Abstract
Flower-like CuO hierarchical nanostructures were synthesized on copper foil substrate through a simple wet chemical route in alkaline media at room temperature. SEM images collected at different reaction times revealed the transformation of initially formed Cu(OH)2 nanowires to flower-like CuO nanostructures. The hierarchical structure of the as-prepared CuO showed high electrocatalytic activity towards the oxidation of glucose making it a promising electrode material for the development of non-enzymatic glucose sensor. The amperometric sensor exhibited a wide linear response to glucose ranging from 4.5 × 10−5 to 1.3 × 10−3 mol L−1 (R 2 = 0.99317) at fixed potential of 0.3 V. The detection limit was 6.9 × 10−6 mol L−1 (LOD = 3σ/s) with a sensitivity of 1.71 μA μmol−1 cm−2. Moreover, the developed sensor offers a fast amperometric response, good selectivity and stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal oxide nanostructures have attracted considerable attention because of their outstanding properties and numerous technological applications [1–3]. They exhibit size-dependent features, i.e. at the nanometer scale, their properties significantly differ from their bulk material, conferring them new optical, magnetical and electronic characteristics. Among these metal oxides, copper oxide (CuO) is an important p-type semiconductor that exhibits a narrow band gap (1.2–2.6 eV) [4, 5]. Thanks to its unique properties such as high stability and excellent electrical conductivity, CuO finds applications in the fields of catalysis, fuel cells, electronics, batteries and sensors [6–8]. Based on their high surface-to-volume ratio, efficient electron transport properties and low-cost, CuO nanostructures are extensively investigated as electrode material for the development of non-enzymatic glucose sensors [9–12].
The Cu(III)/Cu(II) redox couple mediates the electrocatalytic oxidation of glucose. The effect of the morphologies on the electrochemical performance has stimulated the development of many different approaches to synthesize a great variety of CuO nanostructures. For example, Wang et al. electrodeposited copper nanoparticles/poly(o-phenylenediamine) composite onto glassy carbon electrode [11]. Ibupoto et al. prepared CuO nanosheets on a gold-coated glass substrate by a hydrothermal method [12]. Yang et al. showed the formation of a hollow CuO polyhedron using a Cu2O-templated growth approach [13]. Kim et al. reported the synthesis of CuO and single-walled carbon nanotubes (SWCNTs) nanocomposite via simultaneous arc discharging of Cu and graphite followed by oxidation [14]. Another simple approach consists in the synthesis of CuO on copper foil substrates via Cu oxidation in an alkaline medium by a simple wet chemical route at room temperature. By following this method, Chattopadhyay et al. obtained CuO with different morphologies, nanoneedles, nanoflowers and staked flake-like structures depending on the pH solution [15]. These are just some examples among the many possibilities for the development of CuO nanostructures.
In spite of their good accuracy and precision, enzymatic biosensors for glucose detection still suffer some limitations resulting from the dependency of enzyme’s activity on the temperature, humidity, pH, etc. Thus, in the last decades, considerable attention has been directed towards the development of non-enzymatic glucose sensors [16, 17]. This way, metal oxides, hybrid metal oxides, noble metals, graphene, carbon nanotubes, polymers and other composites have been explored for application in glucose sensors.
Although that graphene-, noble metals- and carbon nanotubes-based sensors are highly sensitive, selective, stable and reproducible, they are expensive compared to other materials, especially copper. This metal is low-cost, and as mentioned above, CuO nanostructures can be readily formed on its surface making it a suitable material for glucose electrooxidation.
In this study, flower-like hierarchical nanostructures of CuO were prepared on a copper foil via a template-free wet chemical method in an alkaline medium. The as-synthesized material showed excellent electrocatalytic properties towards the oxidation of glucose, pointing out its potential usefulness as working electrode for the development of glucose sensor.
Experimental
Materials and reagents
All reagents were of analytical grade and used as received without any further purification: hydrochloric acid, absolute ethanol, ammonium hydroxide solution and sodium hydroxide. Copper foil was purchased from Sigma-Aldrich, with 99.98 % purity and 0.25 mm of thickness. All solutions were prepared using a deionized water of resistivity not less than 18.2 MΩ cm (Milli-Q purification system, Millipore). The electrochemical studies were performed using 0.1 mol L−1 NaOH as the supporting electrolyte. Glucose standard solutions were freshly prepared before each measurement.
Synthesis of flower-like CuO hierarchical nanostructures
The in situ oxidation process allows the formation of flower-like CuO hierarchical nanostructures on copper foil, as previously described [15]. In a typical procedure, the copper foil was firstly pre-treated to remove the impurities from the metal surface, as well as oxide layers. Cu (0.5 cm × 1.0 cm) was washed with 4 mol L−1 HCl solution for 15 min and then with ultra-pure water and absolute ethanol. The treated copper foil was then immersed in a solution consisting of 0.0125 mol L−1 NaOH and 0.0975 mol L−1 NH3·H2O (pH = 11.5). The reaction proceeds for a period ranging from 6 to 96 h at 22 °C. Two replicate experiments were conducted to assess the reproducibility. After the reaction time, the sample was removed, washed with deionized water and dried in air at 60 °C for 3 h. For the electrodes, the 96 h reaction time was chosen due to the homogeneous and complete coverage. Also, a larger piece of copper (0.5 cm × 2.0 cm) was used for the electrode to make the electric contact and the experiment was performed in triplicate. The geometric area of the CuO electrode was 0.5 × 0.5 cm.
Characterization
The morphologies of the as-grown CuO were investigated by scanning electron microscopy (SEM) using a JEOL JSM-6060 instrument operated at 20 kV. X-ray diffraction (XRD) pattern was obtaining by a Rigaku Dengi D-Max 2000 equipment operated at 30 kV voltage and 15 mA current using a monochromatic Cu Kα radiation (λ = 1.5406 Å) in the range 20–70°. The electrochemical measurements were performed using a potentiostat (Autolab, PGSTAT302N model). The cell consists of a system of three electrodes, the synthesized working electrode, a platinum wire as the counter electrode and saturated calomel electrode as the reference electrode.
Results and discussion
Characterization of the flower-like CuO structures
SEM images were registered at different reaction times to monitor the evolution in the formation of the flower-like structures on the copper foil. Fig. 1(a–f) reveals the morphology of the copper surface before the immersion in the alkaline solution (a) and after 6, 15, 24, 68 and 96 h of reaction times (b–f). After the 6 h of reaction, the metal is covered but not completely by grass-like nanowires (Fig 1b). As the reaction proceeds, a full coverage is achieved, but at this time concomitantly with the nanowires starting to appear another kind of arrangement (Fig. 1c). It seems that thinner and shorter nanowires start branching out and forming agglomerates. At 24 h of reactions, these structures become predominant (Fig. 1d). Subsequent growth of these agglomerated structures leads to the formation of 3D flower-like architectures (Fig. 1e–f). Based on this time-dependent experiment, a growth mechanism of CuO is proposed in Fig. 1f, drawing the different stages starting from the formation of nanowires until the achievement of the flower-like hierarchical structures. According to the reported references [15, 18], the chemical reactions involved in the growth of CuO by the treatment of copper foils with NH3·H2O and NaOH is as follows:
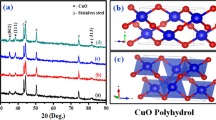
It is known that the oxidation of copper in the air or under humid conditions proceeds very slowly, but the presence of alkali solutions dramatically accelerates the reactions. In the initial stage of the reaction, the copper foil releases Cu+2 ions into the solution forming the [Cu(NH3)4]+2 complex (Eq. 1). The high −OH concentration causes the formation of Cu(OH)2 which subsequently dehydrates producing CuO (Eq. 2–3). As a precursor, the presence of Cu(OH)2 may become apparent at short reaction times. Thus, the observed nanowires can be strongly associated with the layered structure of orthorhombic Cu(OH)2. According to previous studies, Cu(OH)2 has a preferred growth direction and usually form one-dimensional nanostructures [19, 20]. Since the reaction also depends on the temperature, another reaction was performed at 4 °C, attempting this time to obtain the Cu(OH)2 nanowires exclusively. Figure 2 depicts the SEM image and XRD diffraction pattern of the obtained material. Low-intensity diffraction peaks are observed at 2Ɵ values of 23.89°, 33.9°, 35.8°, 39.7° and 64.6°. They are indexed to (021), (002), (111), (130) and (152) copper hydroxide orthorhombic planes (JCPDS 13-420). The lattice constants were calculated as a = 2.88 Å, b = 11.03 Å and c = 5.03 Å, which are also in good agreement with the standard card. Based on these evidences, therefore, the nanowires structures correspond to Cu(OH)2.
Figure 2 also displays the XRD patterns of the flower-like CuO grown on copper foil. The peaks located at 2Ɵ values of 35.6°, 38.7°, 67.2° and 67.9° correspond to the planes (002), (111), (311) and (113), respectively. Those belong to CuO monoclinic phase (JCPDS 45-937), with calculated lattice parameters is c = 5.11 Å. Furthermore, the grain size of 25 nm was calculated by using the Scherrer equation, D hkl = 0.89λ/βcosθ, where λ is the wavelength of Cu Kα line (λ = 0.15406 nm), θ is the Bragg diffraction angle of measured plane and β is the full width at half maximum (FWHM) of the (002) peak of CuO [21].
Figure 3 shows enlarged SEM images of the side view (a) and top view (b) where the hierarchical structure of the as-synthesized CuO can be seen clearly. At the bottom, assembly of thick branches constituted of stacked nanowires is developed. Over this structure, small and short branches highly interconnected arise leading to the formation of the 3D structure observed from the top view. As indicated by arrowheads, it seems that CuO also presents a preferential orientation growth.
Electrocatalytic activity of CuO electrode towards glucose oxidation
The electrocatalytic activity of the synthesized CuO nanostructures was evaluated by using cyclic voltammetry (CV). Figure 4a shows the CV curves of the electrode in 0.1 mol L−1 NaOH solution with the concentrations of glucose ranging from 0 to 2 × 10−3 mol L−1 at a scan rate of 10 mV s−1. The signals ascribed to the Cu(III)/Cu(II) redox couple that handles the glucose oxidation appear broad and poorly defined in the absence of glucose (dotted line). But as expected, a remarkable catalytic peak resulted by increasing the glucose concentration. The decrease in the cathodic current that occurs in the backward swept constitutes another evidence of the electrocatalytic activity of CuO towards glucose oxidation. Figure 4b exhibits the response of the untreated copper electrode in the absence and the presence of glucose as a comparison. There are differences in the voltammetric profiles; the oxidation potential of CuO electrode (dotted line) is less positive than the copper electrode as a consequence of the electrocatalytic effect while the current is similar. But it must take into consideration that this comparison is not entirely accurate because the measurement is carried out in an alkaline medium, which can promote the formation of the oxide on the surface of the copper electrode during the positive scan. The alkaline solution is necessary to ensure the electrocatalytic activity of CuO as shown in Fig. 5. The shape of the voltammetric curve greatly depends on the NaOH concentration; the peaks become more pronounced as the electrolyte concentration increased, which confirmed that the electrocatalytic oxidation of glucose involves the hydroxyl group (−OH). A maximum peak current is reached at 0.1 mol L−1 NaOH, so this concentration was chosen for subsequent experiments. This fact can be explained considering the following reactions [9, 16]:
a CVs for the CuO electrode recorded in the absence (dotted line) and the presence (solid line) of glucose (0.25, 0.50, 0.75, 1.0, 1.5 and 2.0 × 10−3 mol L−1). b CVs for the CuO electrode (dotted line) and untreated copper electrode (solid line) recorded in the absence and the presence of 5.0 × 10−3 mol L−1. 0.1 mol L−1 NaOH and scan rate of 10 mV s−1
Amperometric determination of glucose
The amperometric response was preferred to assess the analytical performance of the CuO electrode. This technique is easy to use and furthermore shows low background current that is important to achieve higher sensitivity. Here, the applied potential is an important parameter that must be carefully selected to achieve a higher current at lower potential value; the latter is important to reduce the interferences from other oxidizable species. Figure 6a shows the current responses registered as a function of the applied potential from 0 to 0.45 V in the presence of 1.0 × 10−3 mol L−1 glucose. A strong electrocatalytic activity becomes evident as the potential is shifted to more positive values, reaching a maximum current response at +0.3 V, so this potential was taken as the optimum potential. This value is quite similar to previously described CuO-based electrodes, but at least 100 to 200 mV less positive when compared with many others as shown in Table 1. The decrease in the overpotential suggests that the 3D hierarchical structure enhance the electron transfer reactivity of CuO.
Figure 6b shows the step-like amperometric responses of the CuO electrode with successive addition of glucose at an applied potential of +0.3 V; the average response of three repeated measurements was used to establish the calibration curve with 3 % RSD. As shown in Fig. 6c, the oxidation current is proportional to glucose concentration up to 1.33 × 10−3 mol L−1. The current response equation is i (A) = 3.55 × 10−5 + 0.853 [Glu] with a correlation coefficient of 0.99317. The estimated limit of detection (LOD) is 6.9 × 10−6 mol L−1 based on 3σ/s, where σ is the standard deviation of the blank currents (n = 8), and s is the slope of the calibration curve. The detection limit is lower than the normal blood glucose level (6.1 × 10−3 mol L−1), indicating that the as-prepared CuO electrode is suitable for monitoring glucose in blood. The sensitivity of the CuO electrode is 1.71 μA μmol−1 cm−2.
Stability, reproducibility and anti-interference of the CuO electrode
Three CuO electrodes prepared under the same conditions gave a 2.7 % RSD by measuring the peak current using cyclic voltammetry and 2.9 % taking the current registered by chronoamperometry, indicating that the proposed method produced reliable and reproducible electrodes. Also, successive amperometric measurements yielded reproducible currents with the RSD of 3 %. Figure 7 shows the amperometric response of the CuO electrode in the presence of 5.0 × 10−3 mol L−1 of glucose throughout an experiment prolonged for 10 min. The response remains constant during the experiment, a decrease of less than 2 % observed in the current indicates good stability. CuO is a highly stable electrode material and preserves its activity by storing in air at room temperatures.
The presence of some oxidizable biomolecules in blood that commonly co-exist with glucose may affect the electrode response. Thus, the anti-interference test was performed to evaluate the selectivity of the glucose sensor. Figure 8a shows the amperometric response of 1.0 × 10−3 mol L−1 glucose, followed by the additions of 0.1 × 10−3 mol L−1 of dopamine (DA), uric acid (UA) and ascorbic acid (AA). These concentrations were taken considering that the concentration of glucose in the healthy human blood is higher than these others. The small response of DA, UA and AA compared to glucose indicates that this electrode can selectively determine the glucose concentration in the coexistence of these interfering agents. The addition of 0.1 × 10−3 mol L−1 of NaCl also does not cause interference. The CuO electrode exhibited similar electrocatalytic responses for other carbohydrates like fructose and galactose; this result is not surprising considering the mechanism involved in the oxidation. However, the electrode was less efficient for mannose and also showed only a slight catalytic current after the addition of sucrose; thereby exhibiting its ability for selective oxidation of sugars as shown in Fig. 8b.
a) Anti-interference test of the CuO electrode in 0.1 mol L−1 NaOH at an applied potential of +0.30 V in the presence of 1.0 × 10−3 mol L−1 followed by the addition of 1.0 × 10−4 mol L−1 DA, UA, AA and NaCl. b Amperometric response of 3 × 10−4 mol L−1 glucose in the absence and presence of 3 × 10−4 mol L−1 of fructose, sucrose, mannose and galactose
Real Sample analysis
To evaluate the ability of the proposed sensor for routine analysis, 50 μL of serum samples were diluted with 25 mL of 0.1 mol L−1 NaOH solutions. The amperometric response of glucose was recorded at a potential of +0.30 V and determined by the standard addition method. The results presented in Table 2 reveal good agreement with the glucose concentration determined by a routine enzymatic method in a regional hospital, with RSD of 7.3 and 2.9 %. Recovery studies also showed an insignificant matrix effect of the serum components in the glucose determination.
Conclusions
The oxidation of copper foil in NH3·H2O and NaOH solution is a simple, low-cost and reliable method that allowed the formation of flower-like CuO hierarchical nanostructures at room temperature. The as-prepared material was successfully applied as working electrode for electrochemical glucose detection. The high electrocatalytic activity, low limit of detection, high sensitivity and easy fabrication make this material a promising electrode in the development of a non-enzymatic glucose sensor. Also, the prepared electrode showed good stability and selectivity towards common interfering biological species.
References
Sun YF, Liu SB, Meng FL, Liu JY, Jin Z, Kong LT, Liu JH (2012) Sensors 12:2610–2631
Hahn YB, Ahmadwa R, Tripathyw N (2012) Chem Commun 48:10369–10385
Liu S, Sun S, You XZ (2014) Nanoscale 6:2037–2045
Koffyberg FP, Benko FA (1982) J Appl Phys 53(2):1173–1177
Ghijsen J, Tjeng LH, Van Elp J, Eskes H, Westerink J, Sawatzky GA (1988) Phys Rev B 38(16):11322–11330
Yang Z, Wang D, Li F, Liu D, Wang P, Li X, Yue H, Peng S, He D (2013) Mater Lett 90:4–7
Zhang Q, Zhang K, Xu D, Yang G, Huang H, Nie F, Liu C, Yang S (2014) Prog Mater Sci 60:208–337
Zoolfakar AS, Rani RA, Morfa AJ, O’Mullane AP, Zadeh KK (2014) J Mater Chem C 2:5247–5270
Huang F, Zhong Y, Chen J, Li S, Li Y, Wang F, Feng S (2013) Anal Methods 5:3050–3055
Li X, Wei C, Fu J, Wang L, Chen S, Li P, Li H, Song Y (2014) RSC Adv 4:52067–52073
Liu L, Chen Y, Lv H, Wang G, Hu X, Wang C (2015) J Solid State Electr 19:731–738
Ibupoto ZH, Khun K, Beni V, Liu X, Willander M (2013) Sensors 13:7926–7938
Kong C, Tang L, Zhang X, Sun S, Yang S, Song X, Yang Z (2012) Anal Methods 4:3174–3179
Dung NQ, Patil D, Jung H, Kim D (2013) Biosens Bioelectron 42:280–286
Jana S, Das S, Das NS, Chattopadhyay KK (2010) Mater Res Bull 45:693–698
Tian K, Prestgard M, Tiwari A (2014) Mater Sci Eng C 41:100–118
Toghill KE, Compton RG (2010) Int J Electrochem Sci 5:1246–1301
Wen X, Zhang W, Yang S (2003) Langmuir 19:5898–5903
Liu J, Huang X, Li Y, Li Z, Chi Q, Li G (2008) Solid State Sci 10:1568–1576
Wang Y, Jiang T, Meng D, Kong J, Jia H, Yu M (2015) RSC Adv 5:16277–16283
Singh DP, Ojha AK, Srivastava ON (2009) J Phys Chem C 113:3409–3418
Zhao Y, Zhao J, Li Y, Ma D, Hou S, Li L, Hao X, Wang Z (2011) Nanotechnology 22:115604
Dong J, Ren L, Zhang Y, Cui X, Hu P, Xu J (2015) Talanta 132:719–726
Jiaojiao J, Yangyang G, Gangying Z, Yanping C, Wei L, Guohua H (2015) Food Chem 175:485–493
Zhang J, Ma J, Zhang S, Wang W, Chen Z (2015) Sensors Actuators B Chem 211:385–391
Zhang J, Zhu X, Dong H, Zhang X, Wang W, Chen Z (2013) Electrochim Acta 105:433–438
Zhang J, Ding N, Cao J, Wang W, Chen Z (2013) Sensors Actuators B Chem 178:125–131
Acknowledgments
The authors acknowledge the financial supports from CNPQ (Process: 550441/2012-3), INCTBio (CNPq/INCT 573672/2008-3) and Propesq/UFRGS. The authors are also grateful to CAPES for the scholarship granted to the first author. The serum samples were donated by the Laboratory of Clinical Analysis of the Institute of Cardiology of Rio Grande do Sul, Porto Alegre-RS, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medeiros, N.G., Ribas, V.C., Lavayen, V. et al. Synthesis of flower-like cuo hierarchical nanostructures as an electrochemical platform for glucose sensing. J Solid State Electrochem 20, 2419–2426 (2016). https://doi.org/10.1007/s10008-016-3163-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3163-1