Abstract
The results of a systematic study of the light sensitivity and long-term potential stability (30 days) of poly(pyrrole) (PPy), poly(3-octylthiophene) (POT), poly(3,4-ethylenedioxythiophene) (PEDOT), poly(aniline) (PANI) and plasticised poly(vinyl chloride) (PVC) containing 20% (m/m) PANI are reported. Thin films were prepared either electrochemically or by the solution casting technique. This fundamental study is of importance because conducting polymers (CP) are commonly used as ion-to-electron transduction materials in all-solid-state solid contact ion-selective electrodes. The potential stability test done in 0.1 M KCl (pH 7.5) simulates the extreme situation when the CP-based SC becomes in direct contact with water. Films prepared of a nanodispersion of PANI showed both good potential stability and insensitivity to light even under illumination with very intensive light (>105 lx). In contrary, it was observed that POT is very light-sensitive. Upon illumination with intensive light, the potential responses of POT films prepared by solution casting and electropolymerisation were 315 and 590 mV, respectively. A room light sensitivity of approximately −10 to −15 mV was observed for these films. The other CPs in this study were insensitive to room light (∼150 lx), but were light-sensitive under illumination with intensive light. The potential drift of PPy(Cl) is below −10 μV/h (3–30 days), whereas the other most stable CPs in this study had a slightly higher potential drift.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The conducting polymers (CP) were already introduced in 1987 [1] and 1992 [2] as ion-to-electron transduction layers or solid contact materials in all-solid-state ion-selective electrodes (SC-ISE). The SC concept has been widely accepted within the ISE community since the paper by Sutter et al. in 2004 [3], which showed that the detection limit of all-solid-state ISEs could be extended to the subnanomolar range by using electropolymerised poly(pyrrole) (PPy) as the SC between the metal substrate (Pt or Au) and the ion-selective membrane (ISM). Different types of SC materials were recently reviewed by Bobacka [4]. The most commonly used CP-based SC materials are poly(3-octylthiophene) (POT) [5–10], PPy [3, 11–23], poly(3,4-ethylenedioxythiophene) (PEDOT) [24–33] and poly(aniline) (PANI) [34–40].
Although the results in many respects are promising, there are still many open questions that must be solved before the SC concept can be successfully commercialised [40]. The most important aspects are the potential stability of the SC, reproducible preparation of the contact and the possible formation of a water layer at the substrate∣CP and/or CP∣ISM interfaces [41] and good mechanical stability between all interfaces in the electrode structure. It was recently shown by de Marco et al. [42] that a very thin water layer (100 ± 10 Å) is formed at the substrate∣ISM interface in coated-wire ISEs (CWE). In their study, neutron reflectometry, electrochemical impedance spectroscopy and secondary-ion mass spectrometry were used to confirm the presence of a water layer in the CWE structure.
The SC layer has been prepared electrochemically in most SC-ISEs due to the poor processibility and solubility of CPs. In the case of POT, PEDOT and PANI, the SC layer can also be prepared by the solution casting technique from organic solvents [6–10 (POT), 40 (PANI)] or aqueous solutions [31–33 (PEDOT)]. The preparation of the SC layer from organic solvents (POT and PANI) is especially favourable to avoid the SC in coming in contact with water already during the preparation step. Quite recently, different types of aqueous and organic dispersions of CP have also become commercially available (http://www.zipperling.de/; http://www.panipol.com/) [43, 44].
It is often difficult to compare the results reported in the literature even for one specific CP due to the large variety of different experimental parameters and procedures, which have been used in electropolymerisation. In this respect, the comparison of the properties of different types of CPs becomes even more difficult. For example, the surface morphology of the SC layer may play an important role in the adhesion between the SC layer and the ISM, as well as the volume expansion of both of these phases, which takes place upon water uptake. There is obviously a certain need to standardise the preparation procedures for the most commonly used CPs so as to facilitate the comparison of the results obtained by different research groups.
In total, seven different types of PPy, POT, PEDOT and PANI films, which are the most commonly used SC materials, were chosen for this study. In addition, a SC membrane consisting of K+-selective plasticised poly(vinyl chloride) (PVC) and 20% (m/m) PANI (PANI-KPVC) was included in the study [40]. All these films were prepared according to the most commonly used experimental procedures reported in the literature. Obviously, these choices are partly subjective. Electrochemically prepared PANI (in HCl) was excluded from this study because of its strong pH sensitivity [37, 45, 46].
It is of fundamental importance to study the light sensitivity and long-term potential stability of CPs, which are used as SC materials, to optimise the performance of the SC-ISEs and to choose right types of CP for these devices. In this study, no plasticised PVC membranes were applied on top of the CP films. In the long-term potential stability study, the potential drifts measured were therefore not influenced by the outer plasticised PVC membrane, which is used in the SC-ISE setup. The aim of the potential stability study was to simulate the extreme situation when the CP-based SC becomes in direct contact with water. To the best of my knowledge, only one paper has been published dealing partly with the light sensitivity of CPs (PPy) applied in SC-ISEs [19]. There is therefore a clear need for a systematic study of the light sensitivity of the most commonly used SC materials.
Materials and methods
Chemicals
Pyrrole (Py) was freshly distilled before the electropolymerisation, and 3,4-ethylenedioxythiophene (EDOT; colourless) was used as received from Aldrich. 3-Octylthiophene (3OT; >98%) was purchased from Tokyo Kasei and propylene carbonate (PC; ≥99%) from Fluka. The PANI (D1003) dispersion was obtained from Ormecon GmbH in Ammersbek, Germany. According to the manufacturer, the D1003 was doped with an organic sulfonic acid (SA) in a molar ratio of 1 between the SA and the repeat unit of PANI. The D1003 dispersion was stored in a closed glass bottle. The PEDOT (Baytron P) dispersion was from Bayer AG in Germany and the POT powder from AC&T in France.
Electropolymerisation and characterisation of the films
The experimental parameters used in the electropolymerisation of Py, EDOT and 3OT on glassy carbon (GC, d = 3 mm; encapsulated in a PVC body, d = 10 mm) have been summarised in Table 1 and were taken without modifications from the following references: PPy(Cl) [15]; PPy(FeCN) [3, 19]; PEDOT(PSS) [25–27] and POT(BF4) [5]. The GC electrodes were always polished to a mirror image with 0.3 μm Al2O3 powder and kept in an ultrasonic bath for 15 min before the electropolymerisation. The polymerisations were always done in oxygen-free fresh monomer solutions. All potentials given in this work refer to the saturated calomel electrode (SCE). A GC rod was used as the counter electrode (CE) in the electropolymerisation and characterisation of the CP films. Three identically prepared electrodes of each electrode type were always used in all long-term potential stability and light sensitivity tests presented in this work. Only ultrapure water (resistivity, 18.2 MΩcm) was used in all experiments.
The PPy(Cl), PPy(FeCN) and the PEDOT(PSS) films were characterised by cyclic voltammetry (CV; v = 50 mV/s) in an oxygen- and monomer-free 0.1 M KCl solution. The POT(BF4) films were characterised in an oxygen-free PC solution containing only 0.1 M LiBF4. After the characterisation, all CP films were polarised for 180 s at a specific oxidation potential (Table 1: pretreatment; parameters not given in the reference cited above), which was high enough to convert the films into the electrically conducting form. The POT(BF4) films were rinsed with chloroform after the polarisation. The polarisation of all CP films was done just before the potential stability test was started and is important so as to bring the CP films to the same oxidation state and thus improve the potential reproducibility (standard potential) of the individual electrodes.
The film thicknesses were not measured, but were assumed to be in the micrometer range for the PPy(Cl), PPy(FeCN) and the PEDOT(PSS) films and considerably lower for the POT(BF4) films. Especially, the electropolymerisation of Py in the presence of 0.5 M K4Fe(CN)6 results in thick films.
Solution-cast films
General properties of POT (POT(ch)), PANI (D1003), PEDOT (Baytron P) and PANI-KPVC films are given in Table 2. The PANI-KPVC film contains 20% (m/m) PANI (D1003). The rest of the film (80%, m/m) is composed of 33.0% (m/m) of high-molecular-weight PVC plasticised with 65.5% dioctyl sebacate (DOS), 0.5% potassium tetrakis(4-chlorophenyl)borate (KTpClPB) as lipophilic salt and 1.0% valinomycin as the active K+-selective component. The preparation procedure of the PANI-KPVC film was recently described elsewhere [40].
The films were prepared by the solution casting technique on the same type of GC electrodes which were used for electropolymerisation of the CP films. Ten milligrams per millilitre of POT(ch) was completely dissolved by chloroform and was allowed to equilibrate for 2 days. It was reported by AC&T that POT(ch) is in the non-conducting form, but it is still assumed that its electrical conductivity is in the lower semi-conducting range. The POT(ch), PANI (D1003) and PANI-KPVC films covered the GC surface and the entire end of the PVC body. The deposition volumes were adjusted to obtain sufficiently thick films to be sure that the GC surfaces were completely covered by the films. The film thicknesses of POT(ch), PANI (D1003) and PANI-KPVC were estimated by the scanning electron microscope (SEM) to ∼40, ∼500 and ∼260 μm, respectively. The POT(ch), PANI (D1003), PEDOT (Baytron P) and PANI-KPVC films were neither polarised nor electrochemically characterised before starting the potential stability test.
In the case of PEDOT (Baytron P), the films could be deposited mainly on the GC surface due to the high surface tension of PEDOT, which repelled the PEDOT dispersion from the PVC body. One set of PEDOT (Baytron P) films were cross-linked with 0.25 M Ru(NH3)Cl3 according to the procedure described by Vázquez et al. [32] by applying a drop of the ruthenium solution on top of the PEDOT films. However, after a contact time of 30 min, the cross-linked PEDOT films came loose in one piece during washing with ultrapure water and drying of the films. This indicates that the adhesion between the GC substrate and PEDOT is not very good. No further attempts to cross-link PEDOT (Baytron P) were done. In some papers, non-cross-linked PEDOT (Baytron P) has been used in sensor applications [33]. The non-cross-linked form was therefore included in this study in spite of the poor adhesion between PEDOT and GC.
SEM and EDXA measurements
A LEO 1530 Gemini FEG-SEM instrument and a Thermo-Noran Vantage X-Ray Microanalysis System instrument was used in the SEM measurements and the energy dispersive X-ray analysis (EDXA) of the CP films, respectively.
Potential stability test
The long-term potential stability test was done for 30 days in 0.1 M KCl with pH adjusted to 7.5 with 10 mM phosphate buffer (KH2PO4/K2HPO4; total K+ concentration, 0.118 M). This test solution was chosen so as to be able to compare the results with previously published results [37, 40, 47]. KCl (0.1 M) is usually used to for the electrochemical characterisation of PPy(Cl) [15], PPy(FeCN) [3, 19] and PEDOT(PSS) [25–27], which makes this electrolyte suitable for the potential stability test.
The potential stabilities of the different electrode types were always measured daily during the first 3 days of the stability test (RE: SCE; n = 3). The potentials were thereafter measured twice per week during 30 days. Between the measurements, the electrodes were always stored in the test solution of 0.1 M KCl (pH 7.5). The test solution was replaced to a fresh solution before each measurement, which took 15 min. The potential readings of the different electrode types obtained after 15 min were used to calculate the mean potential values and standard deviations (SD) of each measurement. These mean potential values and their SD were then used to obtain the potential stability graphs. The potential stabilities were always measured without stirring in quiescent solutions.
The potential drifts of the CP films that showed the best potential stabilities were calculated separately in two time intervals: (a) 2 h–3 days and (b) 3–30 days. In the first interval, the difference in E max and E min (ΔE) were calculated because the potential drift is usually higher due to equilibration effects in the beginning of the stability tests. In the second interval (3–30 days), the potential drifts were calculated from the slopes of the linear regression lines. Linear regression is a simple method to calculate the potential drift and gives rather reliable results, although the potential drifts of all electrode types were not completely linear within the entire time period of 3–30 days. The potential stability study was conducted at 23 ± 1 °C. The test solutions were not thermostated, which may have a minor influence on the calculated potential drifts.
Light sensitivity test
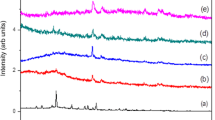
The light sensitivity test was always done with the same polymer films, which had been used in the potential stability test for 30 days. The light sensitivity measurements were done in ∼20 ml of a 0.1 M KCl solution (pH 7.5) directly after the last measurement in the potential stability test. The polymer electrodes (n = 3) were placed in a glass vessel, which was placed on a metal grid. Two optic fiber cables of a Leica CLS 150XE light source, equipped with a tungsten halogen lamp (Philips EKE 150 W, 21 V), were directed in a reproducible manner through the holes of the grid and the glass vessel against the surface of two of the polymer films. The spectrum of the Leica light source is shown in Fig. 1 (spectrum a) and resembles of the spectrum of sunlight in the visible region (400–700 nm; http://en.wikipedia.org/wiki/Image:Solar_Spectrum.png; http://home.twcny.rr.com/geomanagement/ensmingr/spectrum.html). The spectrum (a) in Fig. 1 was measured with an Ocean Optics S2000 fiber optic spectrometer. The spectrometer was calibrated with an Ocean Optics LS-1 light source (tungsten halogen lamp) to correct for wavelength-dependent sensitivity differences of the spectrometer response.
The potentials of the individual polymer electrodes were measured continuously vs SCE during the light sensitivity test. The following measuring sequence was used in the tests: room light (15 min), darkness (no room light; 20 min), illumination with the Leica light source on half effect (∼5 × 104 lx; 20 min), illumination with full effect (>1.6 × 105 lx; 20 min) and darkness (no room light; 25 min). The lux values were measured at a distance of ∼1 cm from the fiber optic cables of the light source with a Lutron LX-105 luxmeter and are therefore only approximate values. For comparison, approximately 3 × 104 to 105 lx corresponds to sunlight during an average day (http://en.wikipedia.org/wiki/Daylight). The spectrum of the room light (fluorescent lamps: Universal Thermo, 36 W/840) is shown in Fig. 1 (spectrum b) and was measured in a similar way as the spectrum of the Leica light source.
In one of the light sensitivity tests, the temperature was continuously monitored in the 0.1 M KCl (pH 7.5) test solution with a Therma 12 Thermometer probe. The probe was placed at the level of the polymer films. The SCE should not be light-sensitive, unlike the Ag∣AgCl reference electrode, but a separate test was done to evaluate the influence on light and temperature on the potential of the SCE (see “Results and discussion”).
Results and discussion
Electropolymerised films
PPy(Cl)
The chronoamperograms measured during electropolymerisation of three PPy(Cl) films showed a certain degree of irreproducibility (Fig. 2a). This problem is usually associated with electropolymerisation due to the large number of parameters that has to be controlled and the random nature of the initiation step of the electropolymerisation process. It is therefore difficult to obtain highly ordered structures with electropolymerisation. The SEM images reveal that the surface morphology of the PPy(Cl) film is relatively rough with large height variations over the electrode surface (Fig. 3a). A more even surface structure could possibly be obtained with CPs, which mainly grow by a two-dimensional layer-by-layer deposition mechanism [48].
The PPy(Cl) films were characterised by CV in 0.1 M KCl (Fig. 4a, solid line). The current magnitude of the CVs of three different electrodes showed some variation, but the broad oxidation and reduction peak was always located at about 200 and about −200 mV, respectively. The last CV in the characterisation of the PPy(Cl) films was completed at −500 mV. To convert the films to the electrically conducting form, they were polarised at 250 mV (t = 180 s) just before the potential stability study was started.
The potential stability test of PPy(Cl) is shown in Fig. 5a. After an initial drift of ∼11 mV during the first 3 days of the stability test, the PPy(Cl) electrodes showed very low potential drift of <10 μV/h and good potential reproducibility of three identical electrodes (SD, ∼1.0 mV) during the rest of the test period (3–30 days; Table 3). It was recently reported by Bühlmann et al. that PVC-based ISEs with three-dimensional macroporous carbon as the SC had a potential drift of only 11.7 μV/h over 70 h and showed the same drift after 1 month of use [47]. The comparison of the potential drifts reported by Bühlmann et al. and those reported in this work is complicated by the fact that no outer plasticised PVC membranes were applied on top of the SCs in this work, whereas this was the case in the paper of Bühlmann et al.
It seems that the irreproducibility observed during the electropolymerisation and characterisation of PPy(Cl) films does not have any bigger influence on the reproducibility of the potentials of the PPy(Cl) electrodes. The high potential stability of the PPy(Cl) electrode in 0.1 M KCl can partially be explained by the fact that the film was polymerised in the presence of KCl. Another reason for the low potential drift of PPy(Cl) is that the Cl− counter ions, which are incorporated in the film during electropolymerisation, are small and mobile. The PPy(Cl) film can therefore quickly reach its equilibrium state (oxidation state) in contact with the sample solution. The potential drift observed during the first 3 days of the stability study indicates that the PPy(Cl) film is slightly reduced (compare with CV in Fig. 4a). This initial potential drift is possibly also partially caused by the polarisation potential of 250 mV, which was applied to the films before the potential stability test was started. A more optimal polarisation potential would have been 100 mV. The influence of oxygen cannot be excluded either. Oxygen is a strong oxidant with a high redox potential and can (depending on pH) oxidise CP films [27, 49]. The long-term potential stability of the GC substrate showed that the potential was stabilised at ∼100 mV after 2 weeks, which is close to the potential value of the PPy(Cl) electrode.
The potential drift pattern of the PPy(Cl) electrode would possibly be different if the potential stability test would be done in a medium containing no Cl−. Ion exchange processes between the PPy(Cl) film and the solution phase would in that case possibly result in a potential drift (oxidation/reduction of the polymer). This is an important aspect when considering the potential stability of the SC-ISEs. After penetrating through the outer membrane, water will reach the SC and bring anions and/or cations (including H+ and OH−) in contact with the SC layer. Depending on the SC, the oxidation state (potential) of the CP film can therefore also be affected by ion exchange processes between the CP film and the cations and/or anions, which are transported to the SC by water.
It is therefore recommended that a long-term potential stability test, similar to the test shown in Fig. 5, should be done for all CP-based SC materials before applying them in all-solid-state SC-ISEs. The potential stability test should be conducted in a medium with a similar chemical composition (redox potential) in which the SC-ISE is aimed to be used, although the ion-selective plasticised PVC membrane (or any other membrane type) will function to a certain extent as a selective barrier, which partially prevents ions from the sample solution in coming in contact with the SC. The result of the potential stability test is therefore not directly transferable to the expected behavior of the SC-ISEs, but can be considered as a simulation of the extreme case when the SC is in entire contact with water.
The results of the light sensitivity test of the PPy(Cl) film is shown in Fig. 6a (curve 2). The room light had no influence on the potential of the PPy(Cl) electrode. A light sensitivity of −25 mV was observed when the PPy(Cl) film was illuminated with light from the Leica light source. The charge conduction mechanism of CPs has been proposed to be more complex than for inorganic semiconductors [50]. When CPs are exposed to light with sufficient energy, electrons from the valence bands are excited to the conduction bands. Holes are simultaneously created in the valence bands. The formed charge carriers consist of electron-hole pairs (excitons), which are strongly bound to each other due to Coulomb interactions. The efficiency of the charge carrier generation is, however, usually low in CPs [51]. It is expected that charge carriers (excitons) are formed in the PPy(Cl) film structure upon exposure to visible light. This would increase the oxidation state of the PPy(Cl) and result in shifting the potential to higher values. However, a negative shift of the potential was observed with the PPy(Cl) film. The reason for this is still unclear and requires a more detailed study. The decrease of the potential indicates that the PPy(Cl) film is reduced, but the explanation is probably much more complex and depends, for example, on the nature of charge carriers (conduction mechanisms) in the PPy(Cl) film and possible photochemical reactions taking place at the CP∣solution interface.
The light sensitivity of the GC substrate is also shown in Fig. 6a (curve 4). A fresh GC electrode, which had not been equilibrated for 30 days in 0.1 M KCl was used for the light sensitivity test. An slight increase of the potential is therefore observed in the beginning of the measurement. As expected, the GC substrate showed no room light sensitivity, but a minor potential response of −6 mV was observed when GC was illuminated with light from the Leica light source.
The influence of light and temperature on the SCE was studied in a separate experiment (Fig. 7). The GC electrode was protected from light with a thick black tubing, but was in contact with the 0.1 M KCl (pH 7.5) solution through a small opening in the tubing. The temperature of the solution increased with 3.7 °C, and the potential decreased simultaneously with 2.5 mV during the illumination with intensive light from the Leica light source. This corresponds very well with the expected potential decrease of 2.5 mV of the SCE when the temperature increases with 3.7 °C [52] (http://www.consultrsr.com/resources/ref/calomeleqns.htm). The potential decrease is therefore caused only by a temperature effect of the SCE and can be considered as a system-dependent “background” potential.
The CV of the PPy(Cl) film measured after the light sensitivity test indicate that the electroactivity of the film has considerably decreased during the test period of 30 days (Fig. 4a, dotted line). This may influence the light sensitivity of PPy(Cl) to a certain extent. It was previously reported that the electroactivity of the PPy(PSS) severely degraded during long-term potential cycling in 0.1 M KCl [27]. In spite of the decrease in electroactivity, the very low potential drift of the PPy(Cl) film during the potential stability test in combination with the good potential reproducibility is advantageous for SC-ISE applications.
PPy(FeCN)
The chronoamperograms measured during electropolymerisation of three PPy(FeCN) films showed minor variations similar to the chronoamperograms of the PPy(Cl) films (Fig. 2b). The electropolymerisation results in an intense PPy(FeCN) film growth due to the high monomer concentration (0.5 M). The cyclic voltammograms measured during characterisation of the PPy(FeCN) films in 0.1 M KCl were very reproducible (Fig. 4b, solid line). The CV of PPy(FeCN) consists probably of two overlapping processes, the oxidation and reduction of the PPy film and the \({\text{Fe}}\left( {{\text{CN}}} \right)_6^{{{3 - } \mathord{\left/ {\vphantom {{3 - } {4 - }}} \right. \kern-\nulldelimiterspace} {4 - }}} \) redox couple.
The SEM image shows that the surface morphology of the PPy(FeCN) film is very rough with a cauliflower like structure (Fig. 3b). The discussion concerning the formation of a water layer at the CP∣ISM interface is therefore slightly misleading for most of the electropolymerised CP films [53], like PPy(Cl) and PPy(FeCN). It would be more correct to talk about the presence of water at this interface because of the difficulty in defining where the water layer is exactly located on rough porous surface structures. If the CP-based SC has a good mechanical strength, the rough surface structure of the CP can be beneficial in anchoring the ISM to the CP layer and thus improving the mechanical strength of this interface. A volume expansion of the plasticised PVC-based ISMs take place during the water uptake. The volume expansion of the ISM can therefore result in mechanical instability of the CP∣ISM interface. The water layer can also be formed at the GC∣CP interface. It was observed that the mechanical stability of the GC∣PPy(FeCN) interface were poor. The PPy(FeCN) films came loose from the GC substrate when the films were dried in contact with air. A water layer can therefore possibly be formed at the GC∣PPy(FeCN) interface.
The PPy(FeCN) electrode showed a relatively high potential drift of ∼35 mV during the first 3 days of the stability test (Fig. 5a, Table 3). A rather constant potential drift of 38 μV/h were observed during the rest of the test period. It seems that the polarisation at 450 mV does not have any influence on the potential stability of PPy(FeCN). Two hours after starting the potential stability test, the potential of the PPy(FeCN) film in 0.1 M KCl (pH 7.5) had dropped to ∼50 mV and increased thereafter during the rest of the stability test. The initial drift (decrease of potential) observed during the first 2 h in the test solution can possibly be attributed to reduction of the PPy(FeCN) films and expulsion of counter ions from the polymer matrix. After the initial potential drift of 2 h, it is most probably oxygen that slowly oxidises the PPy(FeCN) film and the potential therefore increased. The potentials of the PPy(FeCN) electrodes were almost the same as for the PPy(Cl) electrodes after 30 days in contact with the test solution. The reproducibility of the potential readings of the individual PPy(FeCN) electrodes (n = 3) were good throughout the entire test period (SD, ∼2.0 mV). EDXA measurements indicates that \({\text{Fe}}\left( {{\text{CN}}} \right)_6^{{{3 - } \mathord{\left/ {\vphantom {{3 - } {4 - }}} \right. \kern-\nulldelimiterspace} {4 - }}} \) ions stayed in the CP phase and were not ion exchanged to, e.g. Cl−.
No room light sensitivity was observed for the PPy(FeCN) films (Fig. 6a, curve 1). The illumination with intensive light from the Leica light source resulted in a potential decrease of −15 mV, which is slightly lower than for the PPy(Cl) electrodes but still in the same order of magnitude. The light sensitivity test indicates also that the potential of the PPy(FeCN) electrode will reach its starting value quicker than the PPy(Cl) electrode after completing the illumination of the film. The relaxation times were, in both cases, still very long. The cyclic voltammograms of the PPy(FeCN) films measured after the light sensitivity test indicates that the electroactivity of the PPy(FeCN) had considerably decreased, like for PPy(Cl), during the test period of 30 days (Fig. 4b, dotted line).
PEDOT(PSS)
The chronopotentiograms of three PEDOT(PSS) films obtained during electropolymerisation showed a rather good reproducibility (Fig. 8b), but the cyclic voltammograms of the films obtained in 0.1 M KCl (pH 7.5) showed some irreproducibility (Fig. 4d, solid line). The electropolymerisation of EDOT results in rather smooth films compared to PPy(Cl) and PPy(FeCN) (Fig. 3d). This is probably due to the bulky PSS− counter ions, which are incorporated in the film during the electropolymerisation process.
The potential stability of PEDOT(PSS) is shown in Fig. 5a. The polarisation at 250 mV does not have any influence on the potential stability. After an initial potential drift of ∼28 mV during the first 3 days of the stability test, a low potential drift of 30 μV/h was observed during the rest of the test period (3–30 days). A good potential stability was obtained after 1 week of soaking in 0.1 M KCl (pH 7.5). However, the potential reproducibility of three identical PEDOT(PSS) electrodes was not optimal and showed a SD of ∼11 mV.
Changes in the surface morphology of the PEDOT(PSS) films were studied in a separate experiment. The SEM images indicate that the PEDOT(PSS) membranes were not completely stable during the entire test period. After being in contact with the test solution of 0.1 M KCl (pH 7.5) for 1 week, small holes were observed on the PEDOT(PSS) surface. The holes became bigger during the following 7 days in the contact with the test solution. After 30 days, it was observed that minor parts of the outer surface of the PEDOT(PSS) membranes had partially cracked, and the GC surface was therefore exposed to the test solution. EDXA measurements showed that the sulphur and oxygen content of the film decreases slightly with time during the first 2 weeks of the test period. This indicates that PSS− leaches out from the membrane phase to the test solution. This is not surprising because PSS− is water-soluble.
The potential drift of the PEDOT(PSS) electrodes is expected to be caused mainly by oxidation of the films by oxygen, but can be also partially caused by changes in the composition of the PEDOT(PSS) films and the formed pinholes in the film structure. EDXA measurements showed that no Cl− was extracted or ion exchanged to the membrane phase during the stability test of 30 days.
The PEDOT(PSS) films showed no room light sensitivity (Fig. 6a, curve 3). A light sensitivity of −37 mV was observed when the PEDOT film was illuminated with light from the Leica light source. This is considerably higher than the light sensitivity of PPy(Cl) and PPy(FeCN). It seems also that the relaxation time required for the film to reach its initial potential after the illumination with Leica light is very long. In contrast to the PPy(Cl) and PPy(FeCN) membranes, almost no degradation of the electroactivity of the PEDOT(PSS) films could be observed after the light sensitivity test (Fig. 4d, dotted line). This indicates that the PEDOT(PSS) has very good electrochemical stability despite the pinhole formation.
POT(BF4)
The electropolymerisation of three POT(BF4) films showed some irreproducibility (Fig. 8a), although the cyclic voltammograms obtained during characterisation of the films in 0.1 M LiBF4 (PC) were reproducible (Fig. 4c, solid line). Before starting the stability test, the POT(BF4) electrodes were polarised at 950 mV for 3 min and dipped for 1 min in chloroform to wash out remains of PC from the film.
The results of the potential stability test showed a large potential drift during the first week of the test period (Fig. 5b). This is not surprising because the doped form of poly(thiophene) (PT) is very easily reduced to the undoped form in contact with water [49], which results in the lowering of the potential. The potential reproducibility of three identical POT(BF4) electrodes is also poor.
The highest light sensitivity of all CP studied was observed with POT(BF4) (Fig. 6b, curve 1). The room light sensitivity of the POT(BF4) films were −9 mV (Fig. 9, curve 1), and a potential response of ∼590 mV was observed under illumination with light from the Leica light source. The high light sensitivity is a serious drawback for applications of POT(BF4) in all-solid-state SC-ISEs. The potential response of POT(BF4) is fast and takes place immediately after switching on the light source. The positive potential shift is probably due to the photo-oxidation of the POT film, which is one reason for the application of poly(alkythiophenes) in organic solar cell structures [51]. The oxidation state of the POT film increases during photo-oxidation, resulting in the formation of charge carriers in the polymer structure. A more detailed study should, however, be conducted to find out the exact reason for the positive potential shift of the POT(BF4) films. The films were cycled in 0.1 M LiBF4 (PC) after the light sensitivity test. The electroactivity of the POT(BF4) films had decreased considerably during the test period of 30 days. This can partially be due to the reduction of the films in the aqueous test solutions and an effect of soaking of the films for 30 days in an aqueous solution. EDXA measurements showed that the F− content of the POT(BF4) film decreased during the potential stability test, which further supports the assumption of the reduction of the film. Furthermore, no accumulation of Cl− in the POT(BF4) films was observed by EDXA.
Enlargement of Fig. 6b. The influence of light on the potential response of POT(BF4) (1) and POT(ch) (2); RE: SCE
Solution-cast films
Because of the very low electrical conductivities of POT(ch) and PANI-KPVC (Table 3), the solution-cast films were not characterised by CV before starting the potential stability test so as not to alter their initial oxidation state.
PEDOT (Baytron P)
Three identical Baytron P electrodes (not cross-linked) showed very reproducible potentials, but the PEDOT films started to dissolve almost immediately when coming into contact with the aqueous test solution of 0.1 M KCl (pH 7.5). The potential stability and light sensitivity of Baytron P could therefore not be determined. It is indeed rather contradictory to use a water dispersion of PEDOT in applications designed for aqueous solutions, even in the case when Baytron P is stabilised by cross-linking [32].
In general, with some exceptions [37, 40, 42, 54], the usefulness of spectroscopic techniques has not been fully recognised within the ISE research. Especially, a basic characterisation of most of the CP-based SC materials (e.g. cross-linked PEDOT) should be done before applying them in SC-ISEs. Spectroscopic techniques would also provide valuable information about structural changes taking place in the SC during long-term use of the SC-ISEs.
POT(ch)
The SEM measurements showed that the POT(ch), PANI (D1003) and PANI-KPVC films were all very smooth in comparison to the rather rough CP surfaces obtained by electropolymerisation. The potential stability of the POT(ch) film reveals that the day-to-day reproducibility of the potential of POT(ch) is very poor (Fig. 5c). The SD of the potentials of three identical POT(ch) electrodes became also higher with time. The POT(ch) films are in the undoped non-conducting state in the beginning of the stability test. It seems that the potential of the POT(ch) electrodes is slightly drifting towards higher potential values during the potential stability test. This possibly indicates that the reduced POT(ch) films are partially oxidised by oxygen.
It was observed that the potentials of the POT(ch) electrodes always drifted between ∼3 and 13 mV (to higher potentials) during the potential stability measurements (15 min) conducted during the different days of the stability test. One reason for the potential drift is possibly the low redox capacitance of the POT(ch) film due to its non-conducting nature.
The POT(ch) film showed a room light sensitivity of −16 mV (Fig. 9, curve 2) and a very high light sensitivity of ∼315 mV when illuminated with light from the Leica light source (Fig. 6b, curve 2). The room light sensitivity of POT(ch) may possibly be partially responsible for the poor day-to-day reproducibility of the potential of the POT(ch) electrodes (Fig. 5c). The light sensitivity of POT(ch) is obviously a serious drawback in any ISE applications if the POT(ch) electrodes are not protected from the influence of light.
PANI (D1003)
PANI (D1003) has not been used as a SC layer as such, but has been used as a SC material in combination with plasticised PVC [40]. PANI (D1003) was therefore included in this study. Three identical PANI (D1003) electrodes showed a very good potential reproducibility during the entire test period of 30 days (SD, <1.0 mV). After an initial drift of ∼12 mV during the first 3 days of the stability test, an almost constant drift of approximately −37 μV/h was observed during the rest of the test period (Fig. 5c, Table 3). It has been reported that dispersions of PANI nanoparticles form self-organised structures [55]. This may be advantageous in the PANI (D1003) film preparation, resulting in PANI electrodes with reproducible standard potentials.
It was recently shown that the electrically conducting emeraldine salt (ES) form of PANI (D1003) is extremely stable even up to pH 12 [40]. The long-term UV–vis stability test at pH 7.5 (0.1 M KCl) revealed no changes in the oxidation state of the PANI (D1003) film during a time period of 36 days. The ES form of PANI (D1003) was observed to be completely stable between pH 0 and 8, whereas the electrochemically prepared PANI(Cl) film, which has a logarithmic protonation constant of 3.5 [average proton number (n), 0.57], is almost completely in its non-conducting emeraldine base (EB) form at pH 6 [46]. The constant potential drift of approximately −37 μV/h of the PANI (D1003) electrode may, however, be an indication of a minor ES to EB conversion of the PANI films. It is also noteworthy that the potential of the PANI electrodes was not affected by oxygen in the test solution.
The PANI (D1003) film is the only CP in this study that showed no light sensitivity either for room light or light from the Leica light source (Fig. 10a). The minor decrease of the potential of PANI (D1003) electrode observed in the light sensitivity test (−1.9 mV) is due to the temperature effect of the SCE. The absence of the light sensitivity of the PANI electrode shows that there are considerable differences in the light sensitivity of different CPs. It is therefore recommended that a light sensitivity test should be done for any specific CP before using them in any ISE setups.
The PANI(D1003) films showed a rather good mechanical stability and scratch resistance. The films could not be easily removed from the GC-PVC substrates, in contrary to most of the electrochemically prepared CP films, which can relatively easily be removed from the electrode substrate by a household paper.
PANI-KPVC
SC-ISEs with geometrically well-defined SCs consisting of a PANI-KPVC membrane was recently reported [40]. The PANI-KPVC membrane containing 20% (m/m) PANI (D1003) was therefore included in this study. The potential of the PANI-KPVC electrodes showed a continuous drift during 21 days of the potential stability test and stabilised thereafter at ∼90 mV (Fig. 5c). It can be concluded that the potential drift is caused almost entirely by the plasticised PVC part of the membrane matrix due to the good potential stability of PANI (D1003). The potential drift of PANI-KPVC can probably be associated with the water uptake of the PVC phase and a possible formation of a water layer at the GC∣PANI-KPVC membrane interface.
Three identical PANI-KPVC electrodes showed a rather poor potential reproducibility. Two of the electrodes showed almost the same potential, while the potential of the third electrode deviated by ∼30–45 mV from the two others (1–30 days). The exact reason for this deviation is not understood at the moment, but it is reasonable to assume that the potential deviation is connected with the PVC phase.
The light sensitivity of the PANI-KPVC electrodes is shown in Fig. 10b. As expected, the PANI-KPVC showed no potential response to room light, but surprisingly, a potential response of ∼10 mV was observed when the films were illuminated with light from the Leica light source. After the intensive illumination, a slow relaxation process took place, and the potential drifted towards the initial potential value. It can be concluded, by assuming that the interactions of PANI and KPVC can be neglected, that the potential response of the PANI-KPVC membrane must be related to the plasticised PVC phase. Upon illumination of the SC-ISEs, the positive potential shift caused by the PVC phase will probably, to a certain extent, be counterbalanced by the negative potential shift caused by the light sensitivity of the CP. For example, the negative potential shift induced by the light sensitivity of PPy(FeCN) is approximately equal to the positive potential shift of the plasticised PVC membrane. Thus, it can be speculated that upon illumination with intensive light, no potential response will be observed for the PVC based SC-ISE prepared with PPy(FeCN) as the SC.
Conclusions
The CPs studied in this work have been used as SC materials in all-solid-state SC-ISEs. One of the aims of this study was to simulate the extreme situation where the SC is in complete contact with water. It is shown that there are big differences in the potential stability and light sensitivity of CPs in 0.1 M KCl (pH 7.5).
The best potential stability was obtained with PPy(Cl), which showed a potential drift of only −3.8 ± 4.5 μV/h after a short initial period of drift. The PPy(FeCN), PEDOT(PSS) and PANI (D1003) electrodes showed good potential stabilities of 30–40 μV/h (absolute values). Three identical electrodes of PPy(Cl) and the PANI (D1003) showed a very reproducible standard potential (SD, ≤1.0 mV). It should be stressed that the potential stabilities reported apply only to the specific test solution used in this work. It is thus recommended that the potential stability of the CP-based SC materials should always be tested under similar conditions in which the SC-ISE will be used.
All CPs except for PANI (D1003) were light-sensitive when illuminated with intensive light (≥5 × 104 lx). Especially, the POT(BF4) and POT(ch) films were very light-sensitive, which is a serious limitation in the application of POT in all ISE applications. Except for POT, the other CPs showed no sensitivity to room light (150 lx). The light sensitivity of new SC materials should always be measured before using them in any all-solid-state ISE applications.
The formation of pinholes were observed on the PEDOT(PSS) surface, which indicates some instability of the films in 0.1 M KCl. On the other hand, the mechanical stability of the GC∣CP interface of the PPy(FeCN) films is poor. The choice of the CP-based SC material is a compromise of many factors. A large number of parameters must be carefully controlled in the electropolymerisation of the SCs, which easily results in irreproducibility and exposure of the SC to water. A more suitable approach of preparing reproducible SCs is the use of dispersions with nanometre-sized CP particles. This will result in smooth surfaces with a good surface coverage (protection) of the underlying metal substrate. This study shows that there is still a need of new CP-based SC materials.
References
Oyama N, Hirokawa T, Yamaguchi S, Ushizawa N, Shimomura T (1987) Anal Chem 59:258
Cadogan A, Gao Z, Lewenstam A, Ivaska A, Diamond D (1992) Anal Chem 64:2496
Sutter J, Lindner E, Gyurcsányi R, Pretsch E (2004) Anal Bioanal Chem 380:7
Bobacka J (2006) Electroanalysis 18:7
Bobacka J, McCarrick M, Lewenstam A, Ivaska A (1994) Analyst 119:1985
Paciorek R, van der Wal PD, de Rooij NF, Maj-Zurawska M (2003) Electroanalysis 15:1314
Sutter J, Pretsch E (2006) Electroanalysis 18:19
Chumbimuni-Torres KY, Rubinova N, Radu A, Kubota LT, Bakker E (2006) Anal Chem 78:1318
Rubinova N, Chumbimuni-Torres K, Bakker E (2007) Sens Actuators B 121:135
Sutter J, Radu A, Peper S, Bakker E, Pretsch E (2004) Anal Chim Acta 523:53
Hulanicki A, Michalska A (1995) Electroanalysis 7:1
Michalska A, Hulanicki A, Lewenstam A (1994) Analyst 119:2417
Momma T, Yamamoto M, Komaba S, Osaka T (1996) J Electroanal Chem 407:91
Gyurcsányi RE, Nybäck AS, Tóth K, Nagy G, Ivaska A (1998) Analyst 123:1339
Michalska A, Dumanska J, Maksymiuk K (2003) Anal Chem 75:4964
Michalska A, Appaih-Kusi C, Heng LY, Walkiewicz S, Hall EAH (2004) Anal Chem 76:2031
Konopka A, Sokalski T, Michalska A, Lewenstam A, Maj-Zurawska M (2004) Anal Chem 76:6410
Michalska A, Maksymiuk K (2004) Talanta 63:109
Gyurcsányi R, Rangisetty N, Clifton S, Pendley BD, Lindner E (2004) Talanta 63:89
Michalska A (2005) Electroanalysis 17:400
Michalska A, Maksymiuk K (2005) J Electroanal Chem 576:339
Konopka A, Sokalski T, Lewenstam A, Maj-Zurawska M (2006) Electroanalysis 18:2232
Pawlowski P, Michalska A, Maksymiuk K (2006) Electroanalysis 18:1339
Bobacka J (1999) Anal Chem 71:4932
Bobacka J, Lewenstam A, Ivaska A (2001) J Electroanal Chem 509:27
Bobacka J, Lahtinen T, Nordman J, Häggström S, Rissanen K, Lewenstam A, Ivaska A (2001) Electroanalysis 13:723
Vázquez M, Bobacka J, Ivaska A, Lewenstam A (2002) Sens Actuators B 82:7
Michalska A, Ocypa M, Maksymiuk K (2006) Anal Bioanal Chem 385:203
Sundfors F, Bereczki R, Bobacka J, Tóth K, Ivaska A, Gyurcsányi RE (2006) Electroanalysis 18:1372
Ocypa M, Michalska A, Maksymiuk K (2006) Electrochim Acta 51:2298
Vázquez M, Bobacka J, Ivaska A, Lewenstam A (2004) Talanta 62:57
Vázquez M, Danielsson P, Bobacka J, Lewenstam A, Ivaska A (2004) Sens Actuators B 97:182
Michalska A, Maksymiuk K (2004) Anal Chim Acta 523:97
Cui G, Lee JS, Kim SJ, Nam H, Cha GS, Kim HD (1998) Analyst 123:1855
Han WS, Park MY, Chung KC, Cho DH, Hong TK (2000) Anal Sci 16:1145
Han WS, Park MY, Chung KC, Cho DH, Hong TK (2000) Electroanalysis 13:955
Lindfors T, Ivaska A (2004) Anal Chem 76:4387
Han WS, Chung KC, Kim MH, Ko HB, Lee YH, Hong TK (2004) Anal Sci 20:1419
Kholoshenko NM, Ryasenskii SS, Gorelov IP (2006) Pharm Chem J 40:289
Lindfors T, Aarnio H, Ivaska A (2007) Anal Chem 79:8571
Fibbioli M, Morf WE, Badertscher M, de Rooij NF, Pretsch E (2000) Electroanalysis 12:1286
de Marco R, Veder JP, Clarke G, Nelson A, Prince K, Pretsch E, Bakker E (2008) Phys Chem Chem Phys 10:73
Lindfors T, Harju L, Ivaska A (2006) Anal Chem 78:3019
Lindfors T, Ivaska A (2007) Anal Chem 79:608
Lindfors T, Ivaska A (2002) J Electroanal Chem 531:43
Lindfors T, Harju L (2008) Synth Met DOI 10.1016/j.synthmet.2008.01.009
Lai CZ, Fierke MA, Stein A, Bühlmann P (2007) Anal Chem 79:4621
Lindfors T, Bobacka J, Ivaska A (1997) Anal Chim Acta 355:217
Li Y, Qian R (1993) Synth Met 53:149
Wei D (2007) Organic electronic materials based on poly(aniline) derivatives. Doctoral Thesis, Åbo Akademi University (ISBN 978-952-12-1865-1)
Pivrikas A (2006) Charge transport and recombination in bulk-heterojunction solar cells. Doctoral Thesis, Åbo Akademi University (ISBN 952-12-1786-3)
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications. Wiley, New York (back cover)
Wei D, Pivrikas A, Karhu H, Majumdar HS, Lindfors T, Kvarnström C, Österbacka R, Ivaska A (2006) J Mater Chem 16:3014
Gyurcsányi R, Lindner E (2002) Anal Chem 127:4060
Wessling B (2007) Conductive polymers as organic nanometals (Chapter 1). In: Skotheim TA, Reynolds JR (eds) Handbook of conducting polymers, vol 2: conjugated polymers: processing and applications, part 1. Processing of conjugated polymers. CRC, Boca Raton
Acknowledgements
Dr. Jörg Posdorfer at Ormecon GmbH is gratefully acknowledged for the PANI dispersion (D1003). The author is most grateful for discussions with Dr. Pia Sjöberg-Eerola and Prof. Johan Bobacka concerning POT and PEDOT, respectively. Many thanks also to Prof. Ronald Österbacka at Åbo Akademi University (Department of Physics, Centre of Functional Materials) for discussions of the light sensitivity of CPs. Finally, acknowledgments to Mr. Sten Lindholm for measuring the spectra of room light and the Leica light source. This work is part of the activities of the Åbo Akademi Process Chemistry Centre within the Finnish Centre of Excellence Program (Academy of Finland, 2000–2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindfors, T. Light sensitivity and potential stability of electrically conducting polymers commonly used in solid contact ion-selective electrodes. J Solid State Electrochem 13, 77–89 (2009). https://doi.org/10.1007/s10008-008-0561-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0561-z