Abstract
Warming-related growth decrease on southern Fagus sylvatica forests has been observed in different regions; however, whether it is a generalized fact or not remains unclear. Here we investigate the geographical pattern on growth response of the southwestern European beech forests to the warming climate shift which started in the 1980s. We sampled 15 beech forests (215 trees) across four climatically contrasting regions (Mediterranean, Pyrenean, low- and high-elevation Atlantic areas) near the southern distribution limit of the species in the Iberian Peninsula. Dendrochronological analyses were carried out to evaluate the growth of European beech since the 1950s. Growth responses quantified as pointer years, abrupt growth changes and long-term growth trends were compared between periods (before and after the 1980s climate shift), geographical regions and tree sizes. Analyses of the studied variables indicated a growth decrease in basal area increment after the climate shift in three of the four studied regions. Pyrenean stands were not negatively influenced by the climate shift, although an increase in the frequency of negative abrupt growth changes was also found there. Growth after the climate shift presented divergent patterns depending on the geographical region. Although Mediterranean and Atlantic stands presented different indicators of constrained growth, Pyrenean stands showed rising long-term growth trends. Such results suggest that regional characteristics differentially determine the growth response of the southern European beech forests to recent warming periods. Iberian beech forests located at the Pyrenees would benefit from forecasted warming conditions, whereas Atlantic and Mediterranean forests would be more prone to suffer warming-related growth decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Growth decrease in southwestern beech forests after 1980s climate shift.
-
Growth decrease is geographically structured through northeast Iberian Peninsula.
-
Mediterranean and Atlantic stands presented more growth constrains than Pyrenean ones.
Introduction
Tree populations growing in the southernmost distribution limits of the species range (xeric rear edges) face harsh climate conditions, which can compromise their performance and growth (Gazol and others 2015; Sánchez-Salguero and others 2017). Future climate projections forecast warmer climate and an increase of extreme weather events such as droughts and/or heat waves (IPCC 2014), which could trigger local extinctions and/or range shifts of the most vulnerable rear-edge tree populations (Chen and others 2011). Warming-related growth decline and forest dieback triggered by dry spells are a major concern for scientists and managers (Allen and others 2010, 2015), and its influence on forest dynamics and possible retractions of rear-edge tree populations is still under debate (Sánchez-Salguero and others 2017).
European beech (Fagus sylvatica L.) is a drought-sensitive tree species widely distributed across Europe (Köchner and others 2009; Zimmermann and others 2015) which displays productivity and growth reduction (Lendzoin and Leuschner 2008; Thiel and others 2014), and leaf and root trait alteration as a consequence of drought (Knutzen and others 2015). Increase in temperature and water shortage affects beech radial growth across the entire continent, indicating a widespread vulnerability to future warming climate (Hacket-Pain and others 2016). Special attention must be paid at drought-prone rear-edge forests in continental areas such as the Iberian Peninsula, where warm and dry summers constrain beech performance (Gutiérrez 1988; Rozas and others 2015; Dorado-Liñan and others 2017). In the last four decades, a warming trend has been observed in the Iberian Peninsula; particularly, a rapid rise in temperatures has occurred since the 1980s followed by successive severe droughts in the 1990s, 2000s and 2010s (Gonzalez-Hidalgo and others 2015). Such abrupt warming occurred in the transition from the 1970s to the 1980s, and it was partly linked to changes in the winter atmospheric circulation over the northern Atlantic Ocean (Hurrell 1996) and impacted ecosystems worldwide by accelerating climate warming (Reid and others 2016). This climate shift has led to warmer and more arid conditions on several European regions, generating harsher climatic conditions for beech forests. For instance, Jump and others (2006) found a warming-related growth decline in low-elevation beech stands in the Montseny Mountains (NE Spain) due to warming conditions after the 1980s. In a previous study in the same forest, water shortage was suggested as the main constrain of tree growth, indicating that an increase in evapotranspiration could seriously compromise future forest productivity (Gutiérrez 1988). Comparable episodes of warming-induced growth decline have been found at rear-edge stands from Italy (Piovesan and others 2008) and Hungary (Garamszegi and Kern 2014). In the core of its distribution range, beech populations growing at lower altitudes in dry areas have also experienced marked growth declines (Zimmermann and others 2015; Dulamsuren and others 2016; Knutzen and others 2017). Nevertheless, increased climate sensitivity has been also found in mesic stands, while stands from dry sites were not so responsive, suggesting local adaptation to water shortage and extreme conditions in populations from dry sites (Weber and others 2013; Cavin and Jump 2017). Similarly, rising growth trends or absence of a warming-triggered growth decline has been reported for recent decades in other European beech rear-edge forests (Tegel and others 2014; Cavin and Jump 2017; Hacket-Pain and Friend 2017).
The occurrence of warming- and drought-related growth decreases could be modulated by different intrinsic factors (McDowell and others 2008; Galván and others 2012). For instance, tree size has been suggested as a key factor of the resistance to extreme drought events, with larger trees more susceptible to such events than small ones (Bennett and others 2015). In addition, trees of the same species but different size can respond differently to climate (Rozas 2015). Warming- and drought-related growth decline could thus be a consequence of the combined effect of extrinsic (climatic) and intrinsic (size) factors influencing tree growth (Colangelo and others 2017). Therefore, considering individual characteristics as tree size might help to better depict the causes of warming and drought effects on beech radial growth.
Here we study the stem secondary growth of fifteen Iberian beech forests during the period 1950–2008. Our study region covers a large area and includes a wide climatic gradient, from dry Mediterranean to wet Atlantic sites, encompassing the major climatic conditions where beech grows at southwestern Europe. Our aim is to quantify the effect of the 1980s climate shift on radial growth of the studied beech forests. We use a dendrochronological approach, considering tree-ring width as a reliable proxy of tree growth rate and vitality. We evaluate growth variability at different time scales, from short (year to year) to long-term growth changes (30-year periods), to investigate the possible warming- and drought-related effects on tree growth. Furthermore, we also evaluate the effect of tree size (stem diameter at breast height) on the response of trees to a climate shift toward warmer and more arid conditions. Our specific aims are to determine: (1) whether there has been a negative effect on growth of Iberian beech populations caused by the 1980s climate shift; (2) whether there is any biogeographical pattern in the response of these beech populations to climate warming; (3) whether tree size partially modulates the growth response of beech to the 1980s climate shift. We hypothesize that, after the 1980s climate shift, forests from xeric sites will present more growth constraints than those in mesic sites. We expect that Iberian beech forests growth responses to warming climate would vary among regions and would depend on tree size.
Materials and methods
Study Sites and Data Sampling
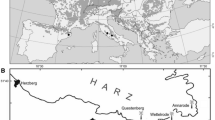
Our study was conducted in the northeast Iberian Peninsula, which represents the southwestern distribution limit of European beech (Figure 1). We sampled 15 forests across a marked climatic gradient, with mean annual temperatures ranging from 6.7 to 15.7 °C and mean annual precipitation ranging from 579 to 1208 mm (CRU TS 4.01 climate data from 1950 to 2008) (Harris and Jones 2017) (Figure 2, Supplementary materials Figure S1). During the growing season (May–September), the mean temperature and precipitation vary considerably depending on the sampled region (Rozas and others 2015). For instance, stands in the Mediterranean and Atlantic areas have higher temperatures (16 °C) than stands found in the Pyrenees (12 °C). In terms of growing season precipitation, Atlantic and Pyrenean stands present higher values than Mediterranean stands, with mean values of 446, 497 and 378 mm, respectively. A different range of altitudes was also present on the sampled stands, going from 300 m to 1530 m a. s. l. More detailed characteristics of each plot are described in Table 1.
(A) Temperature, (B) precipitation and (C) Standardized Precipitation and Evapotranspiration Index (SPEI) on the area during 1950–2008 period. Bold lines represent mean values. Points represent single site values. Vertical dashed lines represent the detected shifts on the mean temperature and SPEI series. Symbols indicate the biogeographical area: Mediterranean (stars), Pyrenean (triangles), low-(circles) and high-elevation Atlantic (rhombus).
Climate Variables and Climatic Areas
Monthly temperature and precipitation series from 1901 to 2010 at 0.5° resolution were downloaded from CRU TS 4.01 dataset (Harris and Jones 2017). Next, mean annual temperature (MAT) and mean annual precipitation (MAP) series for the period 1950–2008 were calculated (Figure 2). To assess the aridity of each site, we calculated De Martonne’s aridity index (hereafter, AI), which is calculated as the ratio of mean annual precipitation and mean annual temperature plus 10 (Tuhkanen 1980). High values of AI indicate wet conditions and low values indicate arid conditions. To estimate drought severity at each site, time series from 1950 to 2008 of the Standardized Precipitation and Evapotranspiration Index (hereafter, SPEI) at 0.5° resolution (Vicente-Serrano and others 2010) were downloaded (Figure 2). We used March to August SPEI to cover the main growth season of beech. We evaluated mean annual precipitation, mean annual temperature and SPEI along the studied period in order to detect possible climate shifts. We looked for shifts in climate variables using the function breakpoints from the strucchange (Zeileis and others 2003) package in R (R core team 2017). This function detects breakpoints that mark separation between segments of time series. Series are divided in segments of constant mean values; thus, years between segments, which we call breakpoint, are years where an increase or decrease of mean values occurs. Furthermore, we calculated trends of spring–summer mean values of temperature and precipitation to evaluate changes on the climate variables during the growing season (Supplementary materials Figure S2).
In order to find groups of stands with similar climatic conditions, we performed a hierarchical cluster analyses based on the Euclidean distance of the climate variables (MAT, MAP and AI for the 1950–2008 period) including the site elevation. These analyses resulted in four different groups that would be used in all the analyses (Supplementary materials Table S1, Figure S3): two stands with low temperatures, high precipitation and situated at high elevation, the Pyrenean stands; six stands situated at high elevations, with warm temperature and low precipitation, the Mediterranean stands; finally, two groups characterized by high precipitation and temperatures, one with two stands located at low elevation and one formed by four stands located at high elevation, the low- and high-Atlantic stands, respectively.
Tree Radial Growth Data
At each stand from 6 to 26 trees (20 in Pyrenean bioregion, 61 in Mediterranean bioregion and 31 and 103 in low- and high-Atlantic bioregions, respectively) were cored at 1.3 m height using Pressler increment borers (Table 2). These cores were air-dried in the laboratory and mounted on wooden supports for further processing. The samples were sanded with progressively finer sandpaper until tree rings were clearly visible. Ring widths were measured at 0.01 mm resolution using measuring device systems (Lintab, F. RinnTech, Germany; Velmex Inc., USA). Visual cross-dating was performed and checked with the program COFECHA (Holmes 1983). Tree-ring widths were transformed to basal area increments (BAIt) (Figure 3), which allows removing the geometrical constraint of adding a volume of wood to a stem of increasing radius (Biondi and Qeadan 2008), using the following formula:
where Rt is the radius of the ring formation year and Rt−1 is the radius of the year preceding the ring formation. BAIt was used to compute different analyses of tree growth variability and determine the 1980s climate shift effects on the studied beech stands. We calculated standardized BAI values by dividing the raw BAI values by the mean BAI value.
Tree-ring width data were detrended to calculate mean series of ring width indices for each site so as to assess the quality of each site chronology. In short, individual horizontal series were fitted using the mean of the series to obtain dimensionless ring width indices. We calculated several dendrochronological statistics based on these chronologies (Table 2). Specifically, we calculated the first-order autocorrelation of ring width data and the mean sensitivity, mean inter-series correlation and expressed population signal (EPS) of ring width indices. Such statistics were calculated on data from 1950 until present. These analyses were carried out using the dplR package (Bunn and others 2018).
To quantify short-term tree growth response to drought, we calculated the number of years with extreme low growth at individual level (hereafter, individual pointer years), from 1950 to 2008. Individual pointer year analyses reflect growth variability at inter-annual scales and may be associated to extreme climate events, such as droughts and frosts (compare Schweingruber and others 1990). To detect individual pointer years, we used the function pointer.norm of the package pointRes (van der Maaten-Theunissen and others 2015). Individual pointer years were calculated using normalized growth deviations in a 5 years length window, with a greater than 0.5 threshold on the so-called Cropper values (Cropper 1979). As we were interested in warming- and drought-related growth decline, we analyzed the individual negative pointer years only. Furthermore, we also defined regional pointer years as those years in which more than 50% of the trees per biogeographical region presented an individual pointer year. With these metrics, we expect to see whether climate shift has changed the pattern of extreme short-term low growth episodes.
To quantify midterm tree growth response to drought, we analyzed abrupt growth changes resulting in longer periods of low growth. This was accomplished using the function breakpoints from the package strucchange (Zeileis and others 2003). We set that minimum distance between breakpoints cannot be shorter than 10% length of the tree-ring series, in order to avoid changes in shorter BAI trends. Therefore, a year with an abrupt growth change indicates a growth deviation that last at least for more than 6, giving an intermediate timescale effect compared with the one offered by pointer years. As in the case of pointer years, we only analyzed negative abrupt growth changes. Finally, we also evaluated long-term growth trends, calculated as the slope of the linear regression between BAI raw data series and calendar years in two different periods (1950–1979 and 1980–2008). These long-term growth trends indicate whether, in overall, growth is increasing or decreasing before and after the 1980s climate shift (Camarero and others 2018).
Statistical Analyses
To evaluate differences between tree growth before and after the climate shift, we used the following linear mixed model:
where Yij represents the growth variable, either negative individual pointer years, negative abrupt growth changes or long-term growth trends. The sub-index i represents the stand level, and the sub-index j represents the tree level; αij represents the random effect for each tree j nested in each stand i; β1 to β6 are the coefficients for every fixed effect and their interactions; εij is the error term for each tree j nested in each stand i. The fixed effects are: period, a factor with two levels, that is, before (1950–1979) or after (1980–2008) the climate shift; biogeography, a factor with four levels determined by the aforementioned cluster analyses (Mediterranean, low- and high-elevation Atlantic and Pyrenean) and diameter at breast height (DBH). Three different models were considered, one per each of the different tree growth variables (negative individual pointer years, negative abrupt growth changes and long-term growth trends). First, we evaluated the individual pointer years, using the total number of individual negative pointer years per tree as a response variable. Second, we analyzed the individual tree growth trends along each period. Finally, to evaluate the abrupt growth changes, we reduced the variability to stand level by calculating the percentage of trees in each stand that suffered an abrupt growth change, thus in this latter model DBH was also calculated at stand level and not at individual level, as well as the random effect that represented only the variability at stand level. The percentage of abrupt growth changes was log-transformed to achieve normality.
The models were fitted using the nlme package (Pinheiro and others 2017). When a significant influence of factors and interactions was found, post hoc comparisons between levels of factors and interactions were done using the package emmeans (Lenth 2018). All statistical analyses were performed in the R environment (R Core Team 2017).
Results
Climate Shift
We detected a significant climate shift on the mean annual temperature series of the studied stands (Figure 2a, Supplementary materials Figure S2). Specifically, two changes were observed on the temperature data, one in 1981 and another one in 1994 (Figure 2a), indicating the 1980s temperature increase and the 1990s warming hiatus. However, no changes were detected in the precipitation series along the studied period (Figure 2b), but a negative shift in August SPEI was detected in 1994 for most of the studied regions (Figure 2c).
Pointer Year Analyses
Fewer individual pointer years were found after the 1980s climate shift than before (Table 3), a pattern independent of the biogeographical region (Table 4). A change in the effect of DBH on the number of pointer years was found after the climate shift (marginally significant interaction period * dbh, Table 4), changing from negative in 1950–1979 (mean ± SE = − 0.03 ± 0.02) to positive in 1980–2008 (0.01 ± 0.02).
Considering regional pointer years (years were > 50% of trees in a region showed an individual pointer year), we found that at Mediterranean and Atlantic forests, the number of regional pointer years increased after the 1980s climate shift (Figure 4). Contrarily, the number of regional pointer years decreased at Pyrenean stands (Figure 4). When analyzing the four regions altogether, no differences were found between regional pointer years before and after the 1980s (p = 0.15).
Percentage of individual pointer years per region (olive-green bars) and percentage of abrupt growth changes (dark-green bars), boxplots of number of individual pointer years detected before and after the 1980s climate shift and percentage of abrupt growth changes before and after the shift for (A) Pyrenean (PYR), (B) low-elevation Atlantic (L-ATL), (C) high-elevation Atlantic (H-ATL), and (D) Mediterranean (MED) stands. Red stars mark the dry years with Standardized Precipitation and Evapotranspiration Index values lower than − 1 (Color figure online).
Abrupt Growth Changes
Abrupt growth changes varied among the two analyzed periods (Table 3), with a higher percentage of trees experiencing abrupt growth changes after the 1980s climate shift (Table 3, Figure 4). Such effect was independent of the biogeographical region or tree size (Table 4, Figure 4).
Long-Term Growth Trends
We found overall positive growth trends before the climate shift that turned negative after the 1980s (Table 3, Figure 5). Such change was related to the biogeographical region (Table 4). Negative post-shift trends were found in forests from Mediterranean and Atlantic areas (Figure 5). Contrarily, positive growth trends were observed in Pyrenean forests (Figure 5). There was no effect of tree size on the long-term growth trends at any of the studied periods (Table 4).
Boxplots of long-term growth trends of basal area increment for the periods before (pre, 1950–1979) and after (post, 1980–2008) the 1980s climate shift. Values represent the coefficients of linear regressions among basal area increment and calendar year calculated for the four biogeographical groups: Pyrenean (yellow), low-elevation Atlantic (L-Atlantic, gray), high-elevation Atlantic (H-Atlantic, purple) and Mediterranean (red) and forests (Color figure online).
Discussion
Understanding the temporal and spatial growth variability among contrasting biogeographical regions in response to recent climate shifts is fundamental to assess long-term changes in forest productivity, growth and adaptability of tree species to forecasted climate warming (Sánchez-Salguero and others 2017). Our results present a marked growth reduction after the 1980s climate shift in most beech stands investigated within southwest Europe, near the species’ rear-edge. We also found a marked increase in the occurrence of negative abrupt growth changes across all biogeographical regions and a generalized decrease in long-term growth trends in many regions. Thus, although the number of individual pointer years per tree did not increase during the second studied period, all the other parameters pointed out a growth decline after the 1980s climate shift. Notably, the Pyrenean stands, which are located at higher elevation and present the low temperature and high precipitation values, were the only forests showing a long-term growth enhancement. These results agree with previous studies observing warming-related growth decreases in southern and xeric edges of beech distribution (Gutiérrez 1988; Jump and others 2006; Piovesan and others 2008; Zimmerman and others 2015; Dulamsuren and others 2016; Knutzen and others 2017; but see Tegel and others 2014; Hacket-Pain and Friend 2016; Cavin and Jump 2017). However, the comparison between regions allowed us to disentangle that regional climate variability modulates warming-induced growth reductions.
Although there was no increase in individual pointer years after the climate shift, a different effect of tree size (DBH) between periods was observed (Table 4). Before the 1980s, larger trees presented a lower number of pointer years, which changed after the climate shift. This change suggests that under warming periods, larger trees experience harsher conditions making them more vulnerable to drought-related growth decline and potential damage (Benett and others 2015). This size-related effect could be explained by physiological constraints related to the higher xylem tension that suffer the hydraulic systems of larger trees (Ryan and others 2006). However, the reverse pattern has also been observed and attributed to a higher hydraulic capacitance due to more sapwood volume in larger trees (Colangelo and others 2017). Moreover, the frequency of individual pointer years differed among periods in some of the biogeographical regions. Regional pointer years (> 50% of trees in a region) increased in three out of the four regions, as they did in temperate beech populations of central Europe (Zimmerman and others 2015; Knutzen and others 2017). Such increase on the number of regional pointer years indicates that, even if the number of individual pointer years is similar between periods, their distribution along the periods is different. Individual pointer years before the 1980s shift were more homogeneously distributed (that is, more years with equal number of trees showing pointer years) and more heterogeneously distributed after the shift (that is, some years with many trees having a pointer year and other years with almost no tree having pointer years, that is, extreme growth responses) (Supplementary materials Figure S4). This could be explained by an increase of growth sensitivity to drought caused by an increase of dry spells as climate warms, thus reducing radial growth rates (van der Werf and others 2007). The fact that the hottest and most extreme droughts of the study period occurred after 1980s supports this hypothesis (Figure 4). Pyrenean forests were the only ones where the number of regional pointer years did not increase. The Pyrenean region is relatively cooler than the other regions; moreover, it also registers high precipitation; thus, droughts there are expected to be less intense, with lower impacts on those forests (Greenwood and others 2017; Gazol and others 2018). Therefore, the climatic conditions of the Pyrenees may benefit beech stands growth and provide them topographic refuges against the climate warming. Other climate extremes (for example, frosts) could explain this biogeographical differentiation. Because Pyrenean forests are located in the coldest conditions, they might be also more prone to suffer early-spring or late-autumn frost damage resulting in narrow rings (Príncipe and others 2017). As Pyrenean stands has equal number of regional pointer years before and after 1980s shift and extreme droughts occurred mostly after 1980s, our results suggest that contrary to the other regions, frost could be limiting growth more than drought on these stands.
The percentage of abrupt growth changes per forest (that is, percentage of trees displaying abrupt growth reductions lasting for more than 6 years) increased after the 1980s, probably as a consequence of the higher number of extreme droughts observed after the 1980s climate shift (Camarero and others 2015). For instance, Vanoni and others (2016) found that higher drought intensity increased the probability to suffer an abrupt growth change. In this case, a similar pattern is shown for the four biogeographical regions, including the Pyrenean stands. Here frost events may not play a relevant role as it was the case in pointer years, as beech can recover quickly from late frosts (Príncipe and others 2017).
The long-term growth trends indicate that tree growth had an increasing tendency before 1980s climate shift, but this changed in the subsequent decades. Consequently, during the 1980–2008 period a decreasing growth trend was observed in all regions with the exception, again, of Pyrenean forests (Figure 4). These results seem to indicate a growth constraint after the climate shift and match with previous studies reporting a growth decline after 1980s for beech in the southern distribution limit of the species (Jump and others 2006; Piovesan and others 2008; but see Tegel and others 2014; Cavin and Jump 2017) and also in central Europe (Zimmerman and others 2015; Dulamsuren and others 2016). However, Pyrenean forests did not show any growth decrease, in terms of long-term growth trends, after the climate shift. These mountain forests present a climate that can buffer the stressing conditions of the warming observed after the climate shift. As summer temperatures strongly limit beech growth at high elevation (Dittmar and others 2003), the warming trend could be positively influencing beech growth in the Pyrenean stands, as observed in high-elevation beech forests in central Europe (Dulamsuren and others 2016). Moreover, the abrupt topography of the Pyrenees generates a wide range of microclimatic conditions, which can alter climate-growth relationships (Adams and others 2014), resulting in a possible range of environments where beech can find more appropriate conditions (for example, lower atmospheric water vapor demand and evapotranspiration, higher soil moisture). Such effects of topography and elevation would be also expected in Mediterranean sites with similar elevation as Pyrenean forest. Nevertheless, precipitation is usually lower in the Mediterranean area, mainly summer, and even if these forests are located at high elevation, negative long-term growth trends were observed. Our results suggest a divergent response in the studied beech forests near the southern distribution limit of the specie. Such divergence is in accordance with the idea that vegetation chronic stress caused by increased drought frequency is higher in drier sites at the Iberian Peninsula (Carnicer and others 2011). Alternatively, legacies of historical forest management, such as removing big and fast-growing individuals through selective logging or former coppicing in some stands (Camarero and others 2011), could have caused reduced growth rates. This explanation does not seem plausible on our results since most sampled forests had similar ages (Table 1).
The decreases of tree growth after the climate shift could be caused by multiple reasons. Drought stress suffered by consecutive drought episodes can promote higher defoliation rates (Carnicer and others 2011), and as a consequence lower photosynthetic carbon gain and a reduction in radial growth and forest productivity (Gazol and others 2018). Increasing temperatures and drought stress may also limit water consumption and enhance water-use efficiency, as seen in declining silver fir populations of the Pyrenees, promoting a long-term growth reduction (Linares and Camarero 2012). Moreover, warming temperatures and drought stress may promote carbon starvation and hydraulic failure, which can compromise tree performance and cause die-off episodes (Adams and others 2009; McDowell and others 2008; Camarero and others 2015; Adams and others 2017). As explained above, the most severe droughts in the study area (for example, 1986, 1994, 2005) occurred after the climate shift, and it is well known that trees can have legacy effects after droughts lasting several years (Anderegg and others 2015; Peltier and others 2016; Gazol and others 2017, 2018) that, together with an increase in the time of recovery (Schwalm and others 2017), could compromise the long-term growth of some beech forests in a nearby future. Even if we did not study tree mortality, we focused on how tree growth can be constrained under a period of high temperatures. Low growth or productivity can be a signal of impending tree death, even several years before the mortality episode occurs (Camarero and others 2015; Rogers and others 2018); thus, our results anticipate the vulnerability of the studied forests if the temperature increase persists. Lastly, our findings are relevant to better understand how warmer and drier climate conditions will impact similar temperate mesic forests dominated by deciduous tree species. These impacts will vary as a function of climatic conditions acting at different spatial scales, and the negative effects of droughts on trees could be buffered in topographic refuges with favorable conditions.
In conclusion, we show that after the 1980s climate shift a divergent response was observed on the studied beech forests near the species’ rear edges. Mediterranean and Atlantic beech stands showed negative growth trends and growth limitations after the 1980s shift, but Pyrenean stands presented a long-term growth enhancement. These biogeographical differences indicate that regional climate characteristics can buffer, at some extent, the negative effects of warmer and drier conditions on beech forests near their southern distribution limits in Europe. Our results suggest a high vulnerability of beech forests in the northeast Iberian Peninsula out of the Pyrenean stands, which agrees with the forecasted beech retraction in the area (Benito-Garzón and others 2008). Therefore, under the projected warming scenario, the future distribution range of beech in the northeast Iberian Peninsula would be likely restricted to cold and wet mountainous areas acting as climatic refuges.
References
Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Camilo Villegas J, Breshears DD, Zou CB, Troch PA, Huxman TE. 2009. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci USA 106:7063–6. https://doi.org/10.1073/pnas.0901438106.
Adams HD, Zeppel MJB, Anderegg WRL, Hartmann H, Landhäusser SM, Tissue DT, Huxman TE, Hudson PJ, Franz TE, Allen CD et al. 2017. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat Ecol Evol 1:1285–91. https://doi.org/10.1038/s41559-017-0248-x.
Adams HR, Barnard HR, Loomis AK. 2014. Topography alters tree growth-climate relationships in a semi-arid forested catchment. Ecosphere 11:1–16. https://doi.org/10.1890/ES14/00296.1.
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell NG, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–84. https://doi.org/10.1016/jforeco.2009.09.001.
Allen CD, Breshears DD, McDowell NG. 2015. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6:1–55. https://doi.org/10.1890/ES15-00203.1.
Anderegg WRL, Schwalm C, Biondi F, Camarero JJ, Koch G, Litvak E, Ogle K, Shaw JD, Shevliakova E, Williams AP et al. 2015. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 349:528–32. https://doi.org/10.1126/science.aab1833.
Bennett AC, McDowell NG, Allen CD, Anderson-Teixeira KJ. 2015. Larger trees suffer most during drought in forests worldwide. Nat Plants 28:1–15139. https://doi.org/10.1038/NPLANTS.2015.139.
Benito-Garzón M, Sánchez de Dios R, Sainz-Ollero H. 2008. Effects of climate change on the distribution of Iberian tree species. Appl Veg Sci 11:169–78. https://doi.org/10.3170/2008-7-18348.
Biondi F, Qeadan F. 2008. A theory-driven approach to tree-ring standardization: defining the biological trend from expected basal area increment. Tree-Ring Res 64:81–96. https://doi.org/10.3959/2008-6.1.
Bunn A, Korpela M, Biondi F, Campelo F, Mérian P, Qeadan F, Zang C, Pucha-Cofrep D, Wernicke J. 2018. dplR: Dendrochronology Program Library in R. R package version 1.6.8. https://r-forge.r-project.org/projects/dplr/.
Camarero JJ, Bigler C, Linares JC, Gil-Pelegrín E. 2011. Synergistic effects of past historical logging and drought on the decline of Pyrenean silver fir forests. For Ecol Manag 262:759–69.
Camarero JJ, Gazol A, Sangüesa-Barreda G, Oliva J, Vicente-Serrano SM. 2015. To die or not to die: early-warning signals of dieback in response to a severe drought. J Ecol 103:44–57.
Camarero JJ, Gazol A, Sangüesa-Barreda G, Cantero A, Sánchez-Salguero R, Sánchez-Miranda A, Granda E, Serra-Maluquer X, Ibáñez R. 2018. Forest growth responses to drought at short- and long-term scales in Spain: squeezing the stress memory from tree rings. Front Ecol Evolut 6:9. https://doi.org/10.3389/fevo.2018.00009.
Carnicer J, Coll M, Ninyerola M, Pons X, Sánchez G, Peñuelas J. 2011. Widespread crown condition decline, food weeb disruption and amplified tree mortality with increased climate change type drought. Proc Natl Acad Sci USA 108:1474–8. https://doi.org/10.1073/pnas.1010070108.
Cavin L, Jump AS. 2017. Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob Change Biol 23:362–79. https://doi.org/10.1111/gcb.13366.
Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–6. https://doi.org/10.1126/science.1206432.
Colangelo M, Camarero JJ, Borghetti M, Gazol A, Gentilesca T, Ripullone F. 2017. Size matters a lot: drought-affected Italian oaks are smaller and show lower growth prior to tree death. Front Plant Sci 8:135.
Cropper JP. 1979. Tree-ring skeleton plotting by computer. Tree-Ring Bull 39:47–59.
Dittmar C, Zech W, Elling W. 2003. Growth variations of common beech (Fagus sylvatica L.) under climatic and environmental conditions in Europe—a dendroecological study. For Ecol Manag 173:63–78. https://doi.org/10.1016/S0378-1127(01)00816-7.
Dorado-Liñán I, Akhmetzyanov L, Menzel A. 2017. Climate threats on growth of rear-edge European beech peripheral populations in Spain. Int J Biometeorol 61:2097–110. https://doi.org/10.1007/s00484-017-1410-5.
Dulamsuren C, Hauck M, Kopp G, Ruff M, Leuschner C. 2016. European beech responds to climate change with growth decline at lower, and growth increase at higher elevations in the center of its distribution range (SW Germany). Trees 31:673–86. https://doi.org/10.1007/s00468-016-1499-x.
Galván JD, Camarero JJ, Sangüesa-Barreda G, Alla AQ, Gutiérrez E. 2012. Sapwood area drives growth in mountain conifer forests. J Ecol 100:1233–44.
Garamszegi B, Kern Z. 2014. Climate influence on radial growth of Fagus sylvatica growing near the edge of its distribution in Bükk Mts, Hungary. Dendrobiology 72:93–102.
Gazol A, Camarero JJ, Gutiérrez E, Popa I, Andreu-Hayles L, Motta R, Nola P, Ribas M, Sangüesa-Barreda G, Urbinati C et al. 2015. Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J Biogeogr 42:1150–62.
Gazol A, Camarero JJ, Anderegg WRL, Vicente-Serrano SM. 2017. Impacts of droughts on the growth resilience of the Northern Hemisphere forests. Glob Ecol Biogeogr 26:166–76.
Gazol A, Camarero JJ, Vicente-Serrano SM, Sánchez-Salguero R, Gutiérrez E, de Luis M, Sangüesa-Barreda G, Novak K, Rozas V, Tíscar PA et al. 2018. Forest resilience to drought varies across biomes. Glob Change Biol 24:2143–58. https://doi.org/10.1111/gcb.14082.
Gonzalez-Hidalgo JC, Peña-Angulo D, Brunetti M, Cortesi N. 2015. Recent trend in temperature evolution in Spanish mainland (1951–2010): from warming to hiatus. Int J Climatol 36:2405–16. https://doi.org/10.1002/joc.4519.
Greenwood S, Ruiz-Benito P, Martínez-Vilalta J, Lloret F, Kitzberger T, Allen CD, Fensham R, Laughlin DC, Kattge J, Bönisch G et al. 2017. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol Lett 20:539–53. https://doi.org/10.10111/ele.12748.
Gutiérrez E. 1988. Dendroecological study of Fagus sylvatica L. in the Montseny Mountains (Spain). Acta Oecol 9:301–9.
Hacket-Pain AJ, Cavin L, Friend AD, Jump AS. 2016. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur J For Res 135:897–909.
Hacket-Pain AJ, Friend AD. 2017. Increased growth and reduced summer drought limitation at the southern limit of Fagus sylvatica L., despite regionally warmer and drier conditions. Dendrochronologia 44:22–30. https://doi.org/10.1016/j.dendro.2017.02.005.
Harris IC, Jone PD. 2017. CRU TS4.01: Climatic Research unit (CRU) Time Series (TS) version 4.01 of high resolution gridded data of month-by-month variation in climate (Jan 1901–Dec 2016). Centre for Environmental Data analyses, 04 December 2017. https://doi.org/10.5285/58a8802721c94c66ae45c3baa4d814d0.
Holmes RL. 1983. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 43:69–78.
Hurrell JW. 1996. Influence of variations in extratropical wintertime teleconnections on Northern Hemisphere temperature. Geophys Res Lett 23:665–8.
IPCC. 2014. Climate Change 2014: Synthesis Report. In: Puchauri RK, Meyer LA, Eds. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva: IPCC. p. 151.
Jump AS, Hunt MJ, Peñuelas J. 2006. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob Change Biol 12:2163–74. https://doi.org/10.1111/j.1365-2486.2006.01250x.
Köcher P, Gebauer T, Horna V, Leuschner C. 2009. Leaf water status and stem xylem flux in relation to soil drought in five temperate broad-leaved tree species with contrasting water use strategies. Ann For Sci 66:101.
Knutzen F, Meier IC, Leuschner C. 2015. Does reduced precipitation trigger physiological and morphological drought adaptations in European beech (Fagus sylvatica L.)? Comparing provenances across precipitation gradient. Tree Physiol 35:949–63. https://doi.org/10.1093/treephys/tpv057.
Knutzen F, Dulamsuren C, Meier IC, Leuschner C. 2017. Recent climate warming-related growth decline impairs European Beech in the center of its distribution range. Ecosystems 20:1494–511. https://doi.org/10.1007/s10021-017-0128-x.
Lendzion J, Leuschner C. 2008. Growth of European beech (Fagus sylvatica L.) saplings is limited by elevated atmospheric vapour pressure deficits. For Ecol Manag 256:648–55. https://doi.org/10.1016/j.foreco.2008.05.008.
Lenth R. 2018. emmeans: Estimated marginal means, aka least-square means. R package version 1.1. https://CRAN.R-project.org/package=emmeans.
Linares JC, Camarero JJ. 2012. From pattern to process: linking intrinsic water use efficiency to drought-induced forest decline. GlobChange Biol 18:1000–15. https://doi.org/10.1111/j.1365-2486.2011.02566.x.
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG et al. 2008. Mechanisms of plant survival and mortality during drought: Why do some plants survive, while others succumb to drought? N Phytol 178:719–39. https://doi.org/10.10111/j.1469-8137.2008.02436.x.
Peltier DMP, Fell M, Ogle K. 2016. Legacy effects of drought in the southwestern United States: a multi species synthesis. Ecol Monogr 86:312–26. https://doi.org/10.1002/ecm.1219.
Pinheiro J, Bates D, Debroy S, Sarkar D, R Core Team. 2017. nlme: Linear mixed effects models. R package version 3.1-131. https://CRAN.R-project.org/package=nlme.
Piovesan G, Biondi F, Di Filippo A, Alessandrini A, Maugeri M. 2008. Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob Change Biol 14:1265–81. https://doi.org/10.1111/j.1365.2486.2008.01570.x.
Príncipe A, van der Maaten E, van der Maaten-Theunissen M, Struwe T, Wilmking M, Kreyling J. 2017. Low resistance but high resilience in growth of a major deciduous forest tree (Fagus sylvatica L.) in response to late spring frost in southern Germany. Trees 31:743–51.
R core Team. 2017. R: A language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria. R foundation for Satatistical Computing. Retrieved from https://www.R-project.org/.
Reid PC, Hari RE, Beaugrand G, Livingstone DM, Marty C, Straile D, Barichivich J, Goberville E, Adrian R, Aono Y et al. 2016. Global impacts of the 1980s regime shift. Glob Change Biol 22:682–703. https://doi.org/10.1111/gcb.13106.
Rogers BM, Solvik K, Hogg EH, Ju J, Masek JG, Michaelian M, Berner LT, Goetz SJ. 2018. Detecting early warning signals of tree mortality in boreal North America using multiscale satellite data. Glob Change Biol 24:2284–304. https://doi.org/10.10111/gcb.14107.
Rozas V. 2015. Individual-based approach as useful tool to disentangle the relative importance of tree age, size and inter-tree competition in dendroclimatic studies. iForest 8:187–94. https://doi.org/10.3832/ifor1249-007.
Rozas V, Camarero JJ, Sangüesa-Barreda G, Souto M, García-González I. 2015. Summer drought and ENSO-related cloudiness distinctly drive Fagus sylvatica growth near the species rear-edge in the northern Spain. Agric For Meteorol 201:153–64.
Ryan MG, Phillips N, Bond BJ. 2006. The hydraulic limitation hypothesis revisited. Plant Cell Environ 29:367–81. https://doi.org/10.1111/j.1365-3040.2005.01478.x.
Sánchez-Salguero R, Camarero JJ, Gutiérrez E, González-Rouco F, Gazol A, Sangüesa-Barreda G, Andreu-Hayles L, Linares JC, Seftigen K. 2017. Assessing forest vulnerability to climate warming using a process based model of tree growth: bad prospects for rear-edges. Glob Change Biol 23:2705–19. https://doi.org/10.10111/gcb.13541.
Schwalm CR, Anderegg WRL, Michalak AM, Fisher JB, Biondi F, Koch G, Litvak M, Ogle K, Shaw JD, Wolf A et al. 2017. Global patterns of drought recovery. Nature 548:202–5. https://doi.org/10.1038/nature23021.
Schweingruber FH, Eckstein D, Serre-Bachet F, Bräker OU. 1990. Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia 8:9–38.
Tegel W, Seim A, Hakelberg D, Hoffmann S, Panev M, Westphal T, Büntgen U. 2014. A recent growth increase of European beech (Fagus sylvatica L.) at its Mediterranean distribution limit contradicts drought stress. Eur J For Res 133:61–71. https://doi.org/10.1007/s10342-013-0737-7.
Thiel D, Kreyling J, Backhaus S, Beierkuhnlein C, Buhk C, Egen K, Huber G, Konnert M, Nagy L, Jentsch A. 2014. Different reactions of central and marginal provenances of Fagus sylvatica to experimental drought. Eur J For Res 133:247–60. https://doi.org/10.1007/s10342-013-0750-x.
Tuhkanen S. 1980. Climatic parameters and indices in plant geography. Acta Phytogeogr Suec 67:1–105.
Van der Maaten-Theunissen M, van der Maaten E, Bouriaud O. 2015. pointRes: An R package to analyze pointer years and components of resilience. Dendrochronologia 35:34–8. https://doi.org/10.1016/j.dendro.2015.05.006.
Van der Werf GW, Sass-Klaassen UGW, Mohren GMJ. 2007. The impact of the 2003 summer drought on the intra-annual growth pattern of beech (Fagus sylvatica L.) and oak (Quercus robur L.) On a dry site in the Netherlands. Dendrochronologia 25:103–12. https://doi.org/10.1016/j.dendro.2007.03.004.
Vanoni M, Bugmann H, Nötzli M, Bigler C. 2016. Drought and frost contribute to abrupt growth decreases before tree mortality in nine temperate tree species. For Ecol Manag 382:51–63.
Vicente-Serrano SM, Beguería S, López-Moreno JI. 2010. A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J Clim 23:1696–718.
Weber P, Bugmann H, Pluess AR, Walthert L, Rigling A. 2013. Drought response and changing mean sensitivity of European beech close to the dry distribution limit. Trees 27:171–81. https://doi.org/10.1007/s00468-012-0786-4.
Zeileis A, Kleiber C, Krämer W, Hornik K. 2003. Testing and dating structural changes in practice. Comput Stat Data Anal 44:109–23. https://doi.org/10.1016/S0167-9473(03)00030-6.
Zimmermann J, Hauck M, Dulamsuren C, Leuschner C. 2015. Climate warming-related growth decline affects Fagus sylvatica, but not other broad-leaved tree species in central European forests. Ecosystems 18:560–72. https://doi.org/10.10007/s10021-015-9849-x.
Acknowledgements
We would like to thank Pere Casals who helped us during the fieldwork on the Montsec site. This study was supported by project FunDiver (CGL2015-69186-C2-1-R) funded by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO), INIA (RTA 2006-00117) and CANOPEE (Interreg V-A POCTEFA 2014-2020-FEDER funds). XSM is supported by FPI grant from the Spanish Ministry of Economy, Industry and Competitiveness (BES-2016-077676). GSB and RSS are supported by “Juan de la Cierva” grants (FJCI 2016-30121, IJCI-2015-25845, respectively, FEDER funds). We thank the editorial tasks of two anonymous reviewers and the editor. We also thank all regional administrations for providing sampling permissions (Gobierno de Navarra, Gobierno de Aragón, HAZI, Gobierno de Euskadi, Junta de Castilla y León and Generalitat de Catalunya).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
XSM, AG and JJC conceived the ideas. JJC, GSB, RSS, MC, VR, XSM and EG conducted fieldwork and tree-ring data processing. XSM and AG analyzed the data. XSM led the writing with assistance of all authors. All the authors contributed to the discussion, read and approved the final draft.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Serra-Maluquer, X., Gazol, A., Sangüesa-Barreda, G. et al. Geographically Structured Growth decline of Rear-Edge Iberian Fagus sylvatica Forests After the 1980s Shift Toward a Warmer Climate. Ecosystems 22, 1325–1337 (2019). https://doi.org/10.1007/s10021-019-00339-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-019-00339-z