Abstract
The blood flow velocity waveforms in the middle cerebral artery (MCA) were measured under incremental cycle exercises and following recovery using the transcranial Doppler ultrasound velocimeter. At rest, the flow waveform in the MCA is characterized by a high forward flow at end-diastole, which is as much as half peak-systolic velocity. The peak-systolic velocity V s was found to markedly rise in proportion with exercise intensity under mild and moderate intensities (up to 110 W) of exercise, while it tended to keep constant values under higher exercise intensities (above 110 W). The end-diastolic velocities V d showed no significant change under mild exercise intensity and gradually increased under moderate exercise intensities. It was also found that the V d values decreased more rapidly than V s in the recovery phase. Such nonlinear changes in V s and V d indicate that the influence of exercise on cerebral blood flow is more complicated than previously thought.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The brain consists of highly metabolically active tissues that require approximately 50 ml/100 g tissue/min of blood flow at rest. This makes the brain extremely vulnerable to oxygen deficiency, and an adequate and continuous supply of oxygenated blood is essential for the function of the brain. The middle cerebral artery (MCA) supplies about 80 % of the flow volume required in the cerebral hemisphere. It is generally accepted that MCA velocity waveforms are closely related to cerebrovascular diseases such as atherosclerosis, stenosis, cerebral infarction, cerebral thrombosis, and intracranial hemorrhage. Velocity measurements using a transcranial Doppler velocimeter (TCD) have been widely conducted in clinical examinations [1–4, and references therein].
Most of the clinical data using TCD have been measured under resting conditions. However, cerebral blood flow can easily be disturbed by daily activities such as work, exercise, and posture change [2], as well as changes in environmental factors such as ambient temperature and humidity. The study of brain blood flow has become a recent topic of interest in the brain science field owing to the development of measurement methods, such as the transcranial Doppler, near infrared flowmetry, and functional MRI, and so on [5].
We have measured velocity waveforms in the MCA during activities such as cycle exercise, posture changes, and squat with load, as well as under mental stress [6, 7]. In the present study, we measured the velocity waveforms during cycle exercise in a wide range of intensities and also following recovery, to understand the dynamics of MCA blood flow caused by vigorous physical exercise. Although many studies on MCA blood flow in cycle exercise have been reported, there are still some discrepancies among velocity data under vigorous exercise. Additionally, little information on MCA blood flow in the recovery period after exercise is available [5, 8].
2 Methods
2.1 Subjects
Twelve healthy young male volunteers participated in the present study. None of the subjects had any history of cardiovascular, cerebrovascular, or respiratory disease. All subjects were fully informed about the procedures, risk, and benefits of the study, and written consent was obtained from all subjects before the study. This study was granted approval by the university institutional board.
2.2 Experimental design
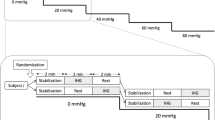
Tests were started at about 10:00 a.m. in all subjects. Prior to the test, the subjects were not allowed to consume any beverages containing caffeine or alcohol after 9:00 p.m. the previous night and vigorous exercise was forbidden for 36 h before the start of testing. Each subject was required to keep his body upright with eyes blindfolded, in a seated position on an electromagnetically braked cycle ergometer during rest, exercise, and recovery periods. A photograph of the present experiment is shown in Fig. 1.
After 3-min rest, the work rate was increased from 50 W to about 190 W in about 20 W increment steps, and then the work rate was decreased to 50 W by about a −50 W step (recovery phase), followed by 3 min rest. The duration of each step was 1 min with a constant pedaling rate of 50 rpm.
2.3 Measurement of blood velocity and pressure
The blood flow velocity in the right middle cerebral artery was obtained using a 2-MHz pulsed Doppler ultrasound system (Intra-view, Rimed, Israel). The pulsed probe was located over the temporal bone and the Doppler signal was optimized by changing the insonation angle. The probe, attached to the skull at a fixed angle, was held in place using headgear with an adjustable positioning system. The blood flow velocity was assessed for 5 s in each exercise step.
The blood pressure in the brachial artery was sphygmo-manometrically measured using a pneumatic arm cuff, which was held at heart level. Mean arterial pressure MAP can be approximately determined from measurements of the systolic pressure SAP and diastolic pressure DAP by following equation [9, 10],
3 Results and discussion
3.1 Velocity waveforms
In Fig. 2, examples of the obtained velocity waveforms are shown. Figure 2a represents the waveform before exercise, which reveals substantial variation of flow velocity during one cardiac cycle. The velocity rises sharply at initial systolic phase of heart (peak-systolic velocity) and decreases gradually during the diastolic phase. The high forward flow at end-diastole, denoted by V d, which is as much as half peak-systolic velocity V s, should be noted. This feature is characteristic of blood flow in intracranial arteries such as the internal carotid artery, anterior cerebral artery, and MCA, and contrasts with the relatively low diastolic flow component in the external carotid artery. This difference is ascribed to the markedly low flow resistance in the cerebral vasculature compared with that in the region of the external carotid artery [3].
At the moderate exercise intensity shown in (B), corresponding work rate = 90 W, both heart rate HR and V s markedly increased, while V d only slightly changed. V s values under moderate exercise were about 1.3 times larger than those under the resting condition. The HR values in the moderate exercise were found to be about 1.4 times larger than those at rest.
At vigorous exercise with a work rate of about 210 W, as indicated in Fig. 2c, V s further increased to about 120 cm/s. V d also increased considerably from 35 cm/s at rest to 60 cm/s. It should be noted that in the cooling down (recovery phase), the corresponding work rate = about 170 W, the average value of V s was 110 cm/s while keeping the speed under vigorous exercise. On the other hand, V d was significantly decreased to about 45 cm/s. Consequently, V s minus V d, called pulse velocity, was extremely large in recovery when compared with vigorous exercise.
3.2 Change in MCA velocity during exercise
Figure 3 shows the obtained V d, V s, and mean velocities V m averaged over one cardiac cycle, which were calculated by the equation,
Here, V i is velocity at time t i , and n number of measured velocity data for one cardiac cycle. Each data point in Fig. 3 represents the average for the 12 subjects.
Peak velocity V s and mean velocity V m rose immediately after onset of exercise. In the early stages of exercise (1–3 min), the corresponding work rate of about 50–90 W, V s rapidly increased as the degree of exercise intensity increased. It should also be noted that V m increased during exercise. The end-diastolic velocity V d showed a minor change.
Under vigorous exercise (5–8 min), the corresponding work rate of about 110–190 W, V s tended to keep a constant value despite the large increase in the exercise rate. Both V d and V m showed a decreasing trend as the work rate was increased.
In the cooling down stage (8–11 min), the three velocities simultaneously decreased, but the rate of velocity change differed. During the last 3 min (rest period), V s values were still on the decrease, while V m and V d seemingly bottomed out.
3.3 Fluctuations in MCA velocity
To see the variations in velocity due to exercise, we calculated the velocity fluctuation index (VF) for measured velocity waveforms, which is defined as,
Here, V i is velocity at time t i , V m mean velocity averaged over one cardiac cycle, and n number of measured velocity data for one cardiac cycle. This index corresponds to the standard deviation in statistics.
Figure 4 shows the VF values averaged for the 12 subjects. As can be seen, the apparent trend of VF was quite similar to that of systolic velocity V s shown in Fig. 3. It should however be noted that during vigorous exercise, VF values continued to increase as exercise intensity increased, which is in contrast to V s in Fig. 3 showing almost no change. It was also noted that during the cooling down, VF was almost constant. This is in contrast to the V s, showing a decreasing trend as noted in Fig. 3. These features of VF can be understood by the definition (Eq. 3), where velocity difference (V i − V m) is the main factor to determine VF.
3.4 Arterial blood pressure
Systemic arterial blood pressures were intermittently measured in the brachial artery. The systolic arterial pressure SAP and diastolic arterial pressure (DAP) averaged for the 12 subjects are graphically represented in Fig. 5.
SAP values rose immediately after the start of exercise, and increased constantly as the exercise intensity increased up to the end of the exercise. In contrast, the diastolic pressure, DAP, showed no statistically significant change during the mild and moderate intensities (up to 4 min). During vigorous exercise, the DAP slightly increased. During cooling down and following rest, the DAP values were smaller than at the initial rest.
3.5 Cerebral blood flow regulation
Although blood flow to the brain is governed by many physical factors, it is fundamental and useful to discuss the flow characteristics using an equation like Ohm’s law. Cerebral blood flow (CBF) can be described by two groups of factors: those affecting the cerebral perfusion pressure (CPP) and those affecting cerebrovascular resistance (CVR) [3].
Here, CPP is calculated as the difference between mean arterial pressure (MAP) and central venous pressure, which could be approximated by intracranial pressure (ICP) (surrounding pressure within the skull).
Any changes in either the MAP or ICP can affect CPP and hence CBF. CPP is known to be approximately 80 mmHg in healthy adults. An increase in CPP is considered to be a result of an increase in MAP [3].
In Figs. 3 and 5, one should note that under vigorous exercise, the mean velocity V m showed a slight decreasing trend, while both systolic and diastolic pressures increased, indicating an increase in CPP. V m is known to be proportional to CBF, since the radius of MCA during exercise usually remains constant [2]. Consequently, the flow resistances in the brain CVR appear to be considerably increased during vigorous exercise.
3.6 Resistance index
The resistance index RI has been introduced to ultrasound Doppler sonography to characterize the peripheral impedance in organ circulation. RI is defined with following equation,
For the normal common carotid artery, RI is reported to be between 0.55 and 0.75. In the arteries that supply high-resistance musculocutaneous beds, RI is known to be greater than 0.75 [3].
The obtained values of RI for each step of experiment are summarized in Table 1. As can be noted, RI values vary increasingly from 0.54 to 0.62 as exercise intensity increased. It is remarkable that RI values during cooling down and following 3-min rest are greater than those during exercise. These features are understood from the change in velocity waveform shown in Fig. 2, where we can note that in the recovery phase, the average value of V s was 110 cm/s with keeping the speed under vigorous exercise. On the other hand, V d was significantly decreased. Consequently, V s minus V d in Eq. (6) becomes markedly large in recovery when compared with vigorous exercise.
The RI is not only a function of flow resistance, but also influenced by vascular compliance [3]. The waveforms of older subjects are characterized by high RI values and steep decrease in velocity in diastole. These changes are considered to result from increased flow resistance due to cerebral atherosclerosis and decreased compliance of vessel walls [3]. The present result disclosed that changes in MCA velocities during rigorous exercise and following cooling down were similar to those due to aging. Moreover, such influence continues more than 3 min after cooling down, indicating that the recovery phase after rigorous exercises should be programmed with considering not only conditions of the heart (heart rate) but also circulatory disturbance occurring in the brain.
4 Conclusion
The velocity waveforms in the middle cerebral artery in young volunteers were measured under exercise across wide intensities and also following a recovery phase. The peak-systolic velocity V s and the end-diastolic velocity V d were obtained from measured waveforms. Calculated hemodynamic variables, velocity fluctuation VF, and resistance index RI showed marked increases under exercise. The RI values in cooling down were markedly higher than in exercise. This fact is first found in the present study and indicates that the cerebral blood flow regulation in exercise is more complicated than previously considered.
References
Aaslid R, Lindegaard KF et al (1989) Cerebral auto-regulation dynamics in humans. Stroke 20:45–52
Willie CK et al (2011) Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. Neurosci Methods 196:221–237
von Reuturn G-M, von Buedingn HJ (1993) Ultrasound diagnosis of cerebrovascular disease, Georg-Thieme Verlag Stuttgart, New York
Hennerici MG, Stephen PM (2001) Cerebrovascular ultrasound: theory, practice and future developments. Cambridge Univ. Press, New York
Willie CK, Ainslie PN et al (2013) Maintained cerebro-vascular function during post-exercise hypotension. Eur J Appl Physiol 113:1597–1604
Watanabe S, Matsuo T et al (2009) Comparison of blood flow velocity in the middle cerebral artery between men and women at rest and during exercise. Ther Res 30:537–544
Matsuo T, Watanabe S, Sorimachi M et al (2011) Blood flow velocity waveforms in the middle cerebral artery at rest and during exercise. Artf Life Robot 16:398–402
Ogoh S, Ainslie P et al (2009) Cerebral blood flow during exercise: mechanism of regulation. J Appl Physiol 107:1370–1380
Pocock G, Richard CD (2006) Human physiology, 3 rd edn. Oxford Univ. Press, translated into Japanese by Okano E et al. (2009), Maruzen, Tokyo
Majima E (1986) Seirigaku, 18th edn., Bunkoudou Tokyo (in Japanese)
Acknowledgments
This study was supported in part by JSPS KAKENHI Grant Number 245000479, and a research fund from Kanagawa Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Matsuo, T., Watanabe, Si., Sorimachi, M. et al. Blood flow velocity waveforms in the middle cerebral artery during cycle exercise and recovery. Artif Life Robotics 20, 336–340 (2015). https://doi.org/10.1007/s10015-015-0235-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10015-015-0235-8