Abstract
Revealing the interactive effects of multiple environmental change drivers (water deficits, nitrogen (N) deposition, land-use change) is crucial for evaluating actual and possible future changes in forest ecosystem functioning. Here, we analyse whether and to what extent combined effects of spring and summer water deficits and variable amounts of N deposition affect radial growth of beech trees growing on forest sites with a different forest history. Dendrochronological data showed that trees growing on ancient forest sites (forest continuity > 200 years) exhibit a higher negative growth response under high N deposition and simultaneous spring water deficits than trees growing on recent (post-agricultural) forest sites. Based on additional analyses of the fine root system and masting behaviour, we propose two different mechanisms to explain differing influences of N deposition and water deficits on negative radial growth responses in recent and ancient forests: (1) for both forest history types, growth reductions during summer water deficits result from the antagonistic effects of elevated N deposition according to the ‘resource optimization hypothesis’. The tendency towards higher negative growth responses in recent forests seems to be caused by a higher fine root mortality and lower standing fine root biomass compared to ancient forests; (2) higher growth reductions in ancient forests during spring water deficits are likely the result of mass fructification, which is enhanced by N deposition. We conclude that nutrient cycling may differ between forests with contrasting forest history, which can modulate the growth trajectories of forests in response to multiple, co-occurring environmental changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
Forest history modulates growth responses to co-occurring environmental changes.
-
N deposition affects resource allocation towards mass fructification of beech.
-
Legacy-mediated nutrient cycles affect growth response to water deficits and N deposition.
Introduction
Both climate change and increasing levels of atmospheric nitrogen (N) deposition are considered important drivers of environmental change that alter key processes in forest ecosystems (Lindner and others 2010; Greaver and others 2016) and impose threats to forest biodiversity (Sala and others 2000). Although there is increasing evidence of the (single) effects of climate shifts and N deposition on tree growth, our understanding of conceivable interaction effects of these drivers of environmental change is still limited. Furthermore, many forest ecosystems of old cultural landscapes, for example in Central Europe, are characterized by a long history of land-use changes, which in turn might affect their response to present changes in environmental conditions (Perring and others 2016). It is therefore key to understand the interactive effects of land-use legacies and multiple drivers of global change to predict future forest responses in terms of important functions such as biomass production and carbon (C) sequestration. In particular, little is known about the interactive effects of forest history and drivers of environmental change on tree growth, such as simultaneous water deficits in the growing season and atmospheric N deposition.
In large parts of Central Europe, beech forest ecosystems represent the potential natural vegetation, and European beech (Fagus sylvatica L.) is considered to be one of the most economically and ecologically important tree species (Leuschner and Ellenberg 2017). Beech is competitively superior to other tree species in many areas of Central Europe, even though it is acknowledged that beech is highly sensitive to drought (Leuschner and Meier 2018), meaning that beech shows higher climate warming-related growth declines compared to other European tree species (Zimmermann and others 2015). The climate-growth response of beech was observed to vary with factors such as precipitation (Müller-Haubold and others 2013), elevation (Di Filippo and others 2007; Dulamsuren and others 2017), forest management history (Mausolf and others 2018a), and tree species composition of the stand (Metz and others 2016).
There is evidence that N deposition (as a single driver of environmental change) has several effects on forest ecosystem functioning. According to Michel and others (2018), atmospheric deposition of reactive N compounds in forest ecosystems enhances the risk of soil acidification, or has profound consequences for forest productivity and plant species composition. N deposition has been found to reduce the diversity and alter the species composition of the forest ground vegetation and of epiphytic lichens in temperate forests (Bobbink and others 1998). On the tree level, N deposition can increase both foliar N content and stand leaf area, thereby promoting C gain and C sequestration (De Vries and others 2014; Schulte-Uebbing and De Vries 2018). Correspondingly, a stimulating effect of moderate N deposition on stem growth increment was found for temperate beech forests (Gentilesca and others 2018).
Fore beech, experimental (Dziedek and others 2016, 2017) and observational (Hess and others 2018) studies demonstrated that the combined effects of multiple environmental change drivers are non-additive, where N deposition enhances a trees’ climate sensitivity. This response was mainly related to an increase in the shoot-to-root ratio. According to the ‘resource optimization hypothesis’, which predicts plants to allocate less C to roots and to increase shoot-to-root ratio with increasing nutrient availability (Ågren and Franklin 2003), an increase in drought sensitivity of fertilized plants can be attributed to both: changes in the fine root system (Dziedek and others 2017; Hess and others 2018) and a higher evaporative demands aboveground (Meyer-Grünefeldt and others 2013). Thus, we can assume a direct non-additive effect on radial growth, when two environmental change drivers (water deficits and N deposition) act together.
Beside this direct effect of environmental change drivers on the radial stem growth of beech, the increased frequency of mast years (for example, years with a high fruit production) in European beech stands has been identified as a cause of periodic growth declines (Hacket-Pain and others 2015). Instead of investing resources such as C and N into radial growth, they are consumed to produce large seed crops, which reduces radial growth in mast years (‘reproduction-growth trade-off’; Hacket-Pain and others 2015). Evidence exists that high temperatures or high solar radiation in the previous summer functions as triggers of high seed production (Müller-Haubold and others 2015), suggesting a second pathway, through which future climate extremes could influence the radial growth of beech (Hacket-Pain and others 2018). However, the impact of N deposition on seed production is still debated (Müller-Haubold and others 2015; Braun and others 2017). It is conceivable that N deposition exerts an indirect, mast-mediated effect on the radial growth of beech as well and thus (non-additively) interacts with climate extremes.
In regions with a long forest use history, the currently acting drivers of environmental change and their effect on forest productivity and stress response may further depend on possible legacies of former land use, which likely act through altered soil nutrient and/or water availability (Bürgi and others 2017; Maes and others 2018). For example, former land use such as past agricultural activity has been found to cause long-lasting shifts in soil chemical properties (Fraterrigo and others 2006; von Oheimb and others 2008; Kopecký and Vojta 2009; Blondeel and others 2018) and soil microbiomes (Fichtner and others 2014; De la Peña and others 2016) in recent forest ecosystems. Altered edaphic conditions due to land-use legacies, in turn, were shown to indirectly affect the susceptibility of tree growth to adverse climatic conditions (von Oheimb and others 2014), mediated by changes in fine root biomass and morphology (Mausolf and others 2018b).
Based on this knowledge, we used dendroecological data of beech trees growing in stands differing in forest history. Additionally we used climate variables, N deposition data, and records of mast intensity to disentangle possible (non-additive) effects on the growth of adult beech trees in a fully factorial approach. We hypothesized that (1) forest history and therefore legacies of former land use alter the response of adult beech trees to the simultaneous acting of water deficits and high N deposition, and (2) mast intensity plays a crucial role in mediating the growth response of beech to water deficits and N deposition.
Materials and Methods
Study Sites and Study Design
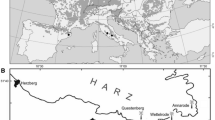
The study was conducted in beech forests (Galio-Fagetum community) near the city of Kiel in northern Germany (Schleswig-Holstein, 54°19′N, 10°7′E). The area is characterized by a sub-oceanic climate with a mean annual precipitation of 777 mm and a mean annual temperature of 8.5°C (DWD Climate Data Center 2017). Elevation ranges from 32 to 81 m a.s.l. Soils originated from deposits of the last (Weichselian) glaciation and consist of till (clay/sandy loam) with varying carbonate content in deeper soil layers. The predominant soil types are (pseudogleyic) Luvisols.
To assess the effect of former land use on the growth response of beech to varying environmental conditions, we identified eight forests dominated by beech (canopy cover of beech > 90%) that differed in former land-use history: ancient forests (n = 4), characterized by a continuity in forest cover of at least 230 years (indicated in historical maps); and recent forests (n = 4), established between 1870 and 1930 on former agricultural land (grassland: n = 3, arable land: n = 1). To avoid confounding effects between land-use history and stand or site characteristics (Fraterrigo 2013), we restricted the analyses to stands that were similar in stand structure (that is, mature, even-aged stands) on sites with similar topography (level terrain) and edaphic conditions (that is (very) good nutrient and water supply). All investigated beech stands have been managed for at least 100 years (see Table 1 for further stand and target tree attributes). Within each stand, we randomly established 2–5 study plots (40 × 40 m), resulting in a total of 28 plots. All trees within a plot with diameter at breast height (DBH; at 1.30 m) above 7 cm were measured in 2014. For each measured tree, DBH and species identity were recorded.
Tree-Ring Analyses
In each plot, we randomly selected ten dominant beech trees of the upper canopy, resulting in a total of 280 target trees. To determine radial growth rates, we cored target trees at 1.30 m height above ground and extracted two bark-to-pith increment cores perpendicular to each other from the southern and eastern side of the trees using a borer of 0.5 cm diameter and 40 cm length (Suunto 400, Vantaa, Finland) in 2014. The preparation and measurement of the wood cores followed the protocol of Mausolf and others (2018b) using a core-microtome of WSL (Birmensdorf, Switzerland) for surface preparation and measuring annual tree-ring width (TRW) from bark to pith with a measuring table (resolution of 0.01 mm; IML GmbH, Wiesloch, Germany) and the IML software T-Tools Pro (version 1.4, IML GmbH, Wiesloch, Germany). Subsequently, single TRW series per tree were cross-dated. We used the cross-dating index provided by TSAP-Win (Version 4.69 k, Rinntech, Heidelberg, Germany) to evaluate matches between the two cores of a tree. A CDI greater than 20 was used as a threshold. Accordingly, the cores of 37 of the 280 trees (13%) were omitted due to inconsistent matching between the two cores of a tree. Afterwards, the averaged TRW series per tree were standardized for size- and age-related differences between trees. We used the moving average standardization procedure provided by the software TSAP-Win to retain as much as possible of the interannual climate signal within the chronologies. First, we calculated the 5-year moving average trend of each chronology. In a second step, tree-ring series were divided by the 5-year moving average trends, resulting in a dimensionless index of tree-ring width (TRI) (Dulamsuren and others 2017). Descriptive dendrochronological statistics were based on individual tree chronologies and calculated using TSAP-Win (Table 1).
Climate, Nitrogen Deposition, and Mast Intensity Data
We used the standardized precipitation-evapotranspiration index (SPEI) to quantify temporal changes in climatic conditions. The SPEI represents a climatic water balance index that comprises both precipitation and potential evapotranspiration (Vincente-Serrano and others 2010) and allows best to analyse the effects of climate change in beech tree-ring chronologies for variable time scales (Bhuyan and others 2017). SPEI data were extracted from the Global SPEI database (http://spei.csic.es/database.html, accessed 14.09.2017) for the nearest 0.5 grid cell (54°45′N, 10°25′E). We selected climate indices for spring and summer conditions, as beech has been shown to be most sensitive to climatic variations during these periods (Lebourgeois and others 2014; Hacket-Pain and others 2015; Bosela and others 2016). For each season, we used aggregated SPEI values based on a 3-month period (that is, SPEIspring for March, April, May; SPEIsummer for June, July, August; Figure S1).
Nitrogen deposition data (Ndep) for the years 2000–2013 were provided by the German Environment Agency (UBA, Dessau, Germany) and based on monthly deposition measurements within a grid of gauging stations across Germany (UBA 2014). Measurements were taken with wet-only samplers (type ARS 721, according to the VDI standard 3870) (LLUR 2010; UBA 2014). Ndep sampling was conducted near the city of Bornhöved in the framework of the Level II permanent monitoring plot network which is part of the International Co-operative Program on the Assessment of Air Pollution Effects on Forests, established to perform ecosystem-related studies on cause–effect relationships (Michel and others 2018). Distance between the Ndep sampling site and the investigated forest sites is 35 km at maximum; we therefore assume that the Bornhöved data describe the deposition climate at our sites well. Ndep-values were calculated as the sum of the amount of N deposited in the form of ammonium (NH4+–N in kg ha−1 a−1) and nitrate (NO3−–N in kg ha−1 a−1). To reduce the number of explanatory variables in our models, we used Pearson correlations to evaluate the linkage between different Ndep-values and annual TRI values of single trees. We tested for correlation between the seasonal (spring and summer) totals of deposited N in the year of ring formation and in the year previous to tree-ring formation, as well as for the totals of deposited N during the entire growing season (April to October) and annual deposition data. The tightest correlation between TRI and Ndep-values was found for values of the current growing season and Ndep for the current summer (r = − 0.37; p < 0.001; r = − 0.38; p < 0.001; Pearson correlation between TRI and deposited N during growing season and summer, respectively). As Ndep in the growing season (NdepGS) and Ndep in summer show a high collinearity; we only used NdepGS as explanatory variable (Figure S1).
Information about the frequency of beech masting was derived from Dammann and others (2016), who give masting intensity as the percentage of beech trees showing high seed production in a given year in the federal state Schleswig-Holstein. Since masting events in beech generally occur synchronously over larger spatial scales (Packham and others 2012), data from Dammann and others (2016) were considered applicable for our study sites (see Hacket-Pain and others 2018 for a similar approach).
Fine Root Data and Soil Chemical Properties
To characterize beech fine root mass at each of the 28 plots, we randomly selected six sampling locations per plot for the fine root inventory in October 2015. Sampling was conducted by using a soil borer (3.5 cm diameter) to a depth of 30 cm of the mineral horizon. The soil cores were divided in two fractions, 0–10 cm depth and 10–30 cm depth. To determine the fine root, biomass and necromass root samples were cleaned from soil residuals above a sieve (mesh size 0.5 mm). Afterwards fine root fractions (rootlets > 10 mm in length, < 2 mm in diameter) were divided by species identity (beech vs. other species) and living and dead rootlets under a stereo-microscope. Selection criteria (that is, colour, root elasticity and cohesion of the cortex, periderm and stele) following Hertel and others (2013). Sorted fine roots were dried at 70°C for 24 h; afterwards dry matter of living and dead beech fine roots was determined for each soil depth separately. As the highest proportion of the fine root system is located in the uppermost soil layers, here we only use the values for 0–10 cm depth.
In addition, soil chemical properties of the 28 plots were analysed in 2015 and published by Mausolf and others 2018b (for a description of the methods see Mausolf and others 2018b). The chemical characterization of the soils showed differences between the stands which are likely caused by former land use. Soils of recent forests were associated with significantly lower carbon to phosphorus (C/P) ratios and a tendency towards a higher base saturation (BS). Soil chemical properties of the uppermost 10 cm of the mineral soil are shown in Table S1.
Data Analysis
The time series of available Ndep data restricted our analyses to the period 2000–2013. In this interval, we found 6 years with positive and 8 years with negative SPEIspring values, whereas 8 years were characterized by positive and six with negative SPEIsummer values. Nitrogen deposition during the growing season (NdepGS) ranged between 5.3 and 10.2 kg N ha-1 a-1 (Figure S1). We applied linear mixed-effects models to test whether NdepGS, shifts in the climatic water balance during spring (SPEIspring) and summer (SPEIsummer), and former land use (forest history) exerts interacting effects on TRI. To account for spatial dependency, ‘study plot’ was used as a random effect. We used a compound symmetry correlation structure to account for temporal autocorrelation among years (‘tree’ nested in ‘plot’; Zuur and others 2009). Competing models were evaluated by sequential comparison (backward selection) based on the Akaike information criterion (AIC) and maximum likelihood. Furthermore, we simplified the model with the lowest AIC value by removing non-significant terms. Parameter estimates of the final model were fitted using the restricted maximum likelihood (REML) method (Zuur and others 2009). We fitted a global model containing climatic conditions during spring and summer to account for both effects simultaneously. All continuous predictors were standardized (mean = 0, SD = 1) before analysis.
To evaluate the linkage of N deposition during the growing season and radial tree growth, we performed confirmatory path analysis by using structural equation model (SEM) techniques (Grace and others 2012; Lefcheck 2016). As the radial growth of beech is greatly influenced by masting which is triggered by high temperatures during the previous summer, we included information on masting intensity and the mean maximum temperature during previous June and July (MaxJJ-1) derived from the CRU TS gridded dataset (v 4.01, Harris and others 2014) to our models (see Hacket-Pain and others 2018 for a related approach). We hypothesized that the effect of N deposition on radial growth consists not only of a fertilizer effect, that is, a direct positive influence on tree growth, but there is also an indirect pathway of N deposition on tree growth mediated through masting intensity. Confirmatory path analysis was performed for each forest history type (ancient forests vs. recent forests) separately, using pooled values of TRI per year and forest history type to reduce all variables to single annual values. To account for temporal correlation among subsequent years, we used generalized least square models with a first-order autoregressive correlation structure. Model fits were evaluated by using the model fit statistics Fisher’s C and p-values. Models were checked for missing paths by using the dSep-function of piecewiseSEM.
Prior to analyses, data exploration was performed following Zuur and others (2010) and model assumptions were visually checked and confirmed according to Zuur and others (2009). All analyses were conducted in R (version 3.5.1) using the packages MASS (Venables and Ripley 2002), nmle (Pinheiro and others 2016), piecewiseSEM (Lefcheck 2018) and vegan (Oksanen and others 2016).
Results
On average, TRW tended to be higher in recent than in ancient forests (2.51 mm vs. 2.17 mm, respectively), but this difference was not significant (Table 1). However, ancient forests showed a significantly lower mean minimum TRW than recent forests (0.88 mm vs. 1.25 mm, p < 0.05, Table 1). The best-fitting growth model revealed positive effects of SPEI and negative effects of NdepGS, with the effect of NdepGS on TRI being stronger than that of SPEI (Table 2).
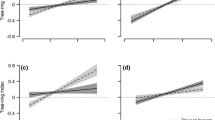
The best-fitting growth model showed a three-way interaction between NdepGS, SPEIspring and forest history type (Figure 1; Table 2; p = 0.006). Under low NdepGS, radial growth in both forest history types was only little influenced by negative climatic water balances during spring; a negative response in radial growth, that is, negative TRI values, due to water deficits was predicted to occur only in the trees of the ancient forests (Figure 1a). In contrast, radial growth of trees growing in recent forests did not show a strong response to water deficits in spring and responded only slightly with increased radial growth rates to a more positive climatic water balance. In the ancient forests, radial growth was significantly promoted by a more positive climatic water balance (Figure 1a). Moreover, the increase in radial growth rates was steeper in ancient forests than in recent forests under slightly negative SPEIspring values. Although deterioration of the climatic water balance did not have a marked negative effect on TRI under low NdepGS rates, high NdepGS caused a strong negative response of radial growth rates in both forest history types even under ample water supply (Figure 1b). The negative response in radial growth rates under negative SPEIspring values was stronger for the trees of ancient than recent forests.
Effect of forest history type (ancient vs. recent forests) on the growth (tree-ring width index, TRI) response of European beech to interannual fluctuations in the climatic conditions during spring (2000–2013) considering (A) years with low nitrogen (N) deposition (30% quantile) and (B) years with high N deposition (70% quantile). The climatic gradient is characterized by the standardized precipitation-evapotranspiration index (SPEI) aggregated for the months March to May. Negative SPEI values display conditions with a tendency of water deficits (negative climatic water balance), positive values display conditions with ample water supply (positive climatic water balance). Lines correspond to the predicted response based on mixed-effects models and shaded areas indicate the 95% confidence interval.
The best-fitting growth model indicated that high NdepGS and negative SPEIsummer values have a negative interactive effect on TRI (Figure S2; p < 0.001), and this effect was consistent across forest history types. Furthermore, radial tree growth responses tended to be more sensitive to water deficits during summer in recent forests, as indicated by the marginal significant interaction between SPEIsummer and forest history type (p < 0.0572). Due to the marginal significance, this interaction term was removed from the best-fitting growth model. In contrast to the effects of SPEIspring, we found no significant three-way interaction between NdepGS, SPEIsummer and forest history type.
For each forest history type, the confirmatory path analyses provided a good fit to the data (Fisher’s C = 1.787, p = 0.409, df = 2 for ancient forests; Fisher’s C = 1.219, p = 0.544, df = 2 for recent forests). Directed separation analysis confirmed no missing paths within the models. The path analyses confirmed a significant indirect effect of NdepGS on radial tree growth through masting intensity. NdepGS was positively related to masting intensity (with 31% of the variation of masting intensity explained), which in turn negatively affected TRI. This effect was only significant for the trees of the ancient forests (Figure 2). A direct effect of NdepGS on TRI was not significant for both forest history types, and it tended to be negative. The explained variation in TRI was slightly higher for ancient forests (R2 = 0.36 and R2 = 0.45 for recent and ancient forests, respectively).
Confirmatory path analyses linking nitrogen (N) deposition and climate conditions, mast intensity and tree growth in (A) ancient forests and (B) recent forests across the years 2000–2013. Black solid, grey solid, and dashed lines indicate significant (p < 0.05), non-significant (p > 0.1) and marginal significant (p < 0.1) relationships, respectively. Positive and negative numbers at arrows are standardized regression coefficients; thus, the magnitude of the coefficients is proportional to their effect size. R2-values for each endogenous variable are given below the boxes. Abbreviations: MI Mast intensity (% of trees showing a high seed production), TRI Tree-ring width index, NdepGS Cumulative amount of N deposited during the growing season (April–October, kg N ha−1 a−1), MaxJJ-1 Mean maximum temperature during June and July of the previous year.
Discussion
Our findings confirm our first hypothesis that forest history, and therefore legacies of former land use, alters the response of adult beech trees to the simultaneous effects of water deficits and high N deposition. The separate analysis of data from spring (March to May) and summer (June to August) produced different results with respect to the role of forest history in modulating the radial growth response to multiple environmental change drivers. On the one hand, high N deposition combined with summer water deficits led to a negative trend in radial increment in both forest history types. On the other hand, sensitivity to high N deposition and water deficits in spring was higher in trees from the ancient forests, as indicated by the three-way interaction between spring climate conditions, N deposition during the growing season, and forest history type.
Direct Effects of N Deposition and Water Deficits on Radial Growth
In general, our results are in line with other studies on the effects of high N deposition, which found antagonistic effects of high N loads and high growing season temperatures on the radial growth of adult beech (that is, Braun and others 2017; Hess and others 2018). Hess and others (2018) suggested that N fertilization triggers an aboveground shift in plant internal resource allocation which is in line with the predictions of the resource optimization hypothesis (Ågren and Franklin 2003) and assumed a possible decline in root productivity. A reduced fine root biomass in N-rich soils as the consequence of high N deposition could explain a lower radial growth rate in the face of water deficits, as the trees might be more susceptible to summer water deficits in both forest history types.
Radial growth of trees in recent forests tended to be more sensitive to water deficits during summer than radial growth of trees in ancient forest stands (interaction SPEIsummer × forest history type; p = 0.0572). This might be the result of differences in the fine root biomass of the investigated stands, which in turn are related to changes in soil chemical properties through former land-use activities (Mausolf and others 2018b, Table S1). Physiologically even more relevant could be the observation that the fine root necromass/biomass ratio was about two times higher in the recent than the ancient forests, pointing at a higher root mortality in the former (Figure S3). Although it is unclear, whether the lower fine root biomass and higher root necromass/biomass ratio in the recent forests is a consequence of the higher P and N availability or is caused by other edaphic factors, it is likely that a reduced fine root biomass/aboveground biomass ratio increases the trees’ susceptibility to water deficits.
N Deposition Effects on Growth Mediated Through Mast Fruiting and Possible Interaction with Water Deficits
Interactive effects of water deficits in spring and elevated N deposition increased the sensitivity of radial growth of trees growing in ancient forests. Confirmatory path analyses clearly suggest that mast intensity plays a crucial role in mediating growth responses of beech trees to water deficits and N deposition, thus confirming our second hypothesis. Beech as a masting tree species produces a large number of nuts every 3–6 years, which alternate with non-seed years (Packham and others 2012). As high seed production comes at a high cost in terms of resource consumption, vegetative growth (that is, radial stem growth) in mast years, and sometimes in subsequent years as well, is lower than in non-mast years (Mund and others 2010; Hacket-Pain and others 2015; Müller-Haubold and others 2015). During recent decades, the frequency of mast events as well as the seed crop itself has increased in many beech stands across Central Europe (Övergaard and others 2007; Paar and others 2011; Müller-Haubold and others 2015), suggesting that climatic or edaphic drivers of fruit production have changed. The mechanisms triggering the synchronous investment of a large amount of resources into reproduction in beech are still a matter of debate. High temperatures and also high radiation intensities during the period of bud formation in previous-year summer were found to be a key driver for the switch from vegetative growth to the investment of resources into reproduction (Övergaard and others 2007; Müller-Haubold and others 2015; Hacket-Pain and others 2018; Lebourgeois and others 2018). Additionally, pollination success during spring is a strong driver for the production of large amounts of seed crop (Pearse and others 2016; Lebourgeois and others 2018; Nussbaumer and others 2018), as beech is a self-incompatible, wind-pollinated species (Packham and others 2012). Because beech nuts are relatively rich in N, nitrogen availability in particular is discussed as a key driver of masting (Smaill and others 2011; Bogdziewicz and others 2017). In a study about the resource consumption with seed crop production in Fagus crenata, Abe and others (2016) found that inner seed maturation highly depends on N availability. Furthermore, Miyazaki and others (2014) showed that N is a key regulator for the expression of various genes responsible for flowering in Fagus crenata, indicating that high N availability promotes flowering and fruit ripening. Hence, the physiological basis for an N deposition effect on the reproduction dynamics of Fagus is quite well understood. The path analyses confirmed a positive effect of N deposition on masting intensity in the Fagus sylvatica trees of our study, which is in agreement with these findings. We are aware of the limitation to generalize results from short-term N deposition time series (that is, N deposition data were only available from 2000 to 2013 in this study). However, our results suggest that simultaneously occurring environmental change drivers may not only affect radial growth responses of beech trees, but may also change their reproductive behaviour.
An interesting finding is that a significant negative effect of masting intensity on TRI was only found for trees growing in ancient forests, but not for those of the recent forests. The shift in resource investment (C and N) from vegetative growth (that is, radial stem growth) to reproductive growth (that is, seed production) thus seems to be stronger in trees growing in ancient forests. We hypothesize that the apparently more pronounced reproduction-growth trade-off in ancient forests is caused by a higher sensitivity of these less disturbed systems to the mast-triggering effect of increased availability of reactive N compounds, which would be in line with the resource matching hypothesis according to which a plant’s resource investment varies with resource availability (Abe and others 2016; Kelly 1994). Given that more research is needed to evaluate the mechanisms underlying the observed differences in radial growth response between forest history types, our findings suggest that recent and ancient forests may be associated with different modes of nutrient acquisition and recycling, which in turn can influence many other ecosystem properties (Lang and others 2016). Consistently lower C/P- and C/N-ratios in the soils of the recent forests might therefore indicate that these forest history types are characterized by more open (acquiring) nutrient cycles. In contrast, ancient forests (associated with lower P availability in the uppermost mineral soil layer and lower N availability in deeper mineral soil layers) likely are characterized by tighter (recycling) nutrient cycles (Lang and others 2016), which should be more responsive in growth to reproduction-mediated effects of additional N input.
Conclusions
Overall, we assume that the different growth responsiveness of beech in ancient and recent forests to N deposition and water deficits is likely a consequence of differences in nutrient cycling and availability, caused by partial interruption of biogeochemical cycles and land-use influences in the past. Water deficits in spring in combination with elevated N deposition have therefore the potential to promote a reproduction-growth trade-off of beech trees primarily growing in ancient forests. Our results indicate that the ‘ecological memory’ of a forest is a crucial component for assessing ecosystem reactions to simultaneously acting environmental change drivers. It should be noted that our data do not allow for exploring forest history-mediated effects of simultaneous long-term N deposition and water deficits on radial tree growth and reproduction behaviour. Thus, it would be valuable in future research to assess the role of forest history in modulating complex relationships between co-occurring shifts in environmental conditions based on long-term observations and larger spatial scales.
References
Abe T, Tachiki Y, Kon H, Nagasaka A, Onodera K, Minamino K, Han Q, Satake A. 2016. Parameterisation and validation of a resource budget model for masting using spatiotemporal flowering data of individual trees. Ecol Lett 19:1129–39.
Ågren GI, Franklin O. 2003. Root: shoot ratios, optimization and nitrogen productivity. Ann Bot 92:795–800.
Bhuyan U, Zang C, Menzel A. 2017. Different responses of multispecies tree ring growth to various drought indices across Europe. Dendrochronologia 44:1–8.
Blondeel H, Perring MP, Bergès L, Brunet J, Decocq G, Depauw L, Verheyen K. 2018. Context-dependency of agricultural legacies in temperate forest soils. Ecosystems. https://doi.org/10.1007/s10021-018-0302-9.
Bobbink R, Hornung M, Roelofs JGM. 1998. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J Ecol 86:717–38.
Bogdziewicz M, Crone EE, Steele MA, Zwolak R. 2017. Effects of nitrogen deposition on reproduction in a masting tree: benefits of higher seed production are trumped by negative biotic interactions. J Ecol 105:310–20.
Bosela M, Štefančík I, Petrás R, Vacek S. 2016. The effects of climate warming on the growth of European beech forests depend critically on thinning strategy and site productivity. Agric For Meteorol 222:21–31.
Braun S, Schindler C, Rihm B. 2017. Growth trends of beech and Norway spruce in Switzerland: the role of nitrogen deposition, ozone, mineral nutrition and climate. Sci Total Environ 599–600:637–46.
Bürgi M, Östlund L, Mladenoff DL. 2017. Legacy effects of human land use: ecosystems as time-lagged systems. Ecosystems 20:94–103.
Dammann I, Paar U, Weymar J, Spielmann M, Eichhorn J. 2016. Waldzustandsbericht 2016 für Schleswig-Holstein. Germany: Printec Offset Kassel.
De la Peña E, Baeten L, Steel H, Viaene N, De Sutter N, De Schrijver A, Verheyen K. 2016. Beyond plant-soil feedbacks: mechanisms driving plant community shifts due to land-use legacies in post-agricultural forests. Funct Ecol 30:1073–85.
De Vries W, Du E, Butterbach-Bahl K. 2014. Short and long-term impacts of nitrogen deposition on carbon sequestration by forest ecosystems. Curr Opin Environ Sustain 9–10:90–104.
Di Filippo A, Biondi F, Cufar K, De Luis M, Grabner M, Maugeri M, Piovesan G. 2007. Bioclimatology of beech (Fagus sylvatica L.) in the Eastern Alps: spatial and altitudinal climatic signals identifies through a tree-ring network. J Biogeogr 34:1873–92.
Dulamsuren C, Hauck M, Kopp G, Ruff M, Leuschner C. 2017. European beech responds to climate change with growth decline at lower, and increase at higher elevations in the center of its distribution range (SW Germany). Trees 31:673–86.
Dziedek C, Härdtle W, von Oheimb G, Fichtner A. 2016. Nitrogen addition enhances drought sensitivity of young deciduous tree species. Front Plant Sci 7:1100.
Dziedek C, Fichtner A, Calvo L, Marcos E, Jansen K, Kunz M, Härdtle W. 2017. Phenotypic plasticity explains response patterns of European beech (Fagus sylvatica L.) saplings to nitrogen fertilization and drought events. Forests 8:91. https://doi.org/10.3390/f8030091.
DWD Climate Data Center (CDC). 2017. Historical monthly station observations (temperature, pressure, precipitation, sunshine duration, etc.) for Germany, version v005.
Fichtner A, von Oheimb G, Härdtle W, Wilken C, Gutknecht JLM. 2014. Effects of anthropogenic disturbances on soil microbial communities in oak forests persist for more than 100 years. Soil Biol Biochem 70:79–87.
Fraterrigo JM. 2013. Landscape legacies. In: Levin SA, Ed. Encyclopedia of biodiversity. Waltham: Academic Press. p 524–30.
Fraterrigo JM, Balser TC, Turner MG. 2006. Microbial community variation and its relationship with nitrogen mineralization in historically altered forests. Ecology 87:570–9.
Gentilesca T, Rita A, Brunetti M, Giammarchi F, Leonardi S, Magnani F, Borghetti M. 2018. Nitrogen deposition outweighs climatic variability in driving annual growth rate of canopy beech trees: Evidence from long-term growth reconstruction across a geographic gradient. Glob Change Biol 24:2898–912.
Grace JB, Schoolmaster Jr. DR, Guntesperger GR, Little AM, Mitchell BR, Miller KM, Schweiger EW. 2012. Guidelines for a graph-theoretic implentation of structural equation modeling. Ecosphere, 3, Article 73
Greaver TL, Clark CM, Compton JE, Vallano D, Talhelm AF, Weaver CP, Haeuber RA. 2016. Key ecological responses to nitrogen are altered by climate change. Nat Clim Change 6:836–43.
Hacket-Pain AJ, Friend AD, Lageard JA, Thomas PA. 2015. The influence of masting phenomenon on growth–climate relationships in trees: explaining the influence of previous summers’ climate on ring width. Tree Physiol 35:319–30.
Hacket-Pain AJ, Ascoli D, Vacchiano G, Biondi F, Cavin L, Conedera M, Hartl C. 2018. Climatically controlled reproduction drives interannual growth variability in a temperate tree species. Ecol Lett 21:1833–44.
Harris I, Jones PD, Osborn TJ, Lister DH. 2014. Updated high-resolution grids of monthly climatic observations—the CRU TS3.10 Dataset. Int J Climatol 34:623–42.
Hertel D, Strecker T, Müller-Haubold H, Leuschner C. 2013. Fine root biomass and dynamics in beech forests across a precipitation gradient—is optimal resource partitioning theory applicable to water-limited mature trees? J Ecol 101:1183–200.
Hess C, Niemeyer T, Fichtner A, Jansen K, Kunz M, Maneke M, Härdtle W. 2018. Anthropogenic nitrogen deposition alters growth responses of European beech (Fagus sylvatica L.) to climate change. Environ Pollut 223:92–8.
Kelly D. 1994. The evolutionary ecology of mast seeding. TREE 9(12):465–70.
Kopecký M, Vojta J. 2009. Land use legacies in post-agricultural forests in the Doupovské Mountains, Czech Republic. Appl Veg Sci 12:251–60.
Lang F, Bauhus J, Frossard E, George E, Kaiser K, Kaupenjohann M, Wellbrock N. 2016. Phosphorus in forest ecosystems: new insights from an ecosystem nutrition perspective. J Plant Nutr Soil Sci 179:129–35.
Lebourgeois F, Eberlé P, Mérian P, Seynave I. 2014. Social status-mediated tree-ring responses to climate of Abies alba and Fagus sylvatica shift in importance with increasing stand basal area. For Ecol Manag 328:209–18.
Lebourgeois F, Delpierre N, Dufrȇne E, Cecchini S, Macé S, Croisé L, Nicolas M. 2018. Assessing the roles of temperature, carbon inputs and airborne pollen as drivers of fructification in European temperate deciduous forests. Eur J For Res 137:349–65.
Lefcheck JS. 2016. PIECEWISESEM: Piecewise structural equation modelling in R for ecology, evolution and systematics. Methods Ecol Evolut 7:573–9.
Lefcheck JS. 2018. piecewiseSEM: Piecewise Structural Equation Modeling in R, version 2.0. http://CRAN.R-project.org/package=piecewiceSEM.
Leuschner C, Ellenberg H. 2017. Ecology of Central European forests: Vegetation ecology of Central Europe, vol. I. Switzerland: Springer.
Leuschner C, Meier IC. 2018. The ecology of Central European tree species: Trait spectra, functional trade-offs, and ecological classification of adult trees. Perspect Plant Ecol Evolut Syst 33:89–103.
Lindner M, Maroschek M, Netherer S, Kremer A, Barbati A, Garcia-Gonzalo J, Marchetti M. 2010. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For Ecol Manag 259:698–709.
LLUR. 2010. Atmosphärische Stoffeinträge in Schleswig-Holstein 2009 – Lufthygienische Überwachung Schleswig-Holstein. Landesamt für Landwirtschaft, Umwelt und ländliche Räume des Landes Schleswig-Holstein, Lufthygienische Überwachung, Itzehoe, Germany. pp. 31.
Maes SL, Perring MP, Vanhellemont M, Depauw L, Van den Bulke J, Brümelis G, Verheyen K. 2018. Environmental drivers interactively affect individual tree growth across temperate European forests. Global Change Biol. 15:17. https://doi.org/10.1111/gcb.14493.
Mausolf K, Wilm P, Härdtle W, Jansen K, Schuldt B, Sturm K, Fichtner A. 2018a. Higher drought sensitivity of radial growth of European beech in managed than in unmanaged forests. Sci Total Environ 642:1201–8.
Mausolf K, Härdtle W, Jansen K, Delory BM, Hertel D, Leuschner C, Fichtner A. 2018b. Legacy effects of land-use modulate tree growth responses to climate extremes. Oecologia 187:825–37.
Meyer-Grünefeldt M, Friedrich U, Klotz M, von Oheimb G, Härdtle W. 2013. Nitrogen deposition and drought events have non-additive effects on plant growth evidence from greenhouse experiments. Plant Biosyst 149:424–32.
Metz J, Annighöfer P, Schall P, Zimmermann J, Kahl T, Schulze E-D, Ammer C. 2016. Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob Change Biol 22:903–20.
Michel A, Seidling W, Prescher A-K, editors. 2018. Forest Condition in Europe: 2018 Technical Report of ICP Forests. Report under the UNECE Convention on long-range transboundary air pollution (Air Convention). BFW-Dokumentation 25/2018. Vienna: BFW Austrian Research Centre for Forests.
Miyazaka Y, Maruyama Y, Chiba Y, Kobayashi MJ, Joseph B, Shimizu KK, Satake A. 2014. Nitrogen as a key regulator of flowering in Fagus crenata: understanding the physiological mechanism of masting by gene expression analysis. Ecol Lett 17:1299–309.
Müller-Haubold H, Hertel D, Seidel D, Knutzen F, Leuschner C. 2013. Climate responses of aboveground productivity and allocation in Fagus sylvatica: a transect study in mature forests. Ecosystems 16:1498–516.
Müller-Haubold H, Hertel D, Leuschner C. 2015. Climatic drivers of mast fruiting in European beech and resulting C and N allocation shifts. Ecosystems 18:1083–100.
Mund M, Kutsch WL, Wirth C, Kahl T, Knohl A, Skomarkova MV, Schulze E-D. 2010. The influence of climate and fructification on the inter-annual variability of stem growth and net primary productivity in an old-growth, mixed beech forest. Tree Physiol 30:689–704.
Nussbaumer A, Waldner P, Aputhin V, Aytar F, Benham S, Bussotti F, Gessler A. 2018. Impact of weather cues and resource dynamics on mast occurrence in the main forest tree species in Europe. For Ecol Manag 429:336–50.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, & Wagner H. 2016. Vegan: Community Ecology Package. R package, version 2.4-0. https://CRAN.R-project.org/package=vegan.
Övergaard R, Gemmel P, Karlsson M. 2007. Effects of weather conditions on mast year frequency in beech (Fagus sylvatica L.) in Sweden. Forestry 80:555–65.
Paar U, Guckland A, Dammann I, Albrecht M, Eichhorn J. 2011. HäuFigurekeit und Intensität der Fruktifikation der Buche. AFZ-DerWald 6(2011):26–9.
Packham JR, Thomas PA, Atkinson MD, Degen T. 2012. Biological flora of the British Isles: Fagus sylvatica. J Ecol 100:1557–608.
Pearse IS, Koenig WD, Kelly D. 2016. Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytol 212:546–62.
Perring MP, De Frenne P, Baeten L, Maes SL, Depauw L, Blondeel H, Carón MM, Verheyen K. 2016. Global environmental change effects on ecosystems: the importance of land-use legacies. Global Change Biol 22:1361–71.
Pinheiro J, Bates D., DebRoy S, Sarkar D, R Core Team. 2016. NLME: linear and nonlinear mixed effects models. R package version 3.1-128, http://CRAN.R-project.org/package=nlme
Sala OE, Chapin FSIII, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Wall DH. 2000. Global diversity scenarios for the year 2100. Science 287:1170–774.
Schulte-Uebbing L, de Vries W. 2018. Global-scale impacts of nitrogen deposition on tree carbon sequestration in tropical, temperate and boreal forests: a meta-analysis. Global Change Biol 24:e416–31.
Smaill SJ, Clinton PW, Allen RB, Davis MR. 2011. Climate cues and resources interact to determine seed production by a masting species. J Ecol 99:870–7.
UBA Umweltbundesamt. 2014. Modelling and mapping of atmospheric nitrogen and sulphur deposition and critical loads for ecosystem assessment of threats to biodiversity in Germany – PINETI (Pollutant INput and EcosysTem Impact). UBA Texte 60, Dessau-Roßlau, Germany. pp 170.
Venables WN, Ripley BD. 2002. Modern applied statistics with S. 4th edn. New York: Springer.
Vincente-Serrano SM, Beguería S, López-Moreno JI, Angulo M, El Kenawy A. 2010. A new global 0.5 gridded dataset (1901–2006) of a multiscalar drought index: comparison with current drought index datasets based on the Palmer Drought Severity Index. J Hydrometeorol 11:1033–43.
Von Oheimb G, Härdtle W, Naumann PS, Westphal C, Assmann T, Meyer H. 2008. Long-term effects of historical heathland farming on soil properties of forest ecosystems. For Ecol Manag 255:1984–93.
Von Oheimb G, Härdtle W, Eckstein D, Engelke H-H, Hehnke T, Wagner B, Fichtner A. 2014. Does forest continuity enhance the resilience of trees to environmental change? Plos One 9:1–18.
Zimmermann J, Hauck M, Dulamsuren C, Leuschner C. 2015. Climate warming-related growth decline affects Fagus sylvatica, but not other broad-leaved tree species in central European mixed forests. Ecosystems 18:560–72.
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, USA: Springer.
Zuur AF, Ieno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evolut 1:3–14.
Acknowledgements
We thank the local forest owners for allowing us to take increment cores and are grateful to Mechthild Stange for assisting with fine root analyses. KM was funded by a doctoral fellowship from the German Federal Environmental Foundation (DBU; AZ20013/279).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author’s Contribution
AF, WH and CL designed the research; DH designed the methodology of the root sampling and analysis; KM collected and compiled the data; KM and AF analysed the data; KM wrote the first draft of the manuscript. All authors substantially contributed to revisions and gave final approval for publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mausolf, K., Härdtle, W., Hertel, D. et al. Impacts of Multiple Environmental Change Drivers on Growth of European Beech (Fagus sylvatica): Forest History Matters. Ecosystems 23, 529–540 (2020). https://doi.org/10.1007/s10021-019-00419-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-019-00419-0