Abstract
Seabirds act as vectors transporting marine nutrients to land by feeding on fish while nesting and roosting on islands. By depositing large amounts of nutrient-rich guano on their nesting islands they strongly affect island soils, vegetation and consumers. However, few studies have investigated how nesting seabirds affect soil communities. In this study, we investigated how cormorant nesting colonies affect soil chemistry, soil microbes and soil and litter fauna on their nesting islands in the Stockholm archipelago, Sweden. We found that cormorant colonies strongly increase organic soil N and P concentrations, and the effect is stronger close to cormorant nests. Microbial communities were studied by extracting phospholipid fatty acids (PLFA) from the soil. The total amounts of PLFA and the amount of PLFA indicating bacterial biomass were lower on active cormorant islands than on reference islands. Furthermore, PLFA structure and thus microbial community structure differed between cormorant and reference islands. Among ten investigated soil and litter arthropod groups three groups (Thysanoptera, Araneae and Oribatida) showed lower densities and one group (Astigmata) showed higher densities in soils on active cormorant than on reference islands. Some arthropod groups showed strong spatial variation on the cormorant islands. Astigmata, Mesostigmata and Diptera showed higher densities in soil samples close to cormorant nests, whereas Oribatida, Collembola and Hemiptera showed lower densities in litter samples close to cormorant nests than in samples taken 3–20 m away from nests. Overall, the cormorant colonies strongly affected soil ecosystems of their nesting islands, but causal correlations between arthropod densities and soil factors were difficult to reveal. One likely reason may be that nesting cormorant islands are very heterogeneous habitats showing large spatial variation in both soil properties as well as fauna densities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seabirds deposit large amounts of nutrient-rich guano and other residues derived from marine organisms while nesting and roosting on islands. These deposits often lead to a noticeable increase in soil nutrient concentrations and a reduced soil pH (Mulder and others 2011b), and thereby indirectly affect soil microbes, island vegetation and invertebrate consumers (Kolb and others 2010, 2012; Wright and others 2010). Close to bird colonies, ammonia concentrations may be very high, reducing plant biomass. Guano deposition, leading to increased plant growth and plant litter production, eventually result in increased microbial biomass in soil (Wright and others 2010). Nutrient addition due to seabird guano typically reduces plant species richness and plant communities, which are modified on seabird islands compared to other islands. The response of invertebrates to seabird colonies is more variable between sites and taxonomic groups (Kolb and others 2010). Invertebrates may respond to changes in plant biomass and also to changes in plant nutrient content, the latter is often increased in the presence of seabird colonies (Kolb and others 2013b).
Although responses of aboveground arthropods to seabirds are fairly well studied, there are only a few studies of soil and litter fauna (Fukami and others 2006; Towns and others 2009; Wardle and others 2009; Wright and others 2010; Zmudczynska and others 2012) and even fewer for one highly abundant and functionally important invertebrate group in the organic horizon: the soil mites (Behan-Pelletier 1999). Among the best studied systems are a group of warm temperate forest islands in Northern New Zealand with burrowing seabirds. On some of these islands, invasive rats drastically reduced the number of burrowing seabirds, which gave rise to a set of studies comparing invertebrate communities between rat invaded and uninvaded islands (Fukami and others 2006; Towns and others 2009; Wardle and others 2009). The results from these studies show that 8 of 18 investigated faunal orders were more abundant (individuals per unit area) in the litter layer of uninvaded islands with nesting seabirds. Five orders—rotifers, enchytraeids and nematodes—were also more abundant on these islands when data were expressed per unit litter weight (Towns and others 2009). Similarly, another study found 5–20 times higher collembolan densities close to compared to further away from seabird colonies (Zmudczynska and others 2012).
The effect of nesting seabirds is likely to vary among systems not only because habitat conditions vary but also because of differences in seabird nesting behaviour (Mulder and others 2011b). For instance, the effects of burrowing seabirds may differ from those of tree-nesting seabirds because the former better redistribute nutrients throughout the soil layer. Because previous studies have mainly involved burrowing seabirds, studies in systems with other types of seabirds are needed for a broader view of the overall impacts of seabirds on soil microbial and faunal communities. In this project, we study effects of nesting cormorants, which nest in trees and on the ground. Their effects on the plant community and the aboveground arthropod community are well documented (Ellis and others 2006; Kolb and others 2010, 2012). Close to cormorant nests, plant growth is suppressed by a toxic load of nutrients and vegetation cover is negatively correlated with cormorant nesting density. Responses by arthropods are quite variable among groups, with both increased and decreased densities on nesting islands (Kolb and others 2010). The variable responses most likely reflect the multiple changes in resource availability and other habitat conditions as a result of cormorant activities. Different taxa of invertebrates may vary their responses as the resources and habitat conditions change. Compared to other seabirds, nesting sites with cormorants have higher turnover and some sites are periodically abandoned. One cause for island abandonment seems to be sea eagles (Haliaeetus albicilla), which are main predators on cormorant chicks. When nesting islands are abandoned, there is rapid plant growth caused by high nutrient availability, and plant biomass on these islands is often higher than on corresponding islands that never had nesting cormorants (Kolb and others 2010). Including abandoned nesting islands in our studies allowed us to study a wider range of cormorant effects on the soil system.
In this study, we investigated how these varying and locally intense effects of cormorant nesting affect microbial and soil arthropod communities. This information is needed to better understand the variable responses to cormorant nesting observed among taxa. Based on previous studies, we hypothesized that nutrient concentrations (N and P) would be higher on islands with actively nesting cormorants and that soil P would be higher on abandoned nesting islands. We then asked if these changes in soil nutrient concentrations also translated into changes in soil microbial and invertebrate communities. To explore these questions, we compared the effects on soil chemistry, soil microbes and soil and litter fauna between islands with and without cormorant nesting colonies in the Stockholm archipelago, Sweden, where cormorants are a main vector transporting nutrients from sea to land. Our hypothesis was that the lower plant productivity, together with high N levels, on cormorant islands would decrease microbial biomass and alter microbial community composition. Nitrogen addition frequently has had these effects in boreal coniferous forests (Treseder 2008). To test this hypothesis, we extracted phospholipid fatty acids (PLFA) from the soil and related them to cormorant presence and soil characteristics both among and within islands. We also expected different responses for different invertebrate communities, and in particular fungal feeders such as collembolan species were expected to be less abundant on active nesting islands. To study responses in invertebrate communities, we compared their densities in both litter and soil between island categories, and related densities to the distance to the nearest cormorant nest and to measured soil parameters.
Materials and Methods
Study Sites
The study was conducted on 18 islands in the Stockholm archipelago, Sweden (N59°20′ E18°03′) in July 2011 (Table 1). The archipelago consists of about 24,000 islands varying in size from less than 1 m2 to several km2. The archipelago is subject to isostatic rebound, at a rate of 0.47 cm y−1, indicating that the islands are relatively young (Jerling 1999). The bedrock consists of granite-gneiss, mostly covered with a very thin soil layer. The first cormorants (Phalacrocorax carbo) recolonized the Stockholm archipelago in 1994 after hundreds of years of absence and increased strongly in numbers until 2007, when population size appeared to stabilize (Hjertstrand 2014). Between April and August, cormorants are largely confined to about 20 colonies spread across the archipelago, with a total of more than 5000 nests on the ground or in trees. For the purposes of this study, we selected seven active and three abandoned cormorant nesting islands—as well as eight reference islands unpopulated by nesting cormorants—in the northern and middle parts of the archipelago. One previously active island (Marskär) was abandoned by cormorants during late June 2011, which may have affected the results. The cormorant islands had been colonized for about 7–13 years, and the islands differ in size (0.3–2.7 ha), distance from the mainland (0.1–18 km) and type of vegetation (forest or open grassland). To reduce the risk of confounding results, we chose reference islands with the same range of sizes and distances to the mainland, and with vegetation types similar to the cormorant islands. Cormorants are sensitive to human disturbance and colonies are typically established on relatively small islands with limited human activity. Cormorant presence or nest density did not correlate with any of the following island characteristics; vegetation structure or position in relation to the mainland or to larger islands.
Sampling Methods
Soil Properties
We collected soil samples on 14 islands (Table 1), but the number of samples varied somewhat between islands. Three larger islands had much higher vegetation heterogeneity than other islands. To catch this heterogeneity, we decided to increase sample sizes on these islands. Thus, we collected three samples on 11 islands, four samples on one reference island (ASK) and five samples on two islands where the effects of cormorants were mainly apparent on parts of the island (MA, BG). When comparing among islands, data within islands were pooled while accounting for the different sample sizes. Cormorants may nest on the ground and in trees. The samples were collected under vegetation representative for each island in spots where soil had accumulated both close to (<3 m) and further away (3–20 m) from the nests. Soil samples were taken down to a depth of 5 cm with a trowel after removal of the litter layer. Each sample consisted of about ten subsamples collected in a 2-m2 area. Samples were sieved through a 4-mm mesh sieve and stored cold in insulated bags for 24–48 h and then frozen until further preparation.
Plant available P was determined by extraction of air-dried samples with 0.5 M NaHCO3 (Olsen and Sommers 1982). For NH4 and NO3 analysis, sieved soils were treated with 2 M KCl (100 g soil per 250 ml liquid) for 2 h (sandy samples) or overnight (clayey soils). Soils were filtered and analysed with flow injection analysis (Foss, Sweden), following the application notes AN 50/84 (Tecator 1984) and ASN 50-01/92 (Tecator 1992). Soil pH was measured by mixing 5 ml dried soil with 25 ml water and shaken for 12 h where after pH was measured. Soil total C and N concentrations were measured in an elemental analyzer (Leco CN 2000). In this analysis, samples were oxidized and reduced to CO2 and N2, respectively, which were measured with a thermal conductivity detector and IR-detection. From each sample 0.5 g dried and ground soil were used for the analysis of soil properties.
Microbial Phospholipid Fatty Acids (PLFA)
From soil samples, PLFA composition for homogenized soil (0.5–1.5 g) was analysed according to Frostegård and others (1993), with modifications (Nilsson and others 2007), in 47 samples. An internal standard (methyl nondecanoate fatty acid 19:0) was added before the methylation step. For each sample, the relative abundances of individual fatty acids were estimated as the proportion of the sum of all fatty acid ratios (mol%). We used the fatty acid nomenclature as described by Frostegård and others (1993) and identified 29 fatty acids in total. The fatty acids i15:0, a15:0, 15:0, i16:0, 16:1ω9, 16:1ω7t, i17:1ω8, i17:0, a17:0, cy17:0, 17:0, 18:1ω7, 18:1ω9 and cy19:0 were chosen to indicate bacterial PLFAs and 18:2ω6 was used as an indicator for fungal biomass (Frostegård and Bååth 1996). The PLFA 16:1ω5 was not included as an indicator for bacterial biomass because it is also found in arbuscular mycorrhizal fungi (Nordby and others 1981). The ratio of bacterial PLFAs to 18:2ω6 was used to estimate the relative bacterial to fungal biomass in soil (Frostegård and Bååth 1996).
Soil and Litter Fauna
To investigate the effect of nesting cormorants on soil and litter fauna we extracted fauna from one litter and one soil sample at two sites on each of the 18 islands. Due to the variability in habitat conditions, we noted coarse habitat type (forest or grassland) on each site. On cormorant islands one sample was collected under or close to nests and the other 3–20 m from the nest. The samples were collected close to soil samples collected for chemical and PLFA analysis. Litter density was estimated using the area needed to collect 2 l of litter (excluding larger branches) as a proxy. When litter was removed, we collected soil from the same spot with a trowel. Soil and litter samples were stored in cool and dark conditions until extraction, but not longer than 5 days. We extracted a subsample (500–950 ml soil or 400–1600 ml litter) in Tullgren funnels for 48 h. After extraction, the volumes and weights of the soil and litter samples were determined. The extracted animals were stored in 80% ethanol until sorting. All animals were identified to order, and mites were further divided into Oribatida, Mesostigmata, Astigmata and Prostigmata. We calculated two density estimates for each taxonomic group and layer; mean number of individuals 100 g−1 soil or litter and mean number of individuals l−1 soil or litter.
Statistics
Soil and Litter Properties
We first compared soil properties [water content in % (WC%), pH, total C, total N, Ninorg [NH4 + + NO3 −], NH4 +, NO3 − and Olsen P] between island categories with linear mixed-effects models (lme’s), using island category (active nesting island, abandoned nesting island and control island) as fixed effect and island as random effect. Within cormorant nesting islands, we then compared soil properties between sites close to and far from (>3 m) nests, using distance to nest as the fixed effect and island as the random effect.
To compare the depth of the litter layer between island categories we calculated the area needed to collect 1 l litter (island mean) in an lme using island category and habitat type (forest or open grassland) as fixed effects and island as random effect. On nesting islands only, we performed a similar test on the depth of the litter layer close to and further away from cormorant nests, again in an lme, using distance to the closest cormorant nest as fixed effect and habitat and island as random effects.
Fatty Acids (PLFA)
To compare overall structure of PLFA, we compared the total abundance of PLFA (nmol) g−1 soil C, the percentages of bacterial and fungal PLFA as well as the ratio of bacterial to fungal PLFA between the island categories with lme’s using island category as fixed effect and island as random effect. Following these tests, we correlated soil properties and the total abundance of PLFA g−1 C, the percentages of bacterial and fungal PLFA as well as the ratio of bacterial to fungal PLFA using soil properties as fixed effects and island as random effect.
To study the PLFA composition (mol%), we first compared the composition between island categories with NP-MANOVA based on Bray–Curtis dissimilarities using the adonis function of the package vegan for R (ver. 2.12-1). Second, we related the PLFA composition to measured soil chemical characteristics at each sampling site using canonical correspondence analysis (CCA). To control for unmeasured variables, we also performed an indirect ordination using NMDS. The pattern was very similar to the CCA suggesting that no unmeasured variables explained additional variation, so the NMDS is not further discussed. As multiple samples were collected on each island, we included island as a cofactor in the analysis. In order to find the best predictors of the PLFA composition, we used both forward and backward selection. Inclusion or exclusion of variables was based on Akaike information criterion (AIC). The significance of variables was tested using permutation test (500 permutations) by applying the ANOVA function in vegan. We also tested for correlation among variables using variance inflation factors (VIF). Finally, we visually inspected and compared the maximum and final models from the forward selection in order to check for the strength of the variables and the effect of variables not included in the final model on variables included in the final model. We checked for correlation between soil properties included in the final model and the CCA axis with the intersector function. All multivariate analyses were conducted in the vegan package (Oksanen and others 2013).
Soil and Litter Fauna
To investigate the effect of nesting cormorants on soil and litter fauna we performed three analyses on the density (individuals 100 g−1 dry weight) of ten abundant arthropod groups (Thysanoptera, Coleoptera, Diptera, Araneae, Collembola, Hemiptera, Oribatida, Mesostigmata, Prostigmata, Astigmata) using separate models for soil and litter samples. First, we compared the soil and litter fauna densities between island categories with one-way ANOVAs, and Tukey HSD test, using the mean of two samples for each island as response variable. Second, we examined the density patterns of arthropods on the cormorant islands as a function of the distance of the sampling point to the nearest cormorant nest. Because some species had zero densities on many islands, we split the analyses. Arthropod groups found in most samples were analysed with linear mixed-effect models with island as random effect. For arthropod groups which were absent in multiple samples, we instead conducted χ 2 tests on presence/absence data. All density data were log-transformed prior to analysis. Third, we related the soil fauna densities to soil chemical characteristics and PLFA indicators at each sampling site by canonical correspondence analysis (CCA). The CCA was checked for the strength of the variables and the effect of variables not included in the final model similar to the analysis of PLFA. Finally, we related predator densities (mesostigmatid mites, spiders) to potential prey taxa at a site level using a stepwise regression analysis. In this analysis, we observed initially that the relationships differed between sites with limited cormorant impact versus those with high cormorant impact. We therefore repeated the analyses for the two site types. Both densities and soil characteristics were log-transformed prior to the analyses. All statistical analyses were conducted in R version 2.12.1.
Results
Soil and Litter Properties
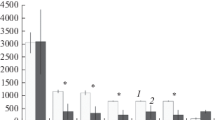
Among the eight investigated soil properties, four (Ninorg, Olsen P, NO3 − and NH4 +) differed between island categories and four did not (WC%, pH, total soil C and total soil N) (Figure 1). Ninorg, NO3 − and NH4 + concentrations were highest on active cormorant islands, whereas P concentrations were higher on both active and abandoned cormorant islands compared to reference islands. Soil NO3 − concentrations on abandoned islands were intermediate of that for reference and active cormorant islands. On the nesting islands, the soil concentrations of Ninorg, P, NO3 − and NH4 + decreased while soil moisture (WC%) increased with increasing distance from cormorant nests (Figure 2; Table 2).
Soil properties (mean ± SE) on reference islands (RF), abandoned (AB) and active (CO) cormorant islands. Different letters refer to significant differences between island categories. For sample sizes see Table 1.
The mean area needed to collect 1 l litter, as a proxy for litter density, did not differ between the island categories but was larger in open habitats than on the forest floor (F = 16.0, P < 0.001). On nesting islands, larger areas were needed to collect 1 l litter (i.e., less litter) close to compared to some meter away from cormorant nests (F = 9.1, P < 0.05).
Fatty Acids (PLFA)
Total PLFA content varied greatly among islands (330–2200 nmol g−1 dry soil; mean 890), and as expected was strongly positively correlated with total C content of the soil (F = 27.7, P < 0.0001). To control for this confounding factor we instead analysed variation in PLFA g−1 soil C, which differed between island categories (F = 4.0, P = 0.05) and was lower on active cormorant islands than on reference islands (t = 2.7, P = 0.02). PLFA g−1 soil C was negatively correlated to total soil C and total soil N (Table 3). Bacterial PLFA indicators (%) also differed between island categories (F = 5.7, P = 0.02), and were slightly lower on abandoned (t = 2.2, P = 0.05) and active (t = 3.2, P = 0.009) cormorant islands than on reference islands. The fungal PLFA indicator did not differ between island categories (F = 1.5, P > 0.2), and neither did the ratio of bacterial to fungal PLFA (F = 1.7, P > 0.2). The percent fungal PLFA was positively correlated with soil P concentrations (F = 38.1, P < 0.0001), whereas the percent bacterial PLFAs was not correlated with any soil property.
The NP-MANOVA showed that the PLFA composition differed between island categories (Pseudo-F = 3.5, P < 0.005, R 2 = 0.39). The CCA with stepwise selection retained two axes that explained 70% of the variation in PLFA and that included three soil properties (Pseudo-F = 6.4, P < 0.01). This model was confirmed by backward selection. Soil pH was negatively correlated with the first axis (Pseudo-F = 32.1, P < 0.01), whereas Olson P (Pseudo-F = 18.0, P < 0.01) and Ninorg (Pseudo-F = 11.0, P < 0.01) were positively correlated with the second axis (Figure 3). Active cormorant islands generally had positive values along the second axis, whereas reference islands had negative values on this axis, with abandoned islands in between. The first axis was characterized by negative values of the unsaturated PLFAs 16:1ω5, 16:1ω9 and 18:1ω7 and positive values for 10:Me16b, 10Me17, br17:0, br18:0 and cy19:0. The second axis was characterized by negative values of the unsaturated PLFAs 16:1ω9 and 18:1ω7 and the branched PLFAs br17:0 and br18:0 and 19:1a, whereas 18:0, a15:0 and the fungal indicator 18:2ω6,9 had high positive values.
Sample (A) and species (B) scores from a canonical correspondence analysis for relative concentrations of PLFAs (mol%) from soil samples collected on reference islands (hollow squares), active (filled squares) and abandoned cormorant islands (no square). Correlated soil properties are included as arrows, where the length indicates the relative importance and the direction of the correlation.
Soil and Litter Fauna
In the soil, the density for five of ten taxonomic groups varied among island categories with a similar pattern for both density estimates (100 g−1 dry soil and l−1 soil), and we therefore only present densities 100 g−1 dry soil (Figure 4). Thysanoptera and Araneae had higher density on reference than on active cormorant islands, oribatid mites had higher density on reference than on both active and abandoned cormorant islands, and astigmatid mites had higher density on active cormorant than on reference islands. Mesostigmatid mites had the lowest density on abandoned cormorant islands. In the litter, none of the ten taxonomic groups responded with density changes to cormorant nesting colonies (Figure 5).
Soil fauna densities (number of individuals 100 g−1 dry weight soil) collected on reference islands (RF), abandoned (AB) and active (CO) cormorant islands in relation to the distance from the nearest cormorant nest. Larger symbols indicate multiple data points for each value. Significant differences (P < 0.05) between island categories are included in the sub-headings.
Litter fauna density (number of individuals 100 g−1 dry weight litter) collected on reference islands (RF), abandoned (AB) and active (CO) cormorant islands in relation to the distance of the sampling point to the nearest cormorant nest. Larger symbols indicate multiple data points for each value. Significant differences (P < 0.05) between island categories are included in the sub-headings.
When focusing on the spatial variation on cormorant islands only, we found that in soil samples, astigmatid and mesostigmatid mites had higher densities close to cormorant nests than 3–20 m from nests (F = 6.7, P = 0.03 and F = 9.7, P = 0.01), whereas dipteran larvae had lower densities close to nests (F = 24.0, P < 0.001) (Figure 4). In litter samples, Thysanoptera were less often found close to cormorant nests than 3–20 m from nests (χ 2 = 7.3, P = 0.007) (Figure 5). Similarly, Collembola, Hemiptera and Oribatida had lower densities in litter samples close to cormorant nests than 3–20 m from nests (F = 17.0, P = 0.003, F = 7.0, P = 0.03 and F = 14 and P = 0.004), whereas Diptera showed an opposite density distribution (F = 7.0 and P = 0.03) (Figure 5). The CCA between soil characteristics and soil fauna densities revealed no significant relations. Finally, the regression analyses on predator densities revealed strong relationships for mesostigmatid mites but not for spiders. When comparing sites with limited cormorant impact, mesostigmatid mite densities were related to densities of two prey groups (Collembola and Oribatida) (Table 4). For Collembola, the relationship to mesostigmatid densities varied between the two substrates (litter vs. soil). The mesostigmatid mite densities had a steeper positive relationship to collembolan densities in litter compared to soil samples (Figure 6). When comparing sites with high cormorant impact, though with a much lower sample size (n = 13), the pattern was similar. Oribatid mite densities were positively related to mesostigmatid mite densities (F oribatid = 6.7, P < 0.04). For Collembola, there was again an interaction where the relationship was steeper for litter than for soil samples (F collembola = 17.8, P < 0.001, F collembola × substrate = 6.3, P < 0.04). All relationships from multiple regressions were verified in regressions with each prey density separately.
Discussion
Soil N and P concentrations greatly increased on islands with nesting cormorants compared with the other sites investigated. Sites with nesting cormorants also had distinctive microbial and invertebrate communities. Soil N mainly increased on active nesting islands, particularly close to cormorant nests, whereas the high soil P concentrations also remained after islands were abandoned by cormorants. This pattern is most likely a consequence of faster leaching of nitrate than phosphate. Fatty acid indicators for microbial communities varied among island categories and particularly bacterial indicators were lower on active nesting islands. Several arthropod groups differed in density among island categories, but only in the soil and not in the litter. Three groups (oribatid mites, Thysanoptera and spiders) had lower densities in soil on all nesting islands than on reference islands, whereas one group (mesostigmatid mites) had lower densities only on abandoned nesting islands. In contrast, astigmatid mites had the highest densities in the soil on active nesting islands.
The observed changes in soil fauna densities combined with soil properties would suggest that oribatid mites, Thysanoptera and spiders responded negatively to soil P, that mesostigmatid mites responded positively to soil P and that astigmatid mites responded positively to soil N. However, when correlating soil properties with invertebrate densities we found that arthropod densities were not correlated with soil properties but rather with resource densities (at least for mesostigmatid mites). The relationship between soil properties and arthropod densities is likely complex and filtered through changes in resources for the microarthropods (see also Hasegawa 2001; Laiho and others 2001; Mulder and Elser 2009). For instance, mesostigmatid mites are known predators on other microarthropods (Koehler 1999) and their densities were also strongly correlated with several potential prey taxa in this study.
Microbial Communities
As hypothesized, total PLFA g−1 soil C was lower on active cormorant islands than on reference islands and tended to negatively correlate with NH4 concentrations, suggesting that the change in PLFA to cormorant nesting is due to the higher N input through guano on these islands. Previous studies similarly suggested that microbial biomass generally declines under N fertilization (Treseder 2008), and studies on cormorant nesting islands suggest a reduced fungal hyphal length and decomposition rate of tree litter (Osono and others 2002, 2006). However, Treseder (2008) pointed out that responses were somewhat inconsistent among studies. In northern forests, total microbial biomass and activity generally decreases in response to fertilization, whereas in areas with bird fertilization it is more common for microbial biomass to increase (Fukami and others 2006; Wright and others 2010). The different responses among studies may arise for several reasons. First, systems differ in habitat conditions other than the nutrient addition, and our study system may be functionally more similar to northern forest systems than to the warm temperate forests on the islands outside New Zealand. Second, guano deposition from cormorants may resemble the addition of artificial fertilizers more than nutrient deposition from burrowing seabirds. Fertilizers and cormorant guano are both deposited on the surface without a direct disturbance of the soil horizon. Burrowing seabirds on the other hand disturb the soil and better distribute nutrients among soil layers (Mulder and others 2011b), which would affect microbial communities deeper into the soil. Finally, the high ammonium concentrations on cormorant islands had direct negative effects on plant biomass, and thereby reduced the amount of plant material in the soil. This effect, in combination with the very shallow soil layer on the islands, may have reduced the capacity of the microbial community to counteract the nitrogen-rich conditions. Not surprisingly, the negative effect on the total microbial community was weaker or absent on abandoned cormorant islands, where plant biomass was as high, or higher, as on reference islands (Kolb and others 2010).
Microbial community structure varied not only between island categories, but also between islands within categories. In the CCA of the PLFA pattern, the first axis (CCA1) was not related to island categories but rather to other habitat differences (pH). CCA1 also had negative loadings for PLFAs 16:1ω5 and 18:1ω7, which have earlier been found to indicate high pH in soils (Nilsson and others 2007; Rousk and others 2010). These PLFAs have also been observed to increase after liming of coniferous forest soils and are considered indicators of gram-negative bacteria (Frostegård and others 1993). Some fatty acids with positive loadings on CCA1, and negative loadings on CCA2, have on the other hand been found to be more abundant in soils with a low pH (forest soils: cy19:0, Nilsson and others 2007; agricultural soils: br18:0, Rousk and others 2010). Other fatty acids with similar values in the CCA were the branched fatty acids br17:0 and the methyl branched PLFAs 10Me16:0 and 10Me17:0. Among these, 10Me16:0, an indicator of gram-positive bacteria, has been found to decrease in liming experiments (Frostegård and others 1993). The close correlation between CCA1 and pH, as well as the expected changes due to pH in the PLFA pattern, confirm that CCA1 is determined by soil pH and not by the presence of cormorants on the islands. Notice that soil pH was not affected by cormorant presence.
The second CCA axis was positively correlated with soil P and Ninorganic concentrations, and active nesting islands had high values while control islands had low values on this axis. Thus, this axis indicated effects of the presence of cormorants. Most of the fatty acids with high loadings on the second axis have also previously been related to the presence of manure. For instance, the anteiso-branched fatty acids a15:0 and a17:0 have been found to dominate in cattle slurry (Frostegård and others 1997), and specifically in anaerobic conditions in hotspots. Although not measured in this study, anaerobic conditions were most likely prevailing in soil directly under active cormorant nests as the guano formed a thick crust on top of the soil and there was a strong smell of putrefaction. Similarly, the fatty acids 16:0, i17:0, 18:0 and cy17:0, with high scores on the second CCA axis, have been found in higher abundance in sewage sludge and manure-treated soil (Börjesson and others 2012).
Invertebrate Communities
Changes in soil microbial communities may affect the soil meso- and macrofauna. However, in our study we found no correlations between microbial communities, as reflected by the PLFA concentration and composition, and soil or litter fauna. One must bear in mind, though, that PLFA is an indicator of biomass, and not growth and activity. The soil fauna will most likely be correlated to production of soil microorganisms, and not standing stock of the microbial biomass. Several animal groups did vary among island categories but we were unable to find solid predictive variables explaining the faunal community structure. Generally, oribatid mites were the group showing the most distinct response to cormorant colonies. They had lower densities on both active and abandoned cormorant islands both close to and away from cormorant nests, suggesting a possible response to soil P. Although these patterns were not verified as an effect in the CCA, the observations match previous studies showing that oribatid mites respond negatively to increased soil P concentrations (Schon and others 2011). This response may be caused indirectly by a reduction in the fungal food resource, which is also likely to have affected the collembolans.
Collembola was another abundant group in litter and soil layers. Other seabird island studies have reported increased collembolan densities close to seabird colonies (Towns and others 2009; Zmudczynska and others 2012), but we have rather found lower collembolan densities in the presence of nesting cormorants in this and in a previous study (Kolb and others 2010). These conflicting results may be explained both by different nesting behaviour of seabirds in different systems and by different habitat conditions. In a tree-nesting species, guano is accumulated immediately below the nests. At high nest densities, few areas remain that are not covered by guano, creating extremely high loads of NO3. Such high NO3 loads have been shown to negatively affect collembolan densities (Birkhofer and others 2012). In burrowing seabirds, as in the study by Towns and others (2009), guano does not accumulate in a similar way and nitrogen is more distributed in the soil layer. As a consequence, the positive indirect effects from a higher nutrient availability on collembolan resources in these systems may be more important than negative direct effects of guano deposition.
Astigmata was the only group with higher densities on active cormorant colonies. This response was expected as many species within this group are parasites on birds and mammals and thus may benefit directly by the presence of cormorants (Proctor and Owens 2000). A similar direct effect of cormorants may also explain the increased densities of dipterans and beetles found close to cormorant nests (Kolb and others 2010, 2012). In a former study on this system (Kolb and others 2010), we found extremely high dipteran densities on active cormorant islands and argued that these densities mainly reflected an increase of bird parasites and carrion-feeding species. Similarly, the higher density of beetles in close vicinity to cormorant nests is likely caused by higher densities of carrion-feeding and fungivorous species (Kolb and others 2012).
Moving up the food chain we observed higher densities of mesostigmatid mites close to cormorant nests, at least in the soil samples, and their densities were correlated with potential prey groups such as oribatid mites and collembolans (Koehler 1999). Even though the mechanisms underlying these patterns are not resolved, it is likely to be a chain of events that connects patterns of cormorant impact to distributions of predator groups such as the mesostigmatid mites. Among other predator groups, we observed fewer ground-living spiders close to nesting cormorants, similar to our former observations (Kolb and others 2010). However, it seems unlikely that the negative effect of cormorants on spiders is an effect of food limitation as, the main food of spiders in this area, dipterans were at higher densities on active cormorant islands than on control islands. Reasons for the negative cormorant effect are still not clear but the current study allows us to exclude some additional factors, such as changed soil pH. It is possible that the mechanism is rather a changed habitat structure, with a lack of ground vegetation close to cormorant colonies.
Conclusion
Cormorant colonies have distinct effects on the vegetation of their nesting islands, an effect that is visible for anyone passing the islands. The changes to the vegetation reflect large inputs of guano together with bird and fish carcasses and create a habitat for soil and litter fauna different to habitats found on non-seabird islands. However, cormorant effects on island ecosystems are complex and the underlying mechanisms are difficult to disentangle. Our results clearly show that nesting cormorants strongly affect several soil properties including N and P concentrations, leading to changes in the microbial and soil arthropod communities. In some cases, such as for mesostigmatid or astigmatid mites, we are able to connect population changes to changes in resource availability. In other cases, such as for spiders and collembola, changes seem to depend more on changes in habitat characteristics. Many of these responses may need other approaches to be resolved. In some cases, more detailed information on consumer diets may provide necessary information to understand density changes. In other cases, a community or food web approach may be needed. For instance, ecological stoichiometry combined with diet data may indicate if taxa are limited by certain nutrients in their diet. We have explored this possibility for larger arthropods in this system and found very limited support of either N or P limitation (Kolb and others 2013a). However, combining allometric scalings and size spectra with soil nutrient conditions may indicate N and P limitations on food web properties (Mulder and Elser 2009; Mulder and others 2011a) that may in turn feed back onto densities of specific groups. In some way, nutrient availability has an important role in explaining responses by invertebrates on cormorant islands but the various ways in which this occurs are yet to be unravelled.
References
Behan-Pelletier VM. 1999. Oribatid mite biodiversity in agroecosystems: role for bioindication. Agric Ecosyst Environ 74:411–23.
Birkhofer K, Schoning I, Alt F, Herold N, Klarner B, Maraun M, Marhan S, Oelmann Y, Wubet T, Yurkov A, Begerow D, Berner D, Buscot F, Daniel R, Diekötter T, Ehnes RB, Erdmann G, Fischer C, Foesel B, Groh J, Gutknecht J, Kandeler E, Lang C, Lohaus G, Meyer A, Nacke H, Nather A, Overmann J, Polle A, Pollierer MM, Scheu S, Schloter M, Schulze ED, Schulze W, Weinert J, Weisser WW, Wolters V, Schrumpf M. 2012. General relationships between abiotic soil properties and soil biota across spatial scales and different land-use types. PLoS One 7:e43292.
Börjesson G, Menichetti L, Kirchmann H, Kätterer T. 2012. Soil microbial community structure affected by 53 years of nitrogen fertilisation and different organic amendments. Biol Fertil Soils 48:245–57.
Ellis JC, Farina JM, Witman JD. 2006. Nutrient transfer from sea to land: the case of gulls and cormorants in the Gulf of Maine. J Anim Ecol 75:565–74.
Frostegård A, Bååth E. 1996. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65.
Frostegård A, Bååth E, Tunlid A. 1993. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty-acid analysis. Soil Biol Biochem 25:723–30.
Frostegård A, Petersen SO, Bååth E, Nielsen TH. 1997. Dynamics of a microbial community associated with manure hot spots as revealed by phospholipid fatty acid analyses. Appl Environ Microbiol 63:2224–31.
Fukami T, Wardle DA, Bellingham PJ, Mulder CPH, Towns DR, Yeates GW, Bonner KI, Durrett MS, Grant-Hoffman MN, Williamson WM. 2006. Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecol Lett 9:1299–307.
Hasegawa M. 2001. The relationship between the organic matter composition of a forest floor and the structure of a soil arthropod community. Eur J Soil Biol 37:281–4.
Hjertstrand G. 2014. Skarvåret 2013 [in Swedish]. Anonymous. Levande skärgårdsnatur 2014. Skärgårdsstiftelsen. pp 16–18.
Jerling L. 1999. Sea shores. Acta Phytogeo Suec 84:169–85.
Koehler HH. 1999. Predatory mites (Gamasina, Mesostigmata). Agric Ecosyst Environ 74:395–410.
Kolb G, Palmborg C, Hambäck PA. 2013a. Ecological stoichiometry and density responses of plant-arthropod communities on cormorant nesting islands. PLoS One 8:e61772.
Kolb GS, Jerling L, Essenberg C, Palmborg C, Hambäck PA. 2012. The impact of nesting cormorants on plant and arthropod diversity. Ecography 35:726–40.
Kolb GS, Jerling L, Hambäck PA. 2010. The impact of cormorants on plant-arthropod food webs on their nesting islands. Ecosystems 13:353–66.
Kolb GS, Palmborg C, Hambäck PA. 2013b. Ecological stoichiometry and density responses of plant-arthropod communities on cormorant nesting islands. PLoS One 8:e61772.
Laiho R, Silvan N, Carcamo H, Vasander H. 2001. Effects of water level and nutrients on spatial distribution of soil mesofauna in peatlands drained for forestry in Finland. Appl Soil Ecol 16:1–9.
Mulder C, Elser JJ. 2009. Soil acidity, ecological stoichiometry and allometric scaling in grassland food webs. Global Change Biol 15:2730–8.
Mulder C, Vonk JA, Den Hollander HA, Hendriks AJ, Breure AM. 2011a. How allometric scaling relates to soil abiotics. Oikos 120:529–36.
Mulder CPH, Jones HP, Kameda K, Palmborg C, Schmidt S, Ellis JC, Orrock JL, Wait DA, Wardle DA, Yang L, Young H, Croll DA, Vidal E. 2011b. Impacts of seabirds on plant soil properties. In: Mulder CPH, Anderson WB, Towns DR, Bellingham PJ, Eds. Seabird islands: ecology, invasion and restoration. Oxford: OUP. p 135–76.
Nilsson LO, Bååth E, Falkengren-Grerup U, Wallander H. 2007. Growth of ectomycorrhizal mycelia and composition of soil microbial communities in oak forest soils along a nitrogen deposition gradient. Oecologia (Heidelb) 153:375–84.
Nordby HE, Nemec S, Nagy S. 1981. Fatty-acids and sterols associated with citrus root mycorrhizae. J Agric Food Chem 29:396–401.
Oksanen J, Guillaume Blanchet F, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2013. Vegan: community ecology package. R package version 2.0-10.
Olsen SR, Sommers LE. 1982. Phosphorous. In: Page AL, Miller RH, Keeney DR, Eds. Methods of soil analysis, part 2, chemical and mineralogical properties. Madison: ASA and SSSA. p 403–30.
Osono T, Hobara S, Fujiwara S, Koba K, Kameda K. 2002. Abundance, diversity, and species composition of fungal communities in a temperate forest affected by excreta of the Great Cormorant Phalacrocorax carbo. Soil Biol Biochem 34:1537–47.
Osono T, Hobara S, Koba K, Kameda K. 2006. Reduction of fungal growth and lignin decomposition in needle litter by avian excreta. Soil Biol Biochem 38:1623–30.
Proctor H, Owens I. 2000. Mites and birds: diversity, parasitism and coevolution. Trends Ecol Evol 15:358–64.
Rousk J, Brookes PC, Bååth E. 2010. The microbial PLFA composition as affected by pH in an arable soil. Soil Biol Biochem 42:516–520.
Schon NL, Mackay AD, Gray RAJ, Minor MA. 2011. Influence of phosphorus inputs and sheep treading on soil macrofauna and mesofauna in hill pastures. N Z J Agric Res 54:83–96.
Tecator F. 1984. Application Note, AN 50/84.
Tecator F. 1992. Application Sub Note, ASN 50-01/92.
Towns DR, Wardle DA, Mulder CPH, Yeates GW, Fitzgerald BM, Parrish GR, Bellingham PJ, Bonner KI. 2009. Predation of seabirds by invasive rats: multiple indirect consequences for invertebrate communities. Oikos 118:420–30.
Treseder KK. 2008. Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–20.
Wardle DA, Bellingham PJ, Bonner KI, Mulder CPH. 2009. Indirect effects of invasive predators on litter decomposition and nutrient resorption on seabird-dominated islands. Ecology 90:452–64.
Wright DG, van der Wal R, Wanless S, Bardgett RD. 2010. The influence of seabird nutrient enrichment and grazing on the structure and function of island soil food webs. Soil Biol Biochem 42:592–600.
Zmudczynska K, Olejniczak I, Zwolicki A, Iliszko L, Convey P, Stempniewicz L. 2012. Influence of allochtonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biol 35:1233–45.
Acknowledgments
Funding for this Project was provided by the Swedish Research Council Formas (to PAH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contribution
GK, CP and PH conceived of and designed the study. GK and CP performed the field research. GK, CP, AT, EB and PH performed analyses of samples and data. EB contributed new methods. GK, CP, AT, EB and PH wrote the paper.
Rights and permissions
About this article
Cite this article
Kolb, G.S., Palmborg, C., Taylor, A.R. et al. Effects of Nesting Cormorants (Phalacrocorax carbo) on Soil Chemistry, Microbial Communities and Soil Fauna. Ecosystems 18, 643–657 (2015). https://doi.org/10.1007/s10021-015-9853-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-015-9853-1