Abstract
The hydrophobic conductive polymer, poly(3-octylthiophene) (POT), is considered as uniquely suited to be used as an ion-to-electron transducer in solid contact (SC) ion-selective electrodes (ISEs). However, the reports on the performance characteristics of POT-based SC ISEs are quite conflicting. In this study, the potential sources of the contradicting results on the ambiguous drift and poor potential reproducibility of POT-based ISEs are compiled, and different approaches to minimize the drift and the differences in the standard potentials of POT-based SC ISEs are shown. To set the potential of the POT film, it has been loaded with a 7,7,8,8-tetracyanoquinodimethane (TCNQ/TCNQ·−) redox couple. An approximately 1:1 TCNQ/TCNQ·−ratio in the POT film has been achieved through potentiostatic control of the potential of the redox couple-loaded conductive polymer. It is hypothesized that once the POT film has a stable, highly reproducible redox potential, it will provide similarly stable and reproducible interfacial potentials between the POT film and the electron-conducting substrate and result in SC ISEs with excellent reproducibility and potential stability. Towards this goal, the potentials of Au, GC, and Pt electrodes with drop-cast POT film coatings were recorded in KCl solutions as a function of time. Some of the POT films were loaded with TCNQ and coated with a K+-selective membrane. The improvement in the potential stabilities and sensor-to-sensor reproducibility as a consequence of the incorporation of TCNQ in the POT film and the potentiostatic control of the TCNQ/TCNQ·−ratio is reported.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
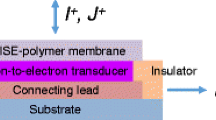
Ion-selective electrodes (ISEs), one of the most successful chemical sensors, are used in many applications including the analysis of clinical and environmental samples [1]. In certain applications, however, conventional ISEs fall short. For example, the measurement of ion activities in very small sample volumes is challenging with conventional macro electrodes. In miniaturized ISEs, the inner filling solution (IFS) of the electrode is eliminated and the ion-selective membrane (ISM) is layered directly over a solid substrate (coated wire electrodes) [2]. The elimination of the IFS permitted the utilization of thin and thick film microfabrication technologies for ISE fabrication [3]. Unfortunately, the apparently minor deviation from the conventional electrode construction (symmetrical with solution contact on both sides of the ISM vs. asymmetrical with solution contact on one side but solid contact on the other side of the ISM) resulted in poor potential stability and sensor-to-sensor potential reproducibility and has been the topic of intensive research in the last more than 40 years [4–6]. In attempts to overcome these limitations while still retaining the SC sensor structure, the surface of the electron-conducting substrate (generally a noble metal, e.g., Au, Pt, or glassy carbon (GC)) has been coated with various materials including self-assembled monolayers (SAMs) of lipophilic redox-active compounds [7], organic compounds [8], conductive polymers (CPs), or some special carbon materials with high double layer capacitance [9]. These materials have been implemented into the SC ISE by layering them between the electron conductive substrate and the ISM. The role of these layers, with generally both ion and electron conduction properties, is to control (stabilize) the interfacial potentials between the electron conductive substrate and the ISE membrane [5].
Several of these approaches have shown very promising results and lead to SC ISEs with improved detection limits [10–14], good potential stability [7, 9, 10, 12, 14–18], and short equilibration time [19]. However, the favorable properties of some of these SC ISEs may be gradually lost if the material sandwiched between the substrate electrode and ion-selective membrane becomes hydrated [20] or with the formation of a thin aqueous film [21] between the ISE membrane and its solid contact. The presence of a water layer between the SC and the ISM may lead to potential instabilities due to changes in the composition of the aqueous layer related to minor ionic fluxes and/or transport of water or other neutral species (CO2, NH3, small organic molecules) across the sensing membrane. Consequently, beyond redox, ion-to-electron transduction properties, and large double layer capacitance, the hydrophobicity of the SC is of utmost importance [15, 22]. The hydrophobicity and the good adhesion properties of the materials implemented between the substrate electrode and the sensing membrane prevent the delamination of the ISM from its SC and deter the formation of a water layer between the two as it has been repeatedly shown [10, 22–26].
Among the most commonly used CPs in SC ISEs, poly(3-octylthiophene) (POT) is the most hydrophobic and due to its excellent adhesion properties, it is considered as uniquely suited to be used in combination with plasticized polymer-based ion-selective membranes. POT is recommended as ion-to-electron transducer because the POT-POTn+ redox couple is assumed to enhance potential stability and reproducibility. However, the reports on the performance characteristics of POT-based SC ISEs are quite conflicting [10, 14] and the search for an optimal hydrophobic ion-to-electron transducer is still active. Most recently, Lindfors suggested the utilization of polyazulenes as a novel SC for ISEs [15].
In this study, we explore the potential sources of the conflicting reports on POT-based SC ISEs and show different approaches to minimize the potential drift and improve the sensor-to-sensor reproducibility of the standard potentials of POT-based SC ISEs. Among the variety of performance characteristics of ISEs, we focus on the potential drift and the reproducibility of the standard potentials of the electrodes because in our view these two sensor characteristics are directly influenced by the properties of the CP as well as the interfaces between the CP and the electron-conducting substrate and the membrane. The conflicting data published in the literature may originate from differences in the (i) POT film itself, (ii) substrate electrode, (iii) ion-selective membrane, and can be the consequence of an unrecognized partial or complete delamination of the ISM from its POT contact due to an aqueous film formation between the ISM and its POT contact. Although some of the reported results may have been distorted by the presence of an aqueous layer, its potential influence on the reported data will not be considered in our overview since some of the data were published before a simple test to trace the formation of an aqueous layer became available [21] or because in the cited papers, this possibility was not even considered.
Drop-cast vs. electrochemically deposited POT
POT can be deposited over the electron conductive substrate by drop-casting or electrochemically [27]. Drop-cast POT [28] is said to be in its undoped or neutral form. However, as it has been shown by FTIR-ATR spectroscopy, the doping level in the commercially available POT for drop-casting (average molecular weight ~34 kD) is not zero. FTIR-ATR spectra of POT films drop-cast over Pt-sputtered ZnSe crystals show bands of both the neutral form of POT and doping-induced bands. The undoped form has low conductivity (1 × 10−6 S/cm) [22] while the reversibly oxidized doped form has high conductivity (0.4 S/cm) [27]. Lindfors reported poor day-to-day reproducibility and positively drifting potentials for drop-cast POT-coated GC electrodes (without ISM) in 0.1 M KCl [29] but negatively drifting potentials in 10−3 M CaCl2 [22]. The positive and negative drifts were interpreted as a consequence of the gradual oxidation or reduction of the POT film, respectively. However, there were significant differences in the experimental conditions, e.g., (i) in the material and surface area of the substrate electrodes, (ii) in the thicknesses of the POT films, (iii) in the composition of the solutions in contact of the POT-coated electrodes during the potential stability measurements, and (iv) in the illumination during the measurements, all of which could also contribute to the inconsistencies in the stability of the POT-based electrodes.
SC ISEs fabricated with POT as an internal solid contact do not show oxygen sensitivity [13, 25]. However, it is well known that POT is light sensitive, which significantly limits its application [29]. In addition, it appears that the light sensitivity is different for the drop-cast and electrochemically deposited POT films [29]. However, it is not clear how much the light sensitivity of POT contributed to the drift values reported for POT-based electrodes. It is also not clear how the drift relates to (i) the conductivity and the redox state of the polymer, (ii) the molecular mass of the drop-cast POT, e.g., 25 vs. 34 kD (Aldrich catalog numbers—682799 and 445711), and (iii) the conditions of the electrochemical deposition (galvanostatic vs. cyclic voltammetry) or the incorporated counter ions [30] during the electrochemical deposition.
In summary, after more than 20 years of the pioneering works of Bobacka et al. [31], due to the differences in the experimental conditions in the diverse studies, it is still not clear how to build robust POT-based SC ISEs with low drift and highly reproducible standard potentials. In other words, the main sources of the inconsistencies in the reported results and the main hurdles towards addressing these inconsistencies remain unclear.
POT-based ISEs on different substrate electrodes
The significance of the substrate electrode is generally not considered as a factor influencing the performance of SC ISEs. Although in most of the studies on SC ISEs with POT as solid contact glassy carbon (GC) is used as a substrate electrode, graphite rods [16], gold disks [13], screen-printed gold over silver [32], platinum sputtered ZnSe [12], and gold sputtered copper [10] have also been used as electrode substrates. In studying the water uptake, impedance characteristics, and potential stability of SC Ca2+ ISEs with POT as SC, Lindfors used Pt as substrate electrode sputtered over a ZnSe crystal for the hyphenated FTIR-ATR/impedance spectroscopy experiments but GC electrodes for the potentiometric measurements [12]. In our view, the selection of the substrate electrode may be significant. It is expected to influence the standard potential and the short and long-term stability (drift) of SC ISEs. In a recent study, we showed that the equilibration time of solid contact sodium, potassium, and hydrogen ion-selective electrodes with poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) as solid contact is significantly shorter with Au and GC substrates compared to Pt as substrate electrode. The difference in the equilibration times has been traced back to the substrate electrode|PEDOT:PSS interface [19].
POT-based ISEs with different ion-selective membranes
POT-based SC ISEs were tested for the measurement of different ions (K+ [10, 15], Ca2+ [10–13, 25], Pb2+ [10, 13, 17], Ag+ and I− [10], Cl− [18, 32], nitrate [16], and carbonate [14]) using a variety of sensing membranes [polyvinyl chloride (PVC) [11, 14–18, 25, 32], silicone rubber (SR) [12], polyurethane (PU) [25, 32], and poly (methyl metacrylate)/poly (decyl methacrylate) (PMMA/PDMA) [10, 13]]. In other studies, the POT film loaded with an ionophore and ion-exchange sites served as the ion-selective membrane [33, 34] or POT was dissolved in the ion-selective membrane cocktail [35] which was deposited over an electron-conducting substrate (termed as single-piece ISEs) [33–35]. The drift values recorded with these electrodes range between −2.9 mV/h (in combination with a Cl−-selective PVC membrane) and ~+4.0 mV/h with a carbonate-selective PVC membrane [14] and almost any value between. For example, 0.0 mV/h drift was reported for POT-based silver ISEs with a PMMA/PDMA membrane in 10−3 M Ag+ ion solution [10]. Unfortunately, a survey of the drift data does not help to trace the source of the recorded drift values, which are not always monotonous and individual electrodes from the same batch may drift in opposite directions [16].
The strategies to manufacture SC ISEs with highly reproducible standard potentials cannot be separated from the efforts to improve their potential stability. Both aim towards the development of robust, maintenance-free sensors, which depending on the task can be used without calibration or need only infrequent single point calibrations. In general, the sensor-to-sensor reproducibility of SC ISEs is significantly worse than those of their liquid contact counterparts. For example, a POT-based SC Ca2+ ISE in combination with a SR membrane had reported sensor-to-sensor reproducibility of ±6.7 mV [22]. But, the standard deviation of the potential values measured in the same solution with a POT-based Ca2+ ISEs using a plasticized PVC membrane (n = 3) was only ±1.0 mV [11]. On the other hand, one should interpret results based on the n = 3 data with caution.
To achieve a sensor-to-sensor reproducibility similar to the conventional ISEs, different redox couples/centers were implemented into the CP layer [36, 37], the ion-selective membrane [38, 39], or applied onto the electron-conducting substrate [7]. However, none of these experiments used POT-based SC ISEs as the model system. Gyurcsanyi [37] and Sutter [36] fabricated K+ and Pb2+-selective electrodes with electrochemically deposited polypyrrole as an inner contact loaded with K4[Fe(CN)6] / K3[Fe(CN)6] as redox couple and achieved drift values less than 1 mV/day. Zou implemented Co(III)/Co(II) into the ion-selective membrane of an electrode with a self-assembled monolayer of 1-hexanethiol as the inner contact layer and achieved ±0.7 mV sensor-to-sensor reproducibility after 1 hour of solution contact [39]. Unfortunately, however, the impressive reproducibility was lost after 24 h and the standard deviation of the recorded potentials increased to ±16.3 mV. Vanamo and Bobacka suggested setting the standard potential of PEDOT:PSS-based ISEs electrochemically by short-circuiting the fully fabricated ISEs with one another or with a reference electrode [40]. The method of Vanamo provided SC ISEs with very similar standard potentials. However, with time, the standard deviation of the potential values increased as the potentials of the disconnected electrodes gradually drifted back towards their original values.
In this study, the ambiguous drift and poor potential reproducibility of POT-based ISEs are addressed. In our view, the key is to control the interfacial potentials between the electron-conducting substrate and the conductive polymer as well as between the conductive polymer and ISM. This requires a POT film with a well-defined redox potential (a constant POT/POTn+ ratio). To set the potential of the POT film, it has been loaded with a 7,7,8,8-tetracyanoquinodimethane (TCNQ/TCNQ·−) redox couple. An approximately 1:1 TCNQ/TCNQ·−ratio in the POT film has been achieved through potentiostatic control of the potential of the redox couple-loaded conductive polymer. It is hypothesized that once the POT film has a stable, highly reproducible redox potential, it will provide similarly stable and reproducible interfacial potentials between the POT film and the electron-conducting substrate and result in SC ISEs with improved potential stability and reproducibility. Towards this goal, the potentials of Au, GC, and Pt electrodes with drop-cast POT film coatings were recorded in KCl solutions as function of time. Some of the POT films were loaded with TCNQ and coated with a K+-selective membrane. The improvement in the potential stabilities and sensor-to-sensor reproducibility as a consequence of the incorporation of TCNQ in the POT film and the potentiostatic control of the TCNQ/TCNQ·−ratio is reported.
Experimental
Chemicals
Poly(vinyl chloride) (PVC, high molecular weight), bis(2-ethylhexyl) sebacate (DOS), potassium tetrakis(p-chlorophenyl)borate (KTpClPB), potassium ionophore I (Valinomycin), 7,7,8,8-tetracyanoquinodimethane (TCNQ), and poly(3-octylthiophene-2,5-diyl) (POT) (Catalog number 445711) were purchased from Sigma Aldrich. Tetrahydrofuran (THF) and chloroform were products of Fisher Scientific. The aqueous solutions were prepared with 18.2 MΩ·cm resistivity deionized (DI) water from Millipore Milli-Q A10 system. The potentiometric responses of the solid contact electrodes were tested using potassium chloride (KCl) standard solutions prepared by serial dilutions. KCl was purchased from Fisher Scientific. Argon and oxygen were obtained from Airgas (Airgas Inc., Radnor, PA, USA).
Electrodes and fabrication
The Au and Pt disk electrodes (Bioanalytical System, Inc. U.S.A.) (d = 1.6 mm, outer body d = 6.4 mm) and Au and GC disk electrodes (MINERAL, Warsaw, Poland) (d = 1.0 mm, outer body d = 6.3 mm) were polished with aqueous dispersions of alumina (1.0 μm (Buehler, Lake Bluff, IL) and 0.3 μm (Electron Microscopy Sciences, Hatfield, PA)) on microfiber polishing cloths. Between polishing steps, the electrodes were rinsed with DI water. Following the final polishing step, the electrodes were cleaned by sonication in DI water for 20 min then rinsed with DI water. The POT-coated Au, GC, and Pt electrodes were fabricated by drop-casting 0.94 or 0.42 μL of 10 mg/mL solutions of POT in chloroform onto the 1.6- or 1.0-mm diameter electrode substrates, respectively (a volume ratio of 2.25 instead of 2.56 corresponding to the areal ratio). The same volumes were used for the deposition of the TCNQ-loaded POT films; however, for this deposition, THF has been selected as solvent with 10 mg/mL POT and 5 mg/mL TCNQ concentrations because larger amounts of TCNQ could be dissolved in THF than in chloroform. The areas of the drop-cast POT and TCNQ-loaded POT films were always significantly larger (between 2.2 and 3.0 times) than the areas of the conducting substrates to ensure full coverage. Due to the uncertainty in the deposited areas (a consequence of the manual drop-casting), the thicknesses of the POT films also varied in a wide range (~1.5 ± 0.6 μm). Consequently, the ~13 % thinner films deposited over the 1.6-mm diameter electrodes (based on the 2.25 volume ratio) did not induce any measurable difference in the results. The electrodes were left to dry for at least 2 h prior to the ion-selective membrane deposition over the POT or POT + TCNQ films to create fully fabricated SC ISEs. The POT-coated or POT + TCNQ-coated electrodes were left to dry overnight in a desiccator if they were tested without an ISM coating.
Membrane cocktails
Potassium-selective SC ISEs were fabricated by drop-casting a K+ ion-selective membrane cocktail over the POT-coated or POT + TCNQ-coated electrode surfaces. The composition of the membrane was 100 mg PVC, 200 mg DOS, 6.1 mg Valinomycin, and 1.3 mg KTpClPB in 1.5 mL THF. From this membrane cocktail, 11 or 5 μL were dispensed over the 1.6- and 1.0-mm diameter electrode surfaces, respectively. The ion-selective membrane cocktail was always spread beyond the borderlines of the drop-cast POT or POT + TCNQ films, i.e., the ISM covered the entire POT or POT + TCNQ film as well as a fraction of the insulating electrode body (~5 mm diameter circular area) to ensure proper adhesion of the ISM. The fully fabricated SC ISEs were left to dry overnight in a desiccator over silica orange beads exposed to dimmed room light.
Methods
Potential stability measurements
The potentials of the electrodes coated with POT and POT + TCNQ (without an ISM) were tested in air-saturated, argon-saturated, and oxygen-saturated 0.1 M KCl solutions over 24 h vs. an Orion Ag|AgCl double junction reference electrode (Model 900200) with 1 M lithium acetate (LiOAc) as salt bridge electrolyte using a 16-channel data acquisition system (Lawson Labs Inc., Malvern, PA), which was connected to a computer equipped with L-EMF Suite 2.0 software. The potential recording started immediately after the electrodes were immersed in the solution. The fully fabricated SC ISEs were tested the same way but only in air-saturated solutions, i.e., the potentials of the completely dry electrodes (just removed from the desiccator) were recorded for 24 h during their equilibration in 0.1 M KCl solution. The potential recording started immediately after the electrodes were immersed in the solution. After the potential stability measurements, the potentiometric response of the ISEs was tested using 10−1 M, 10−2 M, and 10−3 M KCl solutions. When these experiments were performed under regular laboratory lighting (~242 nW measured with Newport 1830–C optical power meter equipped with a Model 818-SL detector), the reproducibility of the potential measurements and consequently, the reproducibility of the parameters of the calibration curves were unacceptable. However, when the measurements were performed in dimmed light in a dark room (26 nW), the reproducibility of the potential measurements significantly improved and the calibration curves lead to Nernstian responses.
Electrode polarization
To minimize the drift of the fully fabricated K+ ISEs with POT or TCNQ-loaded POT (POT + TCNQ) as solid contact, the electrodes were polarized to a predetermined potential value in one or successive steps in a 0.1 M KCl-filled beaker in which the SC ISEs were used as the working electrode, a coiled Pt wire served as counter electrode, and an Orion double junction Ag|AgCl reference electrode completed the cell. The electrodes were polarized for 60 min using an Autolab (Metrohm, Utrecht, The Netherlands) potentiostat/galvanostat running general purpose electrochemical system (GPES) 4.9 software. The potential values for polarization were selected by successive approximation. The first polarization potential was selected based on the “stabilized” potential values measured at the end of the potential stability tests with the POT + TCNQ-based ISEs also considering the standard redox potential of the TCNQ/TCNQ·−redox couple. After 60-min polarization periods, the electrodes were disconnected from the potentiostat, rinsed with DI water, dried with argon for 2 min, and placed in an argon-filled desiccator over silica orange beads exposed to dimmed room light overnight. The next day, the completely dried, previously polarized electrodes were placed again into 0.1 M KCl for determining their potential stability. If the electrode during this second (after polarization) potential stability test still had a negative potential drift, a second polarization potential was applied at a lower potential value. The adjusted polarization potential was also applied to freshly prepared SC ISEs.
Results and discussion
Potential stability of Au, Pt, and GC electrodes with POT and TCNQ-loaded POT (POT + TCNQ) coatings
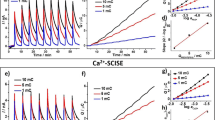
In Fig. 1, the drift values recorded with POT- and POT + TCNQ-coated Au, GC, and Pt electrodes are summarized in a box plot format. The measurements were performed in air, oxygen, and argon-saturated 0.1 M KCl solution for 24 h to study the influence of the substrate electrode (Au, Pt, and GC) and the incorporation of the TCNQ/TCNQ·−redox couple into the POT film on the potential stability of the electrodes. The drift values (mV/h) were calculated from the potential data collected during the last 14 h of the experiment (between 10 and 24 h).
Box plots representing drift values of a POT-coated and b POT + TCNQ-coated Au, GC, and Pt electrodes in air-, argon-, and O2-saturated 0.1 M KCl. The drift values were calculated from the potential data collected between 10 and 24 h of solution contact. The vertical axis of the box plot is the response variable (drift) and the horizontal axis is the factor of interest (different substrate electrodes with coatings in different environments). The top and bottom whiskers represent the maximum and minimum values within the respective category while the box represents the middle 50 % of the data. The horizontal line within each of the individual boxes represents the median value of the data
The POT-coated Au and GC electrodes have large drift values and the values are scattered in a wide range, but apparently, the potential drift is not influenced by the oxygen concentration of the solution (the drift values do not show any trend between the O2, argon, and air-saturated KCl solutions). These results are in agreement with earlier findings [13, 25] that the ambiguous drift and poor potential reproducibility of POT-based ISEs are not related to gradual oxidation of the POT film by dissolved oxygen. Among the POT-coated electrodes, those prepared on Pt as substrate electrode had relatively small drift and the standard deviations of the drift data were also small compared to the other electrodes. The significantly smaller drift values recorded with the POT-coated Pt electrode compared to POT-coated Au and GC electrodes imply that the substrate electrode|POT interface plays an important role in the potential stability. Until recently, the substrate electrode|conductive polymer interface was not considered critical in the design of SC ISEs with short equilibration times and high potential stability [19].
As it is shown in Fig. 1b, the incorporation of TCNQ into the POT film resulted in electrodes with significantly smaller drift in 0.1 M KCl compared to electrodes coated only with POT. The improvements are most significant with the POT + TCNQ-coated Au and GC electrodes. To demonstrate the magnitude of the improvement in the recorded drift, in Fig. 2 we show two representatives of potential-time transients recorded with a POT-coated and a POT + TCNQ-coated Au electrode in air-saturated 0.1 M KCl.
As it is shown in Figs. 1 and 2, the POT-coated Au electrodes have large negative drift in the first 24 h. The negative drift indicates that the POT film becomes gradually reduced (converts further to its undoped, low conductivity form) in the aqueous KCl solution. In contrast, the drift of the POT + TCNQ-coated electrodes is reduced to approximately 1 mV/h which suggests that the incorporation of TCNQ into the POT film defines its potential (prevents the gradual reduction of the film over time) and fixes the interfacial potential between the substrate electrode and the POT film.
Potassium ion-selective electrodes with POT and TCNQ-loaded POT as solid contact
For this part of the study, the POT- or POT + TCNQ-coated Au and Pt electrodes were covered with K+ ISMs and left to dry overnight in a desiccator. The following day, the potential vs. time transients of these fully fabricated ISEs were recorded in 0.1 M KCl for 24 h and the drift values (mV/h) between 10 and 24 h were assessed. Next, the electrodes were polarized for 60 min to a predetermined potential value, removed from the solution, rinsed, dried with argon and kept in a desiccator overnight. The next day the potential stability measurement was repeated.
For example, since at the end of the initial equilibration (after 24 h) the potentials of the POT + TCNQ K+ ISEs approached ~+290 mV, they were first polarized to 285 mV (an extrapolated potential value based on the stabilized potential recorded after 24 h). After a 60 min polarization, the electrodes were removed from the solution, rinsed, and dried overnight, and the potential stability measurements were repeated. The electrodes following this first polarization always had smaller drifts but the ISEs were still drifting negatively. So, using the protocol of successive approximation, the polarization of the electrodes was repeated with polarization potentials of 125 and 0 mV. In other experiments, freshly prepared electrodes were polarized to these adjusted potential values (125 and 0 mV) upon their first exposure to 0.1 M KCl solution then dried overnight before their potential stability was tested. The goal of polarizing the electrodes to different potential values was to set the electrode potential close to the standard redox potential of the TCNQ/TCNQ·−couple where the concentration ratio of the oxidized and reduced forms are close to an optimal 1:1 ratio. The role of the TCNQ/TCNQ·−couple is to act as a redox potential buffer in the SC POT layer and minimize the potential drift so that the optimal 1:1 ratio would allow for maximum buffer capacities. During polarization of the TCNQ-loaded films, since the redox potential of TCNQ is lower than the redox potential of POT, the majority of the current is reducing TCNQ and changes its [ox]/[red] ratio rather than reducing POT. Conversely, during the polarization of POT ISEs without TCNQ, the POT film is further reduced to a state of low conductance. However, since potentiometric measurements are made at “zero current condition,” minimal conductance should be adequate to act as an ion-to-electron transducer. The drift values of the electrodes in 0.1 M KCl without polarization (N/A) and with polarization to different potentials are summarized in Fig. 3.
As it is seen in Fig. 3, despite the expectation based on Fig. 1, the drift of the K+ ISEs with POT and POT + TCNQ as solid contact without polarization are basically the same around −1.4 mV/h. The polarization of the fully fabricated ISEs with POT as solid contact did not result in improvement of the drift values. Only the direction of the drift changed upon polarization. The drift with POT-based ISEs on Au and Pt (Au, Pt|POT|ISM) without polarization was negative but after polarization (60 min at 125 mV), the drift was positive. The change of the sign (the direction) of the drift suggests that with adequate polarization ~0 mV/h drift may be achieved.
In contrast to the electrodes with POT as solid contact, the polarization of the electrodes with POT + TCNQ as solid contact resulted in significant improvements. The drift of the K+ ISEs both on Au and Pt substrate dropped to ~−0.1 mV/h when polarized to 0 mV (Au) or 125 mV (Au and Pt). The standard redox potential of TCNQ/TCNQ·−redox couple in the POT film \( \left({E}_{{\mathrm{TCNQ}/\mathrm{TCNQ}}^{\cdot -}}^o\right) \)has been estimated as ~112 mV from the midpoint potential (the average of the oxidation and reduction peak potentials) of cyclic voltammograms recorded with the POT + TCNQ-coated Au electrodes in 0.1 M KCl. We considered the \( {E}_{\mathrm{TCNQ}/{\mathrm{TCNQ}}^{\cdot -}}^o \)~112 mV only as an estimate due to the large peak separation between the oxidation and reduction peaks (~318 mV) and because it is significantly more positive than the standard potentials reported for the TCNQ/TCNQ·−couple based on cyclic voltammograms recorded in acetonitrile [41]. The very similar low drift values observed following the polarizations of the electrodes with POT + TCNQ as solid contact to 125 or 0 mV suggest that the standard potential of the TCNQ/TCNQ·−couple in the POT film may be between these two values.
In Fig. 4, we show representative examples of the potential-time transients recorded with SC K+ ISEs with POT and POT + TCNQ as solid contacts without (N/A) and with polarizations to different potential values (285, 125, and 0 mV).
In summary, the implementation of TCNQ into the POT film along with polarization improved the potential stability of the SC ISEs built on both Pt and Au substrates. After ~5 h of solution contact, the drift of the polarized POT + TCNQ-based ISEs dropped below 0.1 mV/h. Apparently, the incorporation of a redox couple into the POT film poises the potential of the POT film and the metal|POT interfacial potential. However, the drift could not yet be reduced below −0.1 mV/h by fine-tuning the polarization potential, i.e., the TCNQ/TCNQ·−ratio in the POT film. It is assumed that for further reduction of the potential drift of the electrodes with POT-based SC, the POT|ISM interfacial potential needs to be better controlled. This may be achieved through the incorporation of the appropriate salt of TCNQ into the POT film, e.g., K+TCNQ− in combination with a K+ ion-selective membrane [8].
Based on previous reports [40] and our successes in minimizing the potential drifts of POT-based solid contact electrodes, we assumed that the polarization of the electrodes to predefined potential values would also improve the sensor-to-sensor potential reproducibility of POT-based SC ISEs. In Fig. 5, the potential values recorded after 24-h “conditioning” (measurement of the potential drifts) are shown for POT- and POT + TCNQ-based K+ ISEs without polarization and with 60-min polarization to 125 mV. As it is seen in Fig. 5, the standard deviation of the mean of the measured potentials is very large for the POT-based ISEs without polarization (±36 mV). Although the incorporation of TCNQ into the POT film and the polarization of the electrodes slightly improved the sensor-to-sensor reproducibility of the potential data, the standard deviation of the mean potential values remained quite large. The smallest standard deviation of the potential data (±6 mV) was measured within a single batch of POT + TCNQ-based ISEs (n = 3) following polarization to 125 mV. Similar to our conclusion on the optimization of the potential drift of POT-based SC ISEs for SC ISEs with identical standard potentials, all potential terms contributing to the standard potential of the SC ISEs must be well-defined: (i) substrate electrode/POT interfacial potential; (ii) standard potential of the POT film, i.e., constant POT/POTn+ ratio in the film; and (iii) POT|ISM interfacial potential. The incorporation of TCNQ into the POT film provides well-defined potentials for the POT film and for the substrate electrode|POT interface. However, a well-defined interfacial potential between the POT film and the ISM requires ion-exchange with high exchange current density. In our view, this could be achieved through the incorporation of the appropriate salt of TCNQ into the POT film. We plan to explore this possibility in our future experiments [8].
Box plots of the potential values of K+ ISEs on Au substrate with POT and POT + TCNQ as solid contact after 24-h exposure to 0.1 M KCl solution. POT- and POT + TCNQ-based K+ ISEs are shown without polarization and with polarization at 125 mV. The vertical axis of the box plot is the response variable (potential values recorded after 24-h “conditioning”) and the horizontal axis is the factor of interest (POT- and POT + TCNQ-coated electrodes without and with polarization). The top and bottom whiskers represent the maximum and minimum values within the respective category while the box represents the middle 50 % of the data. The horizontal line within each of the individual boxes represents the median value of the data
Conclusion
Poly(3-octylthiophene) has unique material characteristics to be used as an ion-to-electron transducer in solid contact ISEs. Despite its promising inherent features, the literature on POT-based ISEs is quite contradictory. To trace some of the contradictions, we cataloged the differences in the published works and implemented a redox couple, TCNQ/TCNQ·−, into the POT film of K+-selective SC ISEs. The incorporation of TCNQ along with polarization of the SC ISE improved the potential stability from −1.4 mV/h to −0.1 mV/h, in the range of the smallest drifts published with POT-based SC ISEs. The polarization of the K+ ISEs allowed setting the TCNQ/TCNQ·−ratio close to 1:1 in the POT film. It is believed that the TCNQ/TCNQ·−couple poises the potential of the POT film and substrate|POT interfacial potential which, in summary, lead to the improved potential stability. Since during polarization the drop-cast POT, which is in the undoped, electrically non-conductive form of the polymer, is forced to an even less conductive state, one might ask how long it can act as an ion-to-electron transducer and at which conductance does it become a non-conductive matrix for TCNQ.
Our data suggest that the material of the electron-conducting substrate (therefore, the interfacial potential between the metal and the CP) may have a significant influence on the overall potential stability of SC ISEs. However, once the CP is compounded with a redox couple, differences in the potential stability of SC ISEs built on different substrates decreased.
The incorporation of TCNQ into the POT film and the polarization of the ISEs hardly improved the sensor-to-sensor potential reproducibility. In our view, to optimize both the potential stability and the sensor-to-sensor potential reproducibility, the potential determining primary ion also needs to be present in the POT film for a reproducible POT/ISM interfacial potential. This possibility will be explored through the incorporation of TCNQ as a salt (e.g., K+TCNQ−) into the POT film.
References
Bakker E (2016) Electroanalysis with membrane electrodes and liquid-liquid interfaces. Anal Chem 88:395–413
Cattrall RW, Freiser H (1971) Coated wire ion selective electrodes. Anal Chem 43(13):1905–1906
Lindner E, Buck R (2000) Microfabricated potentiometric electrodes and their in vivo applications. Anal Chem 72(9):336A–345A
Bobacka J (2006) Conducting polymer-based solid-state ion-selective electrodes. Electroanalysis 18(1):7–18
Lindner E, Gyurcsanyi R (2009) Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J Solid State Electrochem 13(1):51–68
Michalska A (2012) All-solid-state ion selective and all-solid-state reference electrodes. Electroanalysis 24(6):1253–1265
Enger O, Nuesch F, Fibbioli M, Echegoyen L, Pretsch E, Diederich F (2000) Photocurrent generation at a fullerene self-assembled monolayer-modified gold electrode cast with a polyurethane membrane. J Mater Chem 10(10):2231–2233
Paczosa-Bator B, Pięk M, Piech R (2014) Application of nanostructured TCNQ to potentiometric ion-selective K+ and Na+ Electrodes. Anal Chem 87:1718–1725
Hu J, Zough X, Stein A, Buhlmann P (2014) Ion-selective electrodes with colloid-imprinted mesoporous carbon as solid contact. Anal Chem 86:7111–7118
Chumbimuni-Torres K, Rubinova N, Radu A, Kubota L, Bakker E (2006) Solid contact potentiometric sensors for trace level measurements. Anal Chem 78:1318–1322
Lindfors T, Sundfors F, Hofler L, Gyurcsanyi R (2011) The water uptake of plasticized poly(vinyl chloride) solid-contact calcium-selective electrodes. Electroanalysis 23:2156–2163
Lindfors T, Hofler L, Jagerszki G, Gyurcsanyi R (2011) Hyphenated FT-IR attenuated total reflection and electrochemical impedence spectroscopy. Anal Chem 83:4902–4908
Sutter J, Radu A, Peper S, Bakker E, Pretsch E (2004) Solid-contact polymeric membrane electrodes with detection limits in the subnanomolar range. Anal Chim Acta 523:53–59
Yuan D, Anthis A, Afshar M, Pankratova N, Cuartero M, Crespo G, Bakker E (2015) All-solid-state potentiometric sensors with a multi-walled carbon nanotube inner transducing layer for anion detection in environmental samples. Anal Chem 87(17):8640–8645
He N, Gyurcsányi R, Lindfors T (2016) Electropolymerized hydrophobic polyazulene as solid-contacts in potassium-selective electrodes. Analyst 141(10):2990–2997
Khripoun G, Volkova E, Liseenkov A, Mikhelson K (2006) Nitrate-selective solid-contact electrodes with poly(3-octylthiophene) and poly(aniline) as ion-to-electron transducers buffered with electron-ion-exchanging resin. Electroanalysis 18(13–14):1322–1328
Michalska A, Wojciechowski M, Bulska E, Maksymiuk K (2010) Experimental study on stability of different solid contact arrangements of ion-selective electrodes. Talanta 82(1):151–157
Sjoberg-Eerola P, Nylund J, Bobacka J, Ivaska A (2008) Soluble semiconducting poly(3-octylthiophene) as a solid-contact material in all-solid-state chloride sensors. Sens Actuat B-Chem 134(2):878–886
Guzinski M, Jarvis J, Pendley B, Lindner E (2015) Equilibration time of solid-contact ion-selective electrodes. Anal Chem 87(13):6654–6659
Veder JP, De Marco R, Clarke G, Jiang SP, Prince K, Pretsch E, Bakker E (2011) Water uptake in the hydrophilic poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) solid-contact of all-solid-state polymeric ion-selective electrodes. Analyst 136(16):3252–3258
Fibbioli M, Morf WE, Badertscher M, de Rooij NF, Pretsch E (2000) Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 12(16):1286–1292
Lindfors T, Hofler L, Jagerszki G, Gyurcsanyi RE (2011) Hyphenated FT-IR attenuated total reflection and electrochemical impedance spectroscopy. Anal Chem 83:4902–4908
Veder J, De Marco R, Clarke G, Chester R, Nelson A, Prince K, Pretsch E, Bakker E (2008) Elimination of undesirable water layers in solid-contact polymeric ISEs. Anal Chem 80:6731–6740
Veder J, Patel K, Clarke G, Grygolowicz-Pawlak E, Silvester D, De Marco R, Pretsch E, Bakker E (2010) Synchrotron radiation/FTIR microspectroscopy study of undesirable water inclusions in solid-contact polymeric ion-selective electrodes. Anal Chem 82:6203–6207
Sutter J, Pretsch E (2005) Response behavior of poly(vinyl chloride)- and polyurethane-based Ca2+-selective membrane electrodes with polypyrrole- and poly(3-octylthiophene)-mediated internal solid contact. Electroanalysis 18(1):19–25
De Marco R, Jee E, Prince K, Pretsch E, Bakker E (2009) Synthesis and characterization of high-integrity solid contact polymeric ion sensors. J Solid State Electrochem 13:137–148
Bobacka J, Grzeszczuk M, Ivaska A (1991) Electrochemical study of poly(3-octylthiophene) film electrodes, I. Electrolyte effects on the voltammetric characteristics of the polymer. Three states of the polymer film. Synth Met 44:9–19
Chen T, Wu X, Rieke R (1994) Regiocontrolled synthesis of poly(3-alkylthiophenes) mediated by Rieke zinc: their characterization and solid-state properties. J Am Chem Soc 117:233–244
Lindfors T (2009) Light sensitivity and potential stability of electrically conducting polymers commonly used in solid contact ion-selective electrodes. J Solid State Electrochem 13:77–89
Ates M, Karazehir T, Arican F, Eren N (2013) Comparison of electrolyte effects for poly(3,4-ethylenedioxythiophene) and poly(3-octylthiophene) by electrochemical impedance spectroscopy and polymerization parameters with morphological analyses on coated films. J Coat Technol Res 10(3):317–330
Bobacka J, McCarrick M, Lewenstam A, Ivaska A (1994) All-solid-state poly(vinyl chloride) membrane ion-selective electrodes with poly(3-octylthiophene) solid internal contact. Analyst 119(9):1985–1991
Paciorek R, van der Wal P, de Rooij N, Maj-Zurawska M (2003) Optimization of the composition of interfaces in miniature planar chloride electrodes. Electroanalysis 15:1314–1318
Bobacka J, Ivaska A, Lewenstam A (1999) Plasticizer-free all-solid-state potassium-selective electrode based on poly(3-octylthiophene) and valinomycin. Anal Chim Acta 385:195–202
Vasquez M, Bobacka J, Ivaska A (2005) Potentiometric sensors for Ag+ based on poly(3-octylthiophene) (POT). J Solid State Electrochem 9:865–873
Mousavi Z, Teter A, Lewenstam A, Maj-Zurawska M, Ivaska A, Bobacka J (2011) Comparison of multi-walled carbon nanotubes and poly(3-octylthiophene) as ion-to-electron transducers in all-solid-state potassium ion-selective electrodes. Electroanalysis 23:1352–1358
Sutter J, Lindner E, Gyurcsányi R, Pretsch E (2004) A polypyrrole-based solid-contact Pb2+−selective PVC-membrane electrode with a nanomolar detection limit. Anal Bioanal Chem 380:7–14
Gyurcsányi R, Rangisetty N, Clifton S, Pendley B, Lindner E (2004) Microfabricated ISEs: critical comparison of inherently conducting polymer and hydrogel based inner contacts. Talanta 63:89–99
Zou X, Cheong J, Taitt B, Bühlmann P (2013) Solid contact ion-selective electrodes with a well-controlled Co(II)/Co(III) redox buffer layer. Anal Chem 85:9350–9355
Zou X, Zhen X, Cheong J, Bühlmann P (2014) Calibration-free ionophore-based ion-selective electrodes with a Co(II)/Co(III) redox couple-based solid contact. Anal Chem 86(17):8687–8692
Vanamo U, Bobacka J (2014) Instrument-free control of the standard potential of potentiometric solid-contact ion-selective electrodes by short-circuiting with a conventional reference electrode. Anal Chem 86:10540–10545
Le TH, Nafady A, Qu XH, Bond AM, Martin LL (2012) Redox and Acid–base Chemistry of 7,7,8,8-Tetracyanoquinodimethane, 7,7,8,8-Tetracyanoquinodimethane Radical Anion, 7,7,8,8-Tetracyanoquinodimethane Dianion, and Dihydro-7,7,8,8-Tetracyanoquinodimethane in Acetonitrile. Anal Chem 84(5):2343–2350
Acknowledgments
The financial support from Instrumentation Laboratories (IL) (Bedford, MA) and the Fedex Institute of Technology through the Sensor Institute of the University of Memphis (SENSORIUM) is gratefully acknowledged. J.J. acknowledges the support of IL to her graduate research assistantship.
Author information
Authors and Affiliations
Corresponding author
Additional information
In honor of my friend Professor György Inzelt on his 70th birthday in recognition of his significant contribution to electrochemistry
Rights and permissions
About this article
Cite this article
Jarvis, J.M., Guzinski, M., Pendley, B.D. et al. Poly(3-octylthiophene) as solid contact for ion-selective electrodes: contradictions and possibilities. J Solid State Electrochem 20, 3033–3041 (2016). https://doi.org/10.1007/s10008-016-3340-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3340-2