichment of contaminants within the PSD is no longer possible. This means that the time to equilibrium depends on the capacity of the collection phase for the contaminants of interest, where capacity is defined by the amount and affinity of the collection material: the larger the amount of collection material and/or the greater its affinity for the contaminants, the greater the capacity. Unfortunately, this implies that the distinction between equilibrium and nonequilibrium sampling is not always clear. This is particularly evident if many contaminants with a broad spectrum of physicochemical characteristics are of interest in the sampled medium. It is also conceivable that some contaminants may be present at equilibrium at the end of the sampling period while others may not yet have reached equilibrium (Bopp 2004, p. 7).
In terms of adsorptive samplers and depending upon the sampler, the receiving phase can be a solvent (e.g., water), chemical reagent, or porous adsorbent (e.g., activated carbon). While there are many different designs for adsorptive PSDs, most have a barrier between the sampled medium and the receiving phase. The barrier determines the sampling rate that contaminants are collected at a given concentration and can be used to selectively permit or restrict various classes of chemicals from entering the receiving phase (Vrana et al. 2005). As further explained by Vrana et al. (2005), the mass transfer of an analyte from water to the sampler includes diffusion, interfacial transport steps across several barriers (compartments), including the stagnant aqueous boundary layer, possible biofilm layer, the diffusion-limiting membrane, and finally the receiving phase. Assuming a rapid equilibrium, the flux of an analyte is constant and equal in each of the individual compartments. This also assumes that sorption equilibrium exists at all compartment interfaces. As such, the resistances of each barrier to the mass transfer of analytes are then additive and independent. The ability of a PSD to act as an equilibrium or nonequilibrium sampler is dependent on the partitioning properties of the contaminants of interest. Some PSDs might act in an equilibrium manner for some environmental pollutants during field sampling, while also acting in a nonequilibrium manner for other compounds (Bopp 2004, p. 8).
Both equilibrium and nonequilibrium methods preconcentrate analytes by acting as a preferred phase for the partitioning of the analyte. The main difference between the two methods is the dimension of time; equilibrium samplers result in time-weighted averages that attenuates the changes in the environmental concentration with a bias towards the current concentration. Alternatively, integrative (nonequilibrium) samplers result in time-integrated average concentration over the whole sampling period (Fig. 2; Roll and Hallden 2016).
Hypothetical example of analyte concentration from samples sorbed onto an equilibrium sampler with an equilibration time of one time period (arbitrary unit), and an integrative sampler operating in an environmental fluid, where the analyte concentration varies between 50 and 150% of the initial (and mean) value. The equilibrium sampler yields a time-weighted average concentration that attenuates and lags the environmental concentration. The integrative (nonequilibrium) sampler yields an average concentration reflecting the entire duration of the sampling period (after Roll and Hallden 2016). A major deficiency with diffusion PSDs is that they tend to achieve equilibrium relatively quickly, which may allow the contaminants to diffuse back into the source water when contaminant concentrations in the source water decline resulting in the reversing of concentration gradients (Bopp 2004, p. 7)
Equilibrium sampling
Equilibrium samplers are characterized by a rapid achievement of equilibrium between the contaminants in the water to be sampled and the contaminants inside the PSD. One consequence of achieving an equilibrium rapidly is that the contaminants are also capable of diffusing back into the surrounding water should aqueous concentrations of contaminants decline (i.e., the concentration gradient reverses). Equilibrium may be generally assumed to be reached within about 1 week (Bopp 2004, p. 7), but times will vary depending on the sampler.
Diffusion passive sampling device
Diffusion (equilibrium) PSDs are devices that rely on diffusion of the analytes to reach equilibrium between the PSD fluid and the aqueous environment (Vrana et al. 2005). Samples are time-weighted toward conditions at the sampling point during the latter portion of the deployment period. The degree of weighting depends on the analyte and device-specific diffusion rates. Typically, conditions during only the last few days of PSD deployment are represented (ITRC 2006, p. 2). Depending upon the contaminant of concern, equilibration times range from a few days to several weeks (ITRC 2006, pp. 21–55). Diffusion PSDs are less versatile than grab samplers insofar as they are not generally effective for all chemical classes but like grab samplers, they can be stacked to obtain multiple samples from various depths.
Nonequilibrium sampling
Nonequilibrium PSDs are those that do not achieve equilibrium with the surrounding aqueous environment within the designated sampling period. These samples are characterized by a high capacity for collecting contaminants of interest. The high capacity ensures that contaminants can be continuously enriched in the PSD throughout the sampling period. This high capacity also means that contaminants are much less likely to diffuse back out of the PSD as a result of decreasing aqueous concentrations. As such, the high capacity of nonequilibrium PSDs for contaminants of interest forms the prerequisite for determining time-weighted average (TWA) contaminant concentrations present in the water over the entire sampling period. Nonequilibrium-type PSDs may be employed for periods of a few weeks to a few months (Bopp 2004, p. 8).
Time-integrating passive sampling device
Integrating, or kinetic PSDs are designed to sequester chemicals over the time they are deployed (Vrana et al. 2005). Analytes are trapped or retained in a suitable medium that can be a solvent, a chemical reagent, or a porous adsorbent. Hence, they produce a total mass of the chemicals that have meet the sampling medium. This total can later be converted to a TWA concentration. Because of the sequestration mechanism, kinetic PSDs can achieve very low detection limits (e.g., ng L−1).
Passive sampling devices tend to smooth contamination curves by integrating over the temporal variability in the concentrations (Poulier et al. 2015; Zhang et al. 2016). The concept of a TWA concentration is most easily understood from a visual perspective (see Fig. 2).
Operational principles
Both the diffusion and time-integrating PSDs depend upon permeation or diffusion through barriers that hold their receiving phase. This diffusion process is chemical and barrier specific. For the diffusion PSDs this sampling rate affects the time they need to be deployed to have the PSD fluid come into equilibrium with the contaminant concentrations in the ambient water. For the integrating PSDs, the sampling rates are used to convert the total concentration found in the receiving phase to a TWA concentration in the kinetic PSDs. Sampling rates for several analytes and barriers can be found in the literature, otherwise they must be determined experimentally in a laboratory. Note that time-integrating PSDs, like diffusion PSDs, may require a minimum number of deployment days before accurate TWA concentrations can be calculated (Vermeirssen et al. 2009).
As the contaminants permeate or diffuse into the PSD, they become trapped or retained in a suitable medium—commonly known as a performance reference compounds (PRCs)—within the PSD. This can be a solvent, chemical reagent, or a porous adsorbent. The receiving phase is exposed to the water phase, but without the aim of quantitatively extracting the dissolved contaminants.
Equilibrium sampling rate
The sampling rate is determined by the transport resistances in the stagnant water boundary layer around the sampler and the resistances in the sampler itself. Which resistance dominates depends on (1) the local water movement that determines the thickness of the aqueous boundary layer, and (2) the diffusion rate in the PSD.
In stagnant water, the water boundary layer is generally thick and so uptake is slow, and the sampling rate is therefore low. When there is more water movement, the water boundary layer will not be as thick and so uptake will be faster, and the sampling rate will be higher (Fig. 3).
Schematic diagram of passive sampling devices (PSDs) operation. a Equilibrium conditions, evident from the equivalent movement of analytes between the aqueous environment and the receiving phase and back. b Nonequilibrium conditions, evident from the greater movement of analytes to the receiving phase relative to the reverse movement of analytes back to the aqueous environment (modified from Bopp 2004, pp. 6–7)
If the diffusion rate in the PSD itself is low, the sampled substances will accumulate on the surface of the PSD and the uptake rate will be slowed down to the rate at which the substances diffuse deeper into the PSD. The sensitivity (limit of detection) of PSDs of this kind is low.
The highest sampling rate is achieved with PSDs in which the compounds being sampled have diffusion coefficients that are so high that the water boundary layer determines the sampling rate. The advantage of PSDs of this kind is that the uptake model is relatively simple, and uptake can be modeled accurately. The sampling rate of the PSD can be accurately determined based on the release of compounds with which the PSD is spiked beforehand (i.e., PRCs; Booij et al. 1998; Huckins et al. 2002). This is because the release rate is determined by the same resistances as the sampling rate, which means that, during the calculation of the concentration, the effect of water movement on the sampling rate is considered. The calculation model developed for this purpose over time is described in Smedes (2010) as cited in Smedes et al. (2010, p. 5).
For PSDs where the uptake is determined by the water boundary layer, the uptake is higher when the flow rate is higher (e.g., in a river). A peak in the flow reduces the size of the boundary layer and will result in more uptake, as will a peak in the concentration. An increase in the flow also leads to greater releases of PRCs and therefore to a higher sampling rate so that the flow will not affect the calculated concentration. The result is a time-integrated measurement in which time-integrated infers both concentration-integrated and flow-integrated. When the transport resistance in the sampler is on the same order or higher than that which is in the water boundary layer, modeling is more problematic and the diffusion coefficient of the compound in the PSD is also needed (Booij et al. 2003). If the water movement changes, the resistances in the water boundary layer and the PSD will determine uptake in turn so that both resistances must be included in the model.
PSD sampler-water partition coefficient
Several process constants have to be known for every compound to be measured with passive sampling. To verify that the uptake process matches the assumed uptake model, it is essential that the diffusion coefficient of the compound be measured and that the sampling material be known. The value of the sampler-water partition coefficient Ksw is also needed to calculate the freely dissolved concentration. Initially, when testing the possibilities for measuring a substance using passive sampling, estimated values are often used. As a rule, each combination of sampler material and compound to be measured has a specific optimal exposure time at which sampling is still time-integrated. However, because sampling with a PSD usually involves several compounds at the same time, the exposure time is selected pragmatically.
Transport from the aqueous phase to the receiving phase within a PSD is, as in the partition samplers, determined by diffusion. However, the difference is that there are three, rather than two, different resistances:
-
1.
The resistance in the water boundary layer
-
2.
The resistance in the filter or membrane
-
3.
The resistance between the parts of the adsorption material itself in the direction of deeper layers in the PSD
Figure 3 depicts these resistances. Little is yet known about which of the three resistances dominates and whether that is the case in all circumstances. As a result, a quantitative calculation of the average water concentration is not yet possible and additional research is necessary for in-situ calibration and conversion to concentrations in the water phase (Smedes et al. 2010, p. 7).
Basic operational theory
Pollutant adsorption or absorption from water into most PSDs generally follow the pattern shown in Fig. 4. As depicted in Fig. 4, the functioning of a PSD is based on an exponential power that can be broken down into three stages (Smedes et al. 2010, pp. 4–5):
Analyte mass uptake profile in passive sampling devices (PSDs) in which three different mass transfer accumulation regimes are shown. Three stages of mass transfer accumulation regimes (linear, kinetic and equilibrium regimes) can be distinguished. Integrative samplers are designed to operate in the kinetic regime, while equilibrium samplers are designed to operate in the equilibrium regime (modified from Roll and Hallden 2016; Roll 2015; Zabiegała et al. 2010; Booij et al. 2000, p. 1; Vrana et al. 2005; Bopp 2004)
-
Stage 1.
Uptake is roughly linear over time and there is no tendency to flow back (i.e., there is no release back into the aqueous environment).
-
Stage 2.
Differences in the concentration between the aqueous environment and the PSD falls and substances are again released into the aqueous phase (i.e., net uptake declines).
-
Stage 3.
Ultimately, uptake and release equilibrate, and equilibrium is reached.
The processes operating during each stage can be complicated. According to Smedes et al. (2010), the following processes occur, beginning with stage 1, during which, uptake is time-integrated and higher or lower temporary concentrations are “registered.” The measured concentration is a time-weighted average concentration during the exposure period. In this instance, there is “one-way traffic” to the sampler. A higher uptake due to a temporarily higher concentration (a peak load) during the exposure period will, therefore, stay in the sampler. To calculate the concentration in the aqueous phase during this first stage, only the sampling rate Rs [L3 T−1] is needed in addition to the measured parameters as shown by Kot-Wasik et al. (2007), Lowman et al. (2012), and Zhang et al. (2016)
where CTWA [M L−3] is the TWA-concentration of the contaminant in the aqueous (water) phase, Ms [M] is the mass of analyte accumulated on the PSD, M0 is the analyte measured on the PSD before deployment [M], Rs is the sampling rate, and t is time [T]. During this stage, the product Rst represents the water volume that is extracted during deployment and, at sufficiently long exposure times, the sampler equilibrates with the water to yield accumulated mass according to (Lowmann et al. 2012)
where ms is the mass of sorbent that is extracted by a specific PSD at equilibrium and Ksw is the sampler-water partition coefficient (also known as the phase-water partition coefficient; Bayen et al. 2009), which is obtained from
where k1 [Mw−1 Ms−1 T−1] and k2 [T−1] are the uptake and offload rate constants, respectively.
Lowmann et al. (2012) further note that Eqs. (1) and (2) are special cases of the general uptake equation
which is valid during the linear uptake phase (t → 0). Equation (4) is easily rearranged to (Novic et al. 2017; Jeong et al. 2018)
Equations (4) and (5) are always exact (as opposed to Eqs. 1 and 2 which are only approximations) and allows for estimating the effectively extracted water volume Vex at deployment time. The quotient Rs/(Kswms) in Eqs. (4) and (5) is a first-order equilibrium rate constant kex, which can be used to estimate the time to equilibrium (Lowmann et al. 2012).
The sampling rate may be estimated from (Kot-Wasik et al. 2007)
where ω0 is the overall mass transfer coefficient, A is the surface area of the membrane, kex is the overall exchange constant, and VD is the volume of the receiving phase.
During stage 2, which follows the linear phase, the release of the measured analyte also starts to play a role. The rate of this release increases as stage 3 is approached. When an analyte is released that has been accumulated earlier during a peak load, the PSD starts to “forget” this peak load. To calculate the concentration in the water both Rs and Ksw are needed, as is the complete model with exponential power.
Lastly, during stage 3, equilibrium is achieved so release and uptake are equal. In this instance, a PSD will “forget” (in part) a temporary increase or decrease in the water concentration from an earlier stage. The concentration in the aqueous phase in this stage can be calculated with the partition coefficient Ksw alone. It should be noted that Fig. 4 exhibits three separate curves (phases), but in actuality the linear curve grades into the nonequilibrium curve which in turn grades into the equilibrium curve.
Hydrophobic compounds generally have a high Ksw, so the PSD capacity (ms Ksw) [M L−1] for these compounds is high and uptake will also generally remain in the linear stage. As a result, these compounds can be sampled on a time-integrated basis. For less hydrophobic compounds (e.g., log Kow < 3), equilibrium time is often shorter than the exposure time and equilibrium will generally be achieved (Smedes et al. 2010, p. 5).
A partition PSD can accumulate several analytes at the same time. Differences in the properties of the compounds mean that one compound may, after a particular exposure period, still be in the linear phase, while another compound will already have attained equilibrium. Competition between the different compounds does not play any role in the uptake of these mixtures of compounds (Smedes et al. 2010, p. 5).
The exchange kinetics between a PSD and water phase may be described by a first-order, two-compartment model (Bayen et al. 2009)
that may be solved as a one-compartmental mathematical model as depicted in Fig. 4 (Vrana et al. 2005; Kot-Wasik et al. 2007; Kaserzon et al. 2012; Persson 2015)
where Cs (t) [M M−3] is the concentration of the contaminant in the PSD at exposure time t. The offload rate constant can be used to derive the 95% equilibrium concentration t95 in the sampler (Bayen et al. 2009)
which represents the time to reach 95% of equilibrium concentration in the sampler. It can be noted that, according to Kot-Wasik et al. (2007), that the concentration of the contaminants in the sampler increase linearly, which allows for estimation of the uptake time to 50% of equilibrium t50 (stage 1) by (Kot-Wasik et al. 2007)
Equation (10) allows for the estimation of the dissolved contaminant concentration. The time to equilibrium t50 may be calculated (stage 3) by (Lowmann et al. 2012)
Noting that the contaminant concentration Cw(t) is constant with respect to time, the combination of Eq. (6) with the diffusion models leads to (Bayen et al. 2009)
and
where δw is the diffusion layer thickness in water [L], r is the radius of curvature of the sampler surface [L], Dw is the diffusion coefficient of the analyte in water [L2/T], and ωt is the mass transfer coefficient in the sampler [T−1].
Bayen et al. (2009) notes for Eqs. (12) and (13) that factors such as waterflow rate, temperature, and (bio)fouling, may affect δw, Dw, and ωt, which in turn could affect uptake and offload rates. Temperature will also affect Ksw. Figure 5 depicts the basic trends of k1, k2, and t95 as a function of Ksw. On the left side of Fig. 5 (lower Ksw where mass transfer is limited by diffusion inside the sampler), k1 theoretically increases with increasing Ksw until a plateau representing maximum uptake kinetics (k1max) is reached, critical (log Kow)c, in a sampler exposed to an aqueous phase that is controlled by the diffusion of the contaminant in the aqueous phase (Bayen et al. 2009). Bayen et al. further point out that, for flat samplers (r ≫ δw), k1max may be obtained from Eq. (10). That is when δw /Dw ≫ (ωt × Ksw)−1 leads to
Relation between uptake rate constant k1, offloading rate k2, and time to 95% equilibrium t95 and the sampler-water partition coefficient Ksw or octanol-water partition coefficient Kow as a basic measure of hydrophobicity (after Bayen et al. 2009)
It is further apparent from Fig. 5 that at lower Ksw, k2 and t95 will remain relatively constant until (log Kow)c is reached at which point, as Ksw continues increasing, k2 rapidly decreases and t95 rapidly increases.
Equilibrium sampling calculation
For equilibrium PSDs, the exposure time is sufficiently long as to permit the establishment of thermodynamic equilibrium between the water and reference phases such that Eq. (6) reduces to (Vrana et al. 2005)
The basic requirements of the equilibrium-sampling methodology are that stable concentrations are reached after a known response time, which requires that the sampler capacity be kept substantially below that of the sample to avoid depletion during extraction. In addition, the device response time must be shorter than any fluctuations in the environmental medium. Passive diffusion bag samplers (PDBSs) have reportedly been used extensively for monitoring volatile organic compounds (VOCs) in water (Vrana et al. 2005).
Nonequilibrium (kinetic) sampling calculation
For kinetic samplers, it is assumed that the rate of mass transfer to the reference/receiving phase is linearly proportional to the difference between the chemical activity of the contaminant in the water phase and that in the reference phase. In the initial phase of sampler exposure, the rate of desorption of the contaminant from the receiving phase to water is negligible, the sampler works in the linear uptake regime, and Eq. (8) reduces to (Vrana et al. 2005)
The advantages of kinetic or integrative sampling are that they sequester contaminants from episodic events commonly not detected with grab sampling and can be used where water concentrations are variable. They permit measurement of ultra-trace, yet toxicologically relevant, contaminant concentrations over extended periods (Vrana et al. 2005). It is exactly these conditions (episodic events, variable concentrations) that are of concern at contaminated karst sites. Details on the appropriate use and application of types of passive sampling devices may be found in Burgess et al. (2016).
Passive sampling instances in karstic terranes
Although the difficulties associated with obtaining representative groundwater-quality samples from karst aquifers, whether sampling from wells or springs and seeps, is widely recognized, it appears that little progress towards alleviating some of the difficulties has been pursued since Quinlan and Alexander (1987) developed their event-based sampling approach. That said, some individuals have begun seriously considering passive sampling methods for obtaining representative groundwater samples from karst aquifers.
An instance when passive sampling in karst terranes is necessary
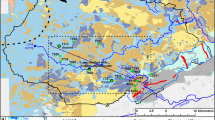
A tracing study was initiated at a highly contaminated site located in the Hagerstown Valley, Maryland (part of the Great Valley) of the Eastern Valley and Ridge Province of the Appalachians with recovery at several locations, details of which may be found in Field (2017). The purpose of the tracer test was to assess the distribution and migration of several pesticides spilled or deliberately released at the site and is now in groundwater in the area. The tracing study consisted of a release of 7.16 kg of fluorescein dye (Colour Index, Acid Yellow 73) into a small sinkhole ~0.5 m in diameter. Various springs and wells were monitored continuously for dye using a Turner Designs Cyclops-7 Logger in situ fluorometer and data logger with recoveries in radial directions as a result of the site overlying a groundwater mound (Field 2017). A breakthrough curve (BTC) for one sampling station is depicted in Fig. 6. Tracer distance from the point of injection to the sampling station for the BTC shown in Fig. 6 was estimated to be 2,000 m but the mean time of travel was calculated to be 35 days (peak time of travel = 34 days), which is quite difficult to visually discern from Fig. 6. The calculated mean time of travel suggests a mean velocity of 86 m/day (0.001 m/s), which is 20× slower than for typical karst aquifers—e.g., 0.02 m/s (Bonacci 1987, p. 9). Such an erratic spiky BTC for such a very slow tracer migration essentially renders an event-driven sampling methodology virtually impossible to effectively implement. Simply stated, which storm event correlates to any instance of pollutants discharging from the spring.
Should an event-driven sampling methodology be implemented at the sampling station depicted in Fig. 6, it would be nearly impossible to relate a precipitation event to the site hydrograph when peak time of travel is estimated to be just over 34 days and mean time of travel is estimated to be approximately 35 days during the wet season when the tracer test was implemented. Tracer migration is likely substantially lower during the dry season. The basic problem is trying to correlate rises in the hydrograph to any specific precipitation event over such a long period, making the timing of the sampling protocol extremely difficult. In addition, the spiky erratic nature of the BTC suggests a potential pulsing of discharges.
Discussion and summary
Although long recognized that basic grab sampling for water-quality analyses at karst springs was problematic, it was not until the paper by Quinlan and Alexander (1987) that a methodology that yields more representative samples was recognized. Unfortunately, only a few instances have been published demonstrating the effectiveness of the methodology. Reasons for not implementing an event-driven sampling methodology in more instances remain obscure but may be due to problems with implementation and cost.
Critically reviewing the difficulties associated with implementing an event-driven sampling methodology and complex BTCs such as is depicted in Fig. 6 suggests that the more effective methodology of passive sampling could be a substantial improvement over event-driven sampling. By allowing deployed PSDs to remain in place anywhere from 14 to 30 days greatly improves the likelihood of obtaining representative mean contaminant concentrations while minimizing the costs associated with collecting multiple samples throughout a precipitation event. The much lower concentrations that can be achieved when employing PSDs also make passive sampling a potentially more appropriate method for the extremely sensitive eco-environments typical of caves. In addition, costs are reduced because fewer man-hours are required.
It is perhaps too early to go so far as to formally recommend passive sampling in all or even just some instances when karst aquifers need to be evaluated for contaminants but passive sampling generally appears to be a valuable compliment to grab sampling and event-driven sampling in most instances. More studies need to be conducted to confirm the potentially valuable addition of adding a passive-sampling methodology to groundwater sampling plans for contaminated karst aquifers. Hopefully, more studies will be conducted in the near future.
References
Bayen S, ter Laak TL, Buffle J, Hermens JM (2009) Dynamic exposure of organisms and passive samplers to hydrophobic chemicals. Environ Sci Technol 43(7):2206–2215. https://doi.org/10.1021/es8029895
Blatnik M, Culver DC, Gabrovšek F, Knez M, Kogovšek B, Liu H, Mayaud C, Mihevc A, Mulec J, Nǎpǎruș-Aljančič M, Otoničar B, Petrič M, Pipan T, Prelovšek M, Ravbar N, Shaw T, Slabe T, Šebela S, Hajna NZ (2020) Water quality monitoring in karst. In: Knez M, Otoničar B, Petrič M, Pipan T, Slabe T (eds) Karstology in the classical karst. Springer, Cham, Switzerland, pp 127–137. https://doi.org/10.1007/978-3-030-26827-5
Benson, RC, Yuhr, LB (2016) Site characterization in karst and pseudokarst terrains: practical strategies and technology for practicing engineers, hydrologists, and geologists. Springer, Heidelberg, Germany
Bidwell JR, Becker C, Hensley S, Stark R, Meyer MT (2010) Occurrence of organic wastewater and other contaminants in cave streams in northeastern Oklahoma and northwestern Arkansas. Archives Environ Contamin Toxicol 58:286–298. https://doi.org/10.1007/s00244-009-9388-6
Bonacci O (1987) Karst hydrology with special reference to the Dinaric karst. Springer, New York
Booij K, Hofmans HE, Fischer CV, van Weerlee, EM (2003) Temperature-dependent uptake rates of nonpolar organic compounds by semipermeable membrane devices and low-density polyethylene membranes. Environ Sci Technol 37:361–366. https://doi.org/10.1021/es025739i
Booij K, Sleiderink HM, Smedes F (1998) Calibrating the uptake kinetics of semipermeable membrane devices using exposure standards. Environ Toxicol Chem 17(7):1236–1245. https://doi.org/10.1002/etc.5620170707
Booij K, Van Weerlee EM, Fischer CV, Hoedemaker J (2000) Passive sampling of organic contaminants in the water phase, final report, Nioz - Rapport 2000 – 5, Netherlands Institute for Sea Research
Bopp SK (2004) Development of a passive sampling device for combined chemical and toxicological long-term monitoring of groundwater. PhD Thesis, Universität Rostock, Rostock, Germany
Burgess RM, Driscoll SBK, Burton GA, Gschwend PM, Ghosh U, Reible D, Ahn S, Thompson T (2016) Laboratory, field, and analytical procedures for using passive sampling in the evaluation of contaminated sediments: user’s manual. Technical report EPA/600/XX-15/071, US Environmental Protection Agency, Washington, DC
Coxon C, Thorn RH (1989) Temporal variability of water quality and the implications for monitoring programmes in Irish limestone aquifers. In: Sahuquillo A, Andreu J. O’Donnell T (eds) Proceedings of the International Symposium on Groundwater Management: Quantity and Quality. IAHS Publ. no. 188, IAHS, Wallingford, UK, pp 111–120
Currens JC (1999) A sampling plan for conduit-flow karst springs: minimizing sampling cost and maximizing statistical utility. Eng Geol 52:121–128. https://doi.org/10.1016/S0013-7952(98)00064-7
Demougeot-Renard H, Bapst A, Trunz C, Fischer L, Renard P (2017) Integrative passive samplers to detect chlorinated hydrocarbon contamination in karst. In: Renard P, Bertrand C (eds) EuroKarst 2016, Neuchâtel. Advances Karst Science, pp 231–241. https://doi.org/10.1007/978-3-319-45465-8_23
Dreybrodt W (2004) Dissolution: carbonate rocks and dissolution—evaporite rocks. In: Gunn J (ed) Encyclopedia of caves and karst science. Fitzroy Dearborn, London
Felton GK, Taraba JL, (1995) The impact of karst water quality sampling frequency on parameter estimates. International American Society of Agricultural Engineers Meeting Paper 95–2426
Fernández D, Vermeirssen ELM, Bandow N, Muñoz K, Schäfer RB (2014) Calibration and field application of passive sampling for episodic exposure to polar organic pesticides in streams. Environ Poll 194:196–202. https://doi.org/10.1016/j.envpol.2014.08.001
Field MS (1990) Transport of chemical contaminants in karst terranes: outline and summary. In: Simpson ES, Sharp Jr JM (eds) Selected papers on hydrogeology, vol 1. 28th International Geological Congress, Washington, DC, pp 17–27
Field MS (1992-1993) Karst hydrogeology and chemical contamination. J Environ Syst 22(1):1–26
Field MS (2017) Tracer-test results for the Central Chemical Superfund Site, Hagerstown, MD, May 2014—December 2015. Technical report EPA/600/R-17/032. US Environmental Protection Agency, Washington, DC
Ford D, Williams P (2007) Karst hydrogeology and geomorphology. Wiley, Chichester, UK
Fox JT, Adams G, Sharum M, Steelman KL (2010) Passive sampling of bioavailable organic chemicals in Perry County, Missouri cave streams. Environ Sci Technol 44:8835–8841. https://doi.org/10.1021/es1019367
Górecki T, Namieśnik J (2000) Passive sampling: trends in. Anal Chem 21(4):276–291. https://doi.org/10.1016/S0165-9936(02)00407-7
Horowitz AJ (2008) Determining annual suspended sediment and sediment-associated trace element and nutrient fluxes. Sci Total Environ 400:315–343. https://doi.org/10.1016/j.scitotenv.2008.04.022
Huckins JN, Petty JD, Lebo JA, Almeida FV, Booij K, Alveraz DA, Cranor WL, Clark RC, Mogensen BB (2002) Development of the permeability/performance reference compound approach for in situ calibration of semipermeable membrane devices. Environ Sci Technol 36:85–91. https://doi.org/10.1021/es010991w
ITRC (2006) Technology overview of passive sampler technologies, technical report DSP-4. The Interstate Technology & Regulatory Council, Diffusion Sampler Team, Washington, DC, http://www.itrcweb.org/Guidance/GetDocument?documentID=26. Accessed 13 November 2016
Jeong Y, Schäffer A, Smith K (2018) A comparison of equilibrium and kinetic passive sampling for the monitoring of aquatic organic contaminants in German rivers. Water Res 145:248–258. https://doi.org/10.1016/j.watres.2018.08.016
Johnes PJ (2007) Uncertainties in annual riverine phosphorus load estimation: impact of load estimation methodology, sampling frequency, baseflow index and catchment population density. J Hydrol 332:241–258. https://doi.org/10.1016/j.jhydrol.2006.07.00
Kačaroğlu F (1999) Review of groundwater pollution and protection in karst area. Water Air Soil Poll 113:337–356
Kaserzon SL, Kennedy K, Hawker DW, Thompson J, Carter S, Roach AC, Booij K, Mueller JF (2012) Development and calibration of a passive sampler for carboxylates and sulfonates in water. Environ Sci Technol 46(9):4985–4993. https://doi.org/10.1021/es300593a
Kilroy G, Coxon C (2004) Temporal variation of phosphorus fraction in Irish karst springs. Environ Geol 47:421–430. https://doi.org/10.1007/s00254-004-1171-4
Kot A, Zabiegeła B, Namieśnik J (2000) Passive sampling for long-term monitoring of organic pollutants in water. Trends Anal Chem 19(7):446–459. https://doi.org/10.1016/S0165-9936(99)00223-X
Kot-Wasik A, Zabiegeła B, Urbaniwicz M, Dominiak E, Wasi A, Namieśnik J (2007) Advances in passive sampling in environmental studies. Anal Chim Acta 602:141–163. https://doi.org/10.1016/j.aca.2007.09.013
Kresic N (2013) Water in karst: management, vulnerability, and restoration. McGraw-Hill, New York
Lerch RN, Erickson JM, Wicks CM (2001) Intensive water quality monitoring in two karst watersheds of Boone County, Missouri. In: Proceedings National Cave Karst Management Symposium, Tucson, AZ, October 2001, pp 157–168
Levy W, Pandelova M, Henkelmann B, Bernhȍft S, Fischer N, Antritter F, Schramm K-W (2017) Persistent organic pollutants in shallow percolated water of the Alps, karst system (Zugspitze summit, Germany). Sci Total Environ 579:1269–1281. https://doi.org/10.1016/j.scitotenv.2016.11.113
Lowmann R, Booij K, Smedes F, Vrana B (2012) Use of passive sampling devices for monitoring and compliance checking of POP concentrations in water. Environ Poll Sci Res 19:1185–1895. https://doi.org/10.1007/s11356-012-0748-9
Malet E, Astrade L, Gauchon C, Jaillet S (2017) Monitorer les milieu naturels entre ambitions et contraintes une affaire de compromise [Monitoring the natural environment between ambitions and constraints a matter of compromise]. In: Collection Edytem Numéro 19, Monitoring en Milieux Naturels − Retours D’expérience en Terrains Difficiles, pp 9–17. https://doi.org/10.3406/edyte.2017.1356
Metcalfe CD, Beddows PA, Bouchot GG, Metcalf TL, Li H, Lavieren HV (2011) Contaminants in the coastal karst aquifer system along the Caribbean coast of the Yucatan Peninsula, Mexico. Environ Poll 159:991–997. https://doi.org/10.1016/j.envpol.2010.11.031
MPCA (2005) Ground water investigations in karst areas. Guidance Document 4–09, Petroleum Remediation Program, Minnesota Pollution Control Agency. https://www.pca.state.mn.us/sites/default/files/c-prp4-09.pdf. Accessed 23 June 2020
Namieśnik J, Zabiegeła B, Kot-Wasik A, Partvka M, Wasik A (2005) Passive sampling and/or extraction techniques in environmental analysis: a review. Anal Bioanal Chem 381:279–301. https://doi.org/10.1007/s00216-004-2830-8
Novic AJ, O’Brien DS, Kaserzon SL, Hawker DW, Lewis SE, Mueller JF (2017) Monitoring herbicide concentrations and loads during a flood event: a comparison of grab sampling with passive sampling. Environ Sci Technol 51:3880–3891. https://doi.org/10.1021/acs.est.6b02858
Palmer AN (2007) Cave geology. Cave Books, Dayton, OH
Persson C (2015) Calibration and application of passive sampling in drinking water for perfluoroalkyl substances. MSc Thesis, Upsala University, Upsala, Sweden
Poulier G, Lissalde S, Charriau A, Buzier R, Cleries K, Delmas F, Mazzella N, Guibaud G (2015) Estimates of pesticide concentrations and fluxes in two rivers of an extensive French multi-agricultural watershed: application of the passive sampling strategy. Environ Sci Poll Res 22(11):8044–8057. https://doi.org/10.1007/s11356-014-2814-y
Preston SD, Bierman VJ, Silliman SE (1992) Impact of flow variability on error in estimation of tributary mass loads. J Environ Eng 118:402–419. https://doi.org/10.1061/(ASCE)0733-9372(1992)118:3(402)
Quinlan JF, Alexander Jr EC (1987) How often should samples be taken at relevant locations for reliable monitoring of pollutants from an agricultural, waste disposal, or spill site in a karst terrane? A first approximation. In: Beck BF (ed) Multidisciplinary Conference on Sinkholes and Environmental Impacts of Karst, Proceedings, Balkema, Dordrecht, The Netherlands, pp 277–293
Quinlan JF, Ewers RO (1985) Ground water flow in limestone terranes: Strategy rationale and procedure for reliable, efficient monitoring of ground water quality in karst areas. In: Fifth National Symposium and Exposition on Aquifer Restoration and Ground Water Monitoring, Columbus, OH. National Water Well Association, Worthington, OH, pp 197–234 http://info.ngwa.org/GWOL/pdf/850138326.pdf. Accessed 23 October 1985
Rabiet M, Margoum C, Gouy V, Carluer N, Coquery M (2010) Assessing pesticide concentrations and fluxes in the stream of a small vineyard catchment: effect of sampling frequency. Environ Poll 158:737–748. https://doi.org/10.1016/j.envpol.2009.10.014
Roll IB (2015) Novel integrative methods for sampling environmental contaminants. PhD Thesis, Arizona State University, Tempe, AZ
Roll IB, Hallden RU (2016) Critical review of factors governing data quality of integrative samplers employed in environmental water monitoring. Water Res 94:200–207. https://doi.org/10.1016/j.watres.2016.02.048
Schleppi P, Waldner PA, Stähli M (2006) Errors of flux integration methods for solutes in grab samples of runoff water, as compared to flow-proportional sampling. J Hydrol 319:266–281. https://doi.org/10.1016/j.jhydrol.2005.06.034
Smedes F (2010) Evaluatie van monitoring met passive sampling: relaties met mosselen, zwevend stof en totaal water [Evaluation of monitoring with passive sampling: relationships with mussels, suspended matter and total water]. Technical report 1202990–000-BGS-0004, Deltares, Enabling Delta Life, Delft, The Netherlands. http://publications.deltares.nl/1202990_000.pdf. Accessed 1 December 2016
Smedes F, Bakker D, de Weert J (2010) The use of passive sampling in WFD monitoring: the possibilities of silicon rubber as a passive sampler. Technical report 1202337–004-BGS-0027, Deltares, Enabling Delta Life, Delft, The Netherlands. http://www.passivesampling.net/utrechtworkshop/pres/1202337-004-BGS-0027-r-The%20use%20of%20passive%20sampling%20in%20WFD%20monitoring.pdf. Accessed 12 November 2016
Schwarz K, Gocht T, Grathwohl P (2011) Transport of polycyclic aromatic hydrocarbons in highly vulnerable karst systems. Environ Poll 159:133–139. https://doi.org/10.1016/j.envpol.2010.09.026
Trček B (2007) How can the epikarst zone influence the karst aquifer hydraulic behavior. Environ Geol 51:761–765. https://doi.org/10.1007/s00254-006-0387-x
Vermeirssen ELM, Bramaz N, Hollender J, Singer H, Escher BI (2009) Passive sampling combined with ecotoxicological and chemical analysis of pharmaceuticals and biocides: evaluation of three Chemcatcher™ configurations. Water Res 43:903–914. https://doi.org/10.1016/j.watres.2008.11.026
Vrana B, Mills GA, Allan IJ, Dominiak E, Svensson K, Knutsson J, Morrison G, Greenwood R (2005) Passive sampling techniques for monitoring pollutants in water. Trends Anal Chem 24(10):845–868. https://doi.org/10.1016/j.trac.2005.06.006
Vrba J (1988) Groundwater quality monitoring as a tool of groundwater resources protection. In: Karst hydrogeology and karst environment protection, 21st Congress of IAH, proceedings, vol XXI, part 2. Geological Publ. House, Beijing, China, pp 88–97
White WB (1988) Geomorphology and hydrology of karstic terrains. Oxford Univ. Press, Oxford, UK
Zabiegeła B, Kot-Wasik A, Urbaniwicz M, Namieśnik J (2010) Passive sampling as a tool for obtaining reliable analytical information in environmental quality monitoring. Anal Bioanal Chem 396:273–296. https://doi.org/10.1007/s00216-009-3244-4
Zhang Z, Troldborg M, Yates K, Osprey M, Kerr C, Hallet PD, Baggaley N, Rhind SM, Dawson JJC, Hough RL (2016) Evaluation of spot and passive sampling for monitoring, flux estimation and risk assessment of pesticides within the constraints of a typical regulatory monitoring scheme. Sci Total Environ 569–570:1369–1379. https://doi.org/10.1016/j.scitoenv.2016.06.219
Acknowledgements
The author appreciates the reviews and comments provided by Dr. Carol Wicks and Dr. William White. Their thoughts and comments greatly improved the manuscript. The author also thanks Dr. Augusto Auler, Dr. Philippe Meus, and two anonymous reviewers for their very helpful thoughts and comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The views expressed in this paper are solely those of the author and do not necessarily reflect the views or policies of the US Environmental Protection Agency. The mention of trade names does not constitute an endorsement.
Additional information
Published in the special issue “Five decades of advances in karst hydrogeology”.
Rights and permissions
About this article
Cite this article
Field, M.S. Groundwater sampling in karst terranes: passive sampling in comparison to event-driven sampling strategy. Hydrogeol J 29, 53–65 (2021). https://doi.org/10.1007/s10040-020-02240-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-020-02240-9