Abstract
Purpose
In patients with terminal ostomies, parastomal hernias (PSHs) occur on a frequent basis. They are commonly associated with various degrees of complaints and occasionally lead to life-threatening complications. Various strategies and measures have been tested and evaluated, but to date there is a lack of published evidence with regard to the best surgical technique for the prevention of PSH development.
Methods
We conducted a retrospective analysis of prospectively collected data of eighty patients, who underwent elective permanent ostomy formation between 2009 and 2014 by means of prophylactic implantation of a three-dimensional (3D) funnel mesh in intraperitoneal onlay (IPOM) position.
Results
PSH developed in three patients (3.75 %). No mesh-related complications were encountered and none of the implants had to be removed. Ostomy-related complications had to be noted in seven (8.75 %) cases. No manifestation of ostomy prolapse occurred. Follow-up time was a median 21 (range 3–47) months.
Conclusion
The prophylactical implantation of a specially shaped, 3D mesh implant in IPOM technique during initial formation of a terminal enterostomy is safe, highly efficient and comparatively easy to perform. As opposed to what can be achieved with flat or keyhole meshes, the inner boundary areas of the ostomy itself can be well covered and protected from the surging viscera with the 3D implants. At the same time, the vertical, tunnel-shaped part of the mesh provides sufficient protection from an ostomy prolapse. Further studies will be needed to compare the efficacy of various known approaches to PSH prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After formation of permanent, terminal enterostomies, parastomal hernia (PSH) occurs in up to 80 % [1] and lead to various symptoms and complications in approximately 80 % of affected patients [2].
Associated medical problems range all the way from repeated leakage from the base of collector bags, abdominal discomfort due to the visceral prolapse and intermittent obstruction to incarceration and occasionally strangulation [3]. According to a registry-based study, PSH is known to lead to acute presentations in 10 % of cases, which in return increase morbidity by 50 % and even mortality to 10 % after the necessary emergency procedures [4]. The high reported rate of surgical emergency interventions may reflect our reluctance towards elective PSH repair—probably caused by the knowledge that the various available operative techniques are associated with sometimes high rates of complications and recurrence [3].
The high rates of PSH, the commonly seen complaints and poor post-operative results after attempted repair shift the focus onto their prevention. A good surgeon alone is not enough and cannot significantly reduce the rate of incisional hernias [5]. PSH is ultimately a subtype of incisional hernia—located in the very vicinity of an enterostomy, which in turn due to its nature resembles a full-thickness defect of the abdominal wall. The fascia gap is usually at least 3 cm wide and must be seen as an obvious weak point which is particulary prone to the development of hernias.
To date, the best strategy for PSH prevention is still not defined. The use of mesh devices, their size, material, possible 3D design and position in relation to the abdominal wall’s layers are also under debate.
In 2004, a randomized controlled trial about PSH management with a partially absorbable mesh device was published. Because of the overwhelmingly convincing results in the group of patients treated with the mesh implant, the trial was stopped by the ethics committee in charge to avoid the disadvantage for the other patient cohort. No implant-related adverse events were reported in the trial [6].
Subsequently, conducted trials and reviews about various mesh devices in sublay-, onlay-, or intraperitoneal position largely supported the initial trial’s results and confirmed significantly reduced rates of PSH after mesh augmentation compared to conventional, sutured ostomy formation—again without morbidity associated with the implanted material [7–11].
Despite these encouraging results, PSH had to be observed in 8–50 % of patients which highlights the need for further attention to the condition and for further improvement of the techniques currently employed. Extraperitoneal ostomy formation has been described as an alternative approach to this pressing issue [12]. It could reduce the rates of PSH significantly but the necessary preparatory steps are demanding. A new technique involving a biological mesh device and a circular stapler is also currently evaluated in a multicenter trial [13]. We see significant advantages for our patients in the use of a 3D inversely funnel-shaped mesh over various flat implants. This preformed device by design protects the notoriously endangered margins of the fascia gaps at the ostomy site not only through local reinforcement but also by means of actual overlap along the diverted bowel and into the abdominal cavity. The only published study on the topic with regard to a 3D funnel mesh did reveal immaculate results but only included 22 patients with a follow-up period of a median 11 months [14]. The aim of the present trial is to evaluate the efficacy of this 3D mesh device in terms of PSH prevention and to analyze the results of the institutions involved in the study.
Materials and methods
We conducted a retrospective analysis of prospectively collected data about patients who underwent permanent, terminal ostomy formation in IPOM technique with augmentation by a 3D inversely funnel-shaped mesh device at the departments of general surgery at either Wilhelminenspital, Vienna or the Sisters of Charity Hospital, Linz, Austria between the years 2009 and 2014.
At both centers, the procedure is routinely carried out in elective cases with either isolated permanent and terminal ostomy formation or definitive Hartmann’s resection. All operations were classified as Class II/Clean-Contaminated according to the Centers for Disease Control Classification (CDC). This is defined as an operative wound, in which the respiratory, alimentary, genital, or urinary tracts are entered under controlled conditions and without unusual contamination.
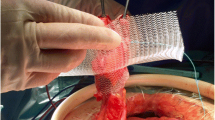
In all documented cases, a prefabricated square mesh device of 15 cm edge length with a central, funnel-shaped channel of 2 cm in diameter and a depth of 2.5 cm was implanted [macro-porous, mono-filamentary Dynamesh™, IPST®, FEG Textiltechnik, Aachen, Germany—designed with a viscera-sided aspect made of polyvinylidene fluoride (PVDF) and a parietal part made of polypropylene (PP)] (Fig. 1).
Surgical preparation was performed in exactly the same manner at the participating departments: in cases with laparoscopically assisted stoma formation the diverted colon was first fed through the future stoma site’s fascial gap. The implant was then slid over the bowel with the funnel-shaped part pointing towards the abdominal cavity (Fig. 2). After these initial steps, the mesh was relocated intra-abdominally. After the mesh was laparoscopically unfolded with atraumatic graspers, it was secured with U-shaped, absorbable tacks (Ethicon™, Vienna, Austria, secure-strap®) in double crown technique (Fig. 3). In case of open surgery with median laparotomy, the bowel was fed through the device’s funnel first and only thereafter moved through the abdominal wall. Parts of the mesh positioned on the lateral side of the ostomy were again secured with absorbable tacks. The implant’s flat parts to the right of the ostomy (i.e., towards the midline and median laparotomy) were armed with nonabsorbable monofilament sutures (Ethicon™, Prolene® 2.0 United States Pharmacopeia) at 2 cm intervals which were passed transfascially and transcutaneously through the abdominal wall at a distance of 4 cm to the right side of the midline incision. By doing so, a secondary benefit could be reached by prophylactically overlapping either the whole midline incision (in case of shorter incisions) or at the very least a length of 15 cm of a longer preexistent incision (Fig. 4).
The implant’s flat parts to the right were armed with sutures which were passed transfascially and transcutaneously through the abdominal wall at a distance of 4 cm to the right side of the midline incision. By doing so, a secondary benefit could be reached by prophylactically overlapping the whole median laparotomy
Evaluated data were retrieved from patients’ files and documents deposited in the hospital’s electronic data systems. Patients with underlying oncological conditions were seen in the outpatient departments at three monthly intervals during the first 2 years and thereafter every 6 months until 5 years after the initial operation. Follow-up examinations comprised the patients’ medical history, clinical examination, blood test relevant for the respective malignant condition (tumor marker), abdominal sonography and plain chest X-ray. As part of the aftercare, the majority of patients also underwent computed tomography (CT) of the abdomen and pelvis 1 year postoperatively. We performed a multislice computed tomographic scanning, including sequences during abdominal press. In patients with inflammatory bowel disease, clinical examination and elective colonoscopy after 3 months and a CT scan of the abdomen and pelvis after 1 year were performed. Symptomatic PSHs that warrant surgical repair could in most cases be diagnosed clinically and are usually evident during Valsalva’s maneuver when patients are standing upright during physical examination. Apart from high attention to demographic, disease- and procedure-specific data, we had a particular focus on PSH formation, ostomy site complications and on potentially present unfavorable, mesh-associated outcomes. Detected PSH (n = 3) were, depending on their clinical and radiological appearance, categorized in accordance with the recommendations of the European Hernia Society [15] and the classification of Moreno-Matias [16]. Statistical analysis was performed with SPSS software version 20 for Windows (SPSS, Chicago, IL, USA). Statistical evaluations were extended to descriptive calculations including percentage, medians and readings of statistical range.
Results
Between 1/2009 and 8/2014, a total of 80 patients could be included in the trial. The mean age was 71.4 years (range 46–91) and 47 (58.7 %) of the patients were of the male persuasion. The body mass index (BMI) was mean 26.4 kg/m2 (range 18.4–36.8). Isolated ostomy formation was mainly carried out laparoscopically (11/13) whereas ostomy formation in combination with bowel resection was more often performed as open, conventional surgical procedures after midline incision (55/67). Due to the patients’ underlying condition, colorectal resections were necessary in 67 of 80 cases (83.7 %) and the indication for the formation of a permanent terminal ostomy arose from abdominoperineal resections due to deep rectal cancer (52/67, 77.6 %). Isolated ostomy formations (n = 13/80, 16.3 %) became necessary as part of patient’s palliative management (n = 8), or because of tumor-related stenosis prior to neo-adjuvant radio-chemotherapy (n = 5). Disease- and procedure-specific data are displayed in Table 1. Median follow-up time was 21 months (3–47) and 51/80 patients (63.8 %) had at least one CT scan of the abdomen not less than 1 year after ostomy formation.
Overall morbidity was 38.8 % in the study and mainly presented as wound healing disturbance-surgical site occurrences (SSO) and surgical site infections (SSI) of the midline incision, the ostomy site or the perineal wound. The overall mortality during the ongoing follow-up affected 17 from 80 patients (21.3 %) and comprised all causes of death during the follow-up period. Two patients died from a rapid progression of their underlying end-stage malignant condition after palliative ostomy formation which resulted in a 30-day mortality rate of 2.5 %. Isolated ostomies for palliative management were created only in 8 of 80 patients (10 %). Three of them are still alive. We included all patients with PSH prevention because life expectancies are can hardly be estimated because of the different tumor biologies and responses to non-surgical oncological treatment modalities.
Ostomy-related complications were identified in 7 of the 80 cases (8.75 %).
Two subcutaneous seromas were treated with needle aspiration because of a volume of more than 50 ml with an associated unpleasant feeling of tension and pressure. Both of them did not require further interventions. Three subcutaneous para-stomal abscesses required incision and drainage outside the stoma appliance under general anesthesia and one case of bowel retraction had to return to theatre after 30 days. One ostomy was found to be too narrow and had to be corrected surgically without mesh removal by widening the incision of the abdominal wall only one day after initial ostomy formation. The incision of the abdominal wall was too narrow. The patient had a powerful rectus muscle. The original implant with its seamless extension into the intestinal cuff offers superb elasticity and flexibility and did not compress the ostomy bowel.
PSHs were found in 3 of 80 cases (3.75 %). Two of them were asymptomatic and could only be detected with CT scans but not from the patients’ histories or through clinical examination. Only one patient had to undergo elective re-operation because of a symptomatic PSH. In this case after multiple previous laparotomies with post-operative disturbances in wound healing, the integrity of the abdominal wall had been compromised to the extent that an extensive hernia defect had formed at the margin of the implant’s flat component. Two years after the initial formation of the ostomy, an open re-do procedure with contralateral stoma translocation and intraperitoneal implantation of a larger 3D mesh device (16 cm side length) was performed. Complications and PSHs are displayed in Table 2. Only six patients had to be re-operated after PSH prevention. 5 patients required an ostomy due to a tumor stenosis prior to neo-adjuvant radio-chemotherapy with subsequent tumor resection and one patient required a reoperation due to a symptomatic PSH. The adhesions to the synthetic mesh concerned mainly omental fat but no dense bowel adhesions to the prosthesis occurred.
Discussion
Various techniques for the prevention of PSH have been described and they are already well supported by published data. To date, however, they are still not firmly included in clinical routine. Reasons for this may be the operating surgeons’ fear of mesh infection and implant-associated complications as well as the fact that preventive measures are often not considered economically justifiable. Studies do document a significant reduction in the occurrence of PSH after mesh augmentation compared to conventional ostomy formations but the rates for PSH remain high between 8 and 50 % [7–11] over all described methods. The laparoscopic‚ keyhole’ technique did help to reduce PSH rates by 50 from 93 %, but results still remain unsatisfactory [10].
Since prevention and repair of an already existing PSH are approached with the very same procedure, the results do not come as a surprise and indeed the recurrence rate in the largest published series is 36.4 % [17].
Most publications on the topic focusing on PSH prevention are based on the sublay technique. Results from a current Dutch multi-center trial in the field will be expected in 2015 [18]. As part of its protocol, too, a flat, incised mesh is used to re-inforce the abdominal wall around the ostomy—an area that does probably not need augmentation. The principle of all techniques involving flat meshes around an ostomy, regardless of their different position, onlay, sublay or intraperitoneal fails to protect the gap between the bowel and the surrounding abdominal wall and reveals non-convincing results in terms of PSH repair [19]. The—per protocol—open surgical approach for the cases involved resembles another weak point of the trial. The international trend is towards laparoscopic colorectal surgery. There are no qualitative differences in the oncological radicality and no longer concerns about safety [20, 21], in particular, since sublay-PSH prevention has been successfully performed and described in a laparoscopically assisted technique [22].
Several studies have aimed to evaluate the value of biological implants in PSH prevention [23]. It is worth noting, however, that according to all trials with a focus on PSH prevention by means of synthetic mesh implantation in sublay, onlay or intraperitoneal position significant mesh-related complications were not detected [6–11]. As for implantation in sublay position, this is even documented in a trial with a follow-up period of 5 years [24]. The clinical value of biological implants, especially when compared to the related financial aspects, therefore appears largely overrated.
The authors of another study fathomed the feasibility of prophylactic implantation of partially absorbable meshes in the heavily contaminated surgical fields of 19 patients. Surgical site infections in these patients frequently occurred around the midline incision but not in the vicinity of the stoma site. PSH prevention by mesh implantation, therefore, might well prove to be a feasible procedure even in the presence of gross peritonitis. This, however, can by no means be declared as a current standard at any of our departments, nor can it be seen as a recommendation [25].
The 3D funnel-shaped implant has proven to be useful not only in the field of PSH prevention but also in the treatment of this particular medical condition [26]. Whenever possible we aim to perform a laparoscopically assisted ostomy repair in the same position. To do so, we excise the pre-existing ostomy directly at its borders with the surrounding skin after laparoscopic adhesiolysis and temporarily closing it with a suture. After mobilization from the abdominal wall, the diverted bowel is passed into the abdominal cavity, which facilitates the removal of the adjacent hernia-sac and, therefore, reduces the risk of seroma formation in the later course. The bowel is eventually slid through the implant outside the abdomen and the mesh is returned into the peritoneal cavity with the funnel-shaped part pointing towards the viscus. The hernia defect in the fascia is sutured to the appropriate diameter and the mesh itself is spread out in position before laparoscopic fixation to the peritoneum with absorbable tacks.
When present a concomitant incisional hernia is treated with an additional flat mesh in laparoscopic IPOM technique. If necessary an often present prolapse of the ostomy is managed by resection of excessive bowel length and eventually the ostomy is created with everting absorbable sutures to the skin only. A modified technique can be applied in open surgical procedures: mesh fixation is then reached with absorbable tacks on the lateral side of the ostomy and with transfascial sutures from the mesh to the subcutaneous tissue on the medial side. As a desirable side effect, the flat part of the mesh thereby prophylactically reinforces the initial operation’s median laparotomy.
There are several beneficial aspects to the technique described: the fascia gaps directly adjacent to the bowel are ideally protected even in the presence of large ostomy diameters and the implant can be used in both open and laparoscopically assisted procedures. The midline incision and the lateral border of the rectus sheath do not define the actual maximum size of the mesh. The accurately fitting funnel-shaped part gives additional protection against the development of stoma prolapses. Mesh placement has proven to be safe and straight forward from a technical point of view. The procedure is, therefore, associated with a steep learning curve and a separation of the abdominal wall’s layers, a potential cause for morbidity, is not necessary.
We measured the time necessary for 3D mesh implantation and fixation in open surgery with 10 min and during the laparoscopic approach with 20 min on average.
An ostomy placement through the rectus muscle seems not to be imperative since the muscle itself does obviously not give relevant long-term protection from either PSH formation or ostomy prolapse as evidenced by the high rates of PSH and prolapse without preventive measures [6–11]. Both complications can essentially be decreased using a 3D funnel mesh [14]. If it is necessary, the ostomy can be brought out through the lateral abdominal wall while the fascia defect remains well covered by a mesh with sufficient overlap.
The technique together with the implant used can be applied not only for the prevention and treatment of left-sided enterostomy complications but also for the management of these conditions in right-sided ostomies and urostomies. An additional lateralization and deformation of the diverted bowel likely to be necessary in the Sugarbaker technique is not needed [27]. This reduces the potential risk of symptomatic bowel obstruction. Despite the fact that Sugarbaker’s technique is associated with a reduced rate of PSH recurrence compared to a simple “keyhole” mesh reinforcement, it still leaves room for improvement with regard to re-herniation—ultimately because the actual space between the fascia’s edge and the bowel wall remains at least partly unprotected from the long-term effects of the intra-abdominal pressure. There are only few studies available regarding Sugarbaker repair with small numbers of patients included and an inconsistent follow-up quality and duration. A meta-analysis published in 2012 including six studies with overall 110 patients reported a recurrence rate of 11.6 % (ranging from 0 to 28.6 %) [28]. However, a recent publication on 61 cases shows a recurrence rate of only 6.6 % after a mean follow-up of 26 months. A CT or MRI scan was performed in 27 of 60 patients during the follow-up period [29]. Viewed in this light, it cannot be ruled out that parastomal hernias had been overlooked. However, we agree that routinely performed medical imaging is expendable, because symptomatic PSHs that, therefore, warrant surgical repair can in most cases be diagnosed clinically and are usually evident during Valsalva’s maneuver when patients are standing upright during physical examination.
The original implant with its seamless extension into the intestinal cuff offers superb elasticity and flexibility. The mesh with a central, funnel-shaped channel of 2 cm in diameter and a depth of 2.5 cm was always rated as suitable. Another square mesh device of 16 cm edge length with a central, funnel-shaped channel of 3 cm in diameter is also available. With funnel meshes, it is possible to make an incision into the anterior or posterior fascia that is large enough to easily bring even a bulky stoma out through the abdominal wall, while the fascia defect remains well covered by a mesh with sufficient overlap. Recently, a mesh with 25 cm edge length with a 2 cm channel diameter has been produced, because the 2 cm channel seems to be appropriate for nearly all ostomies. The different edge lengths may be helpful for parastomal hernia repair in cases of larger fascial defects.
PVDF is designed in a macroporous manner and has shown to cause little adhesion in experimental studies.
It integrates well into the surrounding soft tissue and is hardly prone to shrinkage processes [30].
In a study with 344 cases of intraperitoneally positioned implants, no mesh-related complications had to be documented [31]. However, it has to be stated that another publication described several and at times severely adverse events with regard to this material [32]. To date, we observed no complications in our own patient cohort and no implants had to be removed.
From the economic point of view, PSH prevention seems to be cost-effective in patients with stage I–III rectal cancer undergoing abdominoperineal rectum amputation with prophylactic mesh placement. It might be cost-effective for stage IV disease, but this decision is subject to some uncertainty. Malignancy may decrease life expectancy to the point that PSH might not have time to develop [33].
Symptomatic PSHs that warrant surgical repair can in most cases be diagnosed clinically and are usually evident during Valsalva’s maneuver when patients are standing upright during physical examination. We performed routinely a multislice computed tomographic scanning including sequences during abdominal press. Only 1 of the 80 patients developed a symptomatic PSH (1.25 %). Two hernias were asymptomatic and could only be detected with CT scans but not from the patients’ histories or through clinical examination. Maybe we overlooked some more asymptomatic hernias, because only 2/3 of the patients underwent a CT scan. On the other hand, we could have presented a study with only 1.25 % PSH development (excluding accidentally detected and asymptomatic PSHs in routine CT scan).
The retrospective design of our trial and the lack of a control group are, without doubt, a relative limitation of the present study. Strong points, on the other hand, are the high number of cases included and the fact that data and procedures from two independent surgical centers were evaluated. The comparatively long follow-up period of a median 2 years is also worth mentioning in this context, in particular since to the best of our knowledge the only other published trial applying preshaped 3D funnel meshes merely describes 22 cases with a follow-up of 11 months [14].
In conclusion, the present trial supports that prophylactic implantation of a 3D funnel mesh device in IPOM technique is a very effective, clinically safe procedure to prevent the development of PSHs and to hamper the frequently associated manifestation of prolapses around permanent terminal ostomies. The specifically designed implant with its elastic tunnel that accurately fits to diverted bowel demonstrates significant advantages over flat meshes and implants with mesh incisions. It can be used for both prevention and treatment of PSHs. The results generated in various previous trials show the clear advantage of mesh reinforcement over techniques without the use of implants with regard to PSH prevention already. Future trials should, therefore, no longer compare standard ostomy techniques with other new methods in general. They should rather have a new focus on techniques that include mesh implants, probe their advantages and evaluate the differences in outcome between these strategies.
References
Cingi A, Cakir T, Sever A, Aktan AO (2006) Enterostomy site hernias: a clinical and computerized tomographic evaluation. Dis Colon Rectum 49(10):1559–1563
Moreno-Matias J, Serra-Aracil X, Darnell-Martin A, Bombardo-Junca J, Mora-Lopez L, Alcantara-Moral M, Rebasa P, Ayguavives-Garnica I, Navarro-Soto S (2009) The prevalence of parastomal hernia after formation of an end colostomy. A new clinico-radiological classification. Colorectal Dis 11(2):173–177
Hansson BM (2013) Parastomal hernia: treatment and prevention 2013; where do we go from here? Colorectal Dis 15(12):1467–1470
Gregg ZA, Dao HE, Schechter S, Shah N (2014) Paracolostomy hernia repair: who and when? J Am Coll Surg 218(6):1105–1112
Klinge U, Binnebösel M, Rosch R, Mertens P (2006) Hernia recurrence as a problem of biology and collagen. J Minim Access Surg 2(3):151–154
Jänes A, Cengiz Y, Israelsson LA (2004) Randomized clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. Br J Surg 91(3):280–282
Serra-Aracil X, Bombardo-Junca J, Moreno-Matias J, Darnell A, Mora-Lopez L, Alcantara-Moral M, Ayguavives-Garnica I, Navarro-Soto S (2009) Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Ann Surg 249(4):583–587
Tam KW, Wei PL, Kuo LJ, Wu CH (2010) Systematic review of the use of a mesh to prevent parastomal hernia. World J Surg 34(11):2723–2729
Helgstrand F, Gögenur I, Rosenberg J (2008) Prevention of parastomal hernia by the placement of a mesh at the primary operation. Hernia 12(6):577–582
López-Cano M, Lozoya-Trujillo R, Quiroga S, Sánchez JL, Vallribera F, Martí M, Jiménez LM, Armengol-Carrasco M, Espín E (2012) Use of a prosthetic mesh to prevent parastomal hernia during laparoscopic abdominoperineal resection: a randomized controlled trial. Hernia 16(6):661–667
Gögenur I, Mortensen J, Harvald T, Rosenberg J, Fischer A (2006) Prevention of parastomal hernia by placement of a polypropylene mesh at the primary operation. Dis Colon Rectum 49(8):1131–1135
Lian L, Wu XR, He XS, Zou YF, Wu XJ, Lan P, Wang JP (2012) Extraperitoneal vs. intraperitoneal route for permanent colostomy: a meta-analysis of 1071 patients. Int J Colorectal Dis 27(1):59–64
Williams NS, Nair R, Bhan C (2011) Stapled mesh stoma reinforcement technique (SMART)—a procedure to prevent parastomal herniation. Ann R Coll Surg Engl 93(2):169
Berger D (2008) Prevention of parastomal hernias by prophylactic use of a specially designed intraperitoneal onlay mesh (Dynamesh IPST). Hernia 12(3):243–246
Smietański M, Szczepkowski M, Alexandre JA, Berger D, Bury K, Conze J, Hansson B, Janes A, Miserez M, Mandala V, Montgomery A, Morales Conde S, Muysoms F (2014) European Hernia Society classification of parastomal hernias. Hernia 18(1):1–6
Moreno-Matias J, Serra-Aracil X, Darnell-Martin A, Bombardo-Junca J, Mora-Lopez L, Alcantara-Moral M, Rebasa P, Ayguavives-Garnica I, Navarro-Soto S (2009) The prevalence of parastomal hernia after formation of an end colostomy. A new clinico-radiological classification. Colorectal Dis 11(2):173–177
Hansson BM, Bleichrodt RP, de Hingh IH (2009) Laparoscopic parastomal hernia repair using a keyhole technique results in a high recurrence rate. Surg Endosc 23(7):1456–1459
Brandsma HT, Hansson BM, V-Haaren-de Haan H, Aufenacker TJ, Rosman C, Bleichrodt RP (2012) PREVENTion of a parastomal hernia with a prosthetic mesh in patients undergoing permanent end-colostomy; the PREVENT-trial: study protocol for a multicenter randomized controlled trial. Trials 27;13:226
Kasperk R, Klinge U, Schumpelick V (2000) The repair of large parastomal hernias using a midline approach and a prosthetic mesh in the sublay position. Am J Surg 179(3):186–188
Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100(1):75–82
Bonjer HJ, Deijen CL, Albis GA et al (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372(14):1324–1332
Janson AR, Jänes A, Israelsson LA (2010) Laparoscopic stoma formation with a prophylactic prosthetic mesh. Hernia 14(5):495–498
Hammond TM, Huang A, Prosser K, Frye JN, Williams NS (2008) Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia 12(5):475–81. doi:10.1007/s10029-008-0383-z. (Epub 2008 May 17)
Jänes A, Cengiz Y, Israelsson LA (2009) Preventing parastomal hernia with a prosthetic mesh: a 5-year follow-up of a randomized study. World J Surg 33(1):118–121
Jänes A, Cengiz Y, Israelsson LA (2010) Experiences with a prophylactic mesh in 93 consecutive ostomies. World J Surg 34(7):1637–1640
Köhler G, Koch OO, Antoniou S, Lechner M, Mayer F, Klinge U, Emmanuel K (2014) Parastomal hernia repair with a 3-D mesh device and additional flat mesh repair of the abdominal wall. Hernia 18(5):653–661
Sugarbaker PH (1985) Peritoneal approach to prosthetic mesh repair of paraostomy hernias. Ann Surg 201(3):344–346
Hansson BM, Slater NJ, van der Velden AS, Groenewoud HM, Buyne OR, de Hingh IH, Bleichrodt RP (2012) Surgical techniques for parastomal hernia repair: a systematic review of the literature. Ann Surg 255(4):685–695
Hansson BM, Morales-Conde S, Mussack T, Valdes J, Muysoms FE, Bleichrodt RP (2013) The laparoscopic modified Sugarbaker technique is safe and has a low recurrence rate: a multicenter cohort study. Surg Endosc 27(2):494–500
Klinge U, Klosterhalfen B, Ottinger AP et al (2002) PVDF as a new polymer for the construction of surgical meshes. Biomaterials 23(16):3487–3493
Berger D, Bientzle M (2009) Polyvinylidene fluoride: a suitable mesh material for laparoscopic incisional and parastomal hernia repair! A prospective, observational study with 344 patients. Hernia 13(2):167–172
Fortelny RH, Petter-Puchner AH, Glaser KS, Offner F, Benesch T, Rohr M (2010) Adverse effects of polyvinylidene fluoride-coated polypropylene mesh used for laparoscopic intraperitoneal onlay repair of incisional hernia. Br J Surg 97(7):1140–1145
Lee L, Saleem A, Landry T, Latimer E, Chaudhury P, Feldman LS (2014) Cost effectiveness of mesh prophylaxis to prevent parastomal hernia in patients undergoing permanent colostomy for rectal cancer. J Am Coll Surg 218(1):82–91
Kanters AE, Krpata DM, Blatnik JA et al (2012) Modified hernia grading scale to stratify surgical site occurrence after open ventral hernia repairs. J Am Coll Surg 215(6):787–793
Acknowledgments
The authors thank our hospitals’ stoma care nurses Martina Signer (Steinbeiß), Adelheid Anzinger, and Christa Sorg for their highly valuated support and long-standing empathic care in the management of all our patients with ostomies and stoma-related problems.
Conflict of interest
G. K. declares no conflict of interest. A. H. declares no conflict of interest. M. L. declares no conflict of interest. F. M. declares no conflict of interest. H. W. declares no conflict of interest. K. E. declares no conflict of interest. R. H. F. declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Köhler, G., Hofmann, A., Lechner, M. et al. Prevention of parastomal hernias with 3D funnel meshes in intraperitoneal onlay position by placement during initial stoma formation. Hernia 20, 151–159 (2016). https://doi.org/10.1007/s10029-015-1380-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-015-1380-7