Abstract
Composite materials of Prussian blue–polypyrrole (PB/PPy) on the surface of indium tin oxide (ITO)-coated glasses were obtained via one-step chemical (redox) and one-stage electrochemical procedures in mixed solution of iron (III), hexacyanoferrate (III), and pyrrole with various concentration ratios of components in nitrate supporting electrolyte. Electrochemical stability of composite films depends on the amount of Py in synthetic solution, whereas color contrast coefficient values depend on the type of synthetic procedure. PB/PPy film electrochromic response (tested by spectroelectrochemical potentiodynamic measurements) was compared with response of both pure PB and pure PPy films. It was shown that degradation of composite films occurs due to PB component instability in Prussian white form. The highest value of color contrast coefficient and great electrochemical stability were revealed for composite films obtained via redox-synthesis procedure from solution with 0.1 mM [Fe3+ + Fe(CN)6 3−] and 1.0 mM Ру (PB/PPy-Ch-1:1:10 system).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prussian blue (PB), FeIII 4[FeII(CN)6]3, is a promising material for the creation of electrochromic devices (ECD) [1–3] because of its ability to change the color from dark blue to colorless owing to its redox transformation to the reduced form (Prussian white (PW)). The principal obstacle for such applications of PB-based materials is the fast degradation of its electrochromic properties, mostly due to peeling off fragments of the electroactive film from the substrate [4]. One of the ways to solve this problem is to use a nonaqueous medium as an electrolyte for ECD [5, 6]. Another approach is related to composite or layer structures containing this inorganic component and organic compounds, usually conducting polymers, which are able to act as both the electrolyte [6] and one of electrodes [7, 8]. Synthesis of these composite layers is carried out electrochemically [9] or via chemical (redox reaction) procedure [10], with the use of the solutions of individual components [11] or mixed solutions [12]. However, the stability of electrochromic properties of PB/conducting polymer composites generated via such methods is insufficiently high as yet.
Previously, we reported some results concerning influence of synthetic solution composition on the stability of PB/PPy films formed via one-step and one-pot redox procedure [13]. It was shown that the use of chloride anions in synthetic solution leads to instability in amperometric response of a PB/PPy-modified electrode in H2O2 solution in comparison with films obtained in nitrate solutions due to morphological features of PB/PPy films.
The actual work is aimed to compare the stability of electrochromic response and contrast of color transformation for nanostructured PB/PPy composite films in dependence on synthetic procedure features (electrochemical deposition or chemical redox synthesis from mixed solution of components with various concentration ratios).
Experimental
Our original one-step methods of PB/PPy composite synthesis [12–14] have provided nanostructured materials successfully used as sensors towards hydrogen peroxide with stable amperometric response [14]. It has however not been explored if these methods are appropriate for deposition of composite coatings on the surface of optically transparent electrodes (glassy plates coated with indium tin oxide (ITO)) possessing a high-stability electrochemical response. In the present work, these one-step methods of redox and electrochemical syntheses have been modified to produce PB/PPy coatings with a high adhesion to the surface of such ITO–glass electrodes.
Basic principles of the electrochemical synthesis were described in detail in [14]. It was carried out in a single-compartment three-electrode cell under inert Ar atmosphere with parallelly located ITO–glass plate as a working electrode and Pt plate as a counter electrode while Ag/AgCl-aq.sat.KCl served as a reference electrode (all potential values below are given vs. this RE). Several series of alternate cathodic–anodic potential pulses separated by relaxation periods (under open circuit potential mode) were imposed. In the course of this procedure, the PB/PPy composite film is deposited on the surface of the working electrode from mixed solution containing both ferric salts, Fe(NO3)3 (0.5 mM) and K3[Fe(CN)6] (0.5 mM), as well as Py monomer (1 mM). 0.1 M KNO3 + 0.1 М HNO3 aqueous solution was used as a background electrolyte.
Film deposition was performed with variation of such parameters as the amplitude of voltage pulses, their duration, and number (for optimization of the double-pulsed electrochemical procedure given in [14]). Optimized procedure (used for PB/PPy electrodeposition in this work) includes such steps: +0.745 V for 50 s for internal PPy layer deposition; then, 5 series of 10 double potential pulses: +0.695 V (10 s) and +0.395 V (5 s); series were separated by relaxation periods: 2 cyclic voltammetry (CV) cycles (scan rate 100 mV/s) between −0.005 V and +0.595 V, Е start = +0.545 V; 24 s; final step: +0.745 V for 100 s for external PPy layer deposition. Similar procedure was also used for the formation of pure PPy or PB films (from solution of monomer or iron salts correspondingly with concentration of components mentioned above).
Redox synthesis procedure described in detail in [13] consisted in the immersion of the working electrode into mixed reagent solution of the same composition for 48 h. PB/PPy composite film was deposited on electrode surface as a consequence of the redox reaction between components in solution. Concentration of mixed solution was varied: PB/PPy films were deposited from reaction mixtures with 0.1 mM Fe3+, 0.1 mM [Fe(CN)6]3−, and 0.5 mM Py (system PB/PPy-Ch-1:1:5); 0.1 mM Fe3+, 0.1 mM [Fe(CN)6]3−, and 1.0 mM Py (system PB/PPy-Ch-1:1:10); 0.5 mM Fe3+, 0.5 mM [Fe(CN)6]3−, and 0.5 mM Py (system PB/PPy-Ch-1:1:1); and 0.5 mM Fe3+, 0.5 mM [Fe(CN)6]3−, and 1.0 mM Py (system PB/PPy-Ch-1:1:2).
For both procedures, the adhesion of composite films (their thickness did not exceed 200 nm) to the ITO-coated glass electrode surface was evaluated by their detachment from electrode surface mechanically.
Morphology of PB/PPy composite films was characterized by scanning electron microscopy (SEM) (Zeiss LEO SUPRA 25), atomic force microscopy (AFM), and also transmission electron microscopy (TEM) (Jeol JEM 2100).
Redox and electrochromic properties of such PB/PPy composite films in contact with background nitrate solution were studied by means of electrochemical (cyclic voltammetry (CV)) and spectroscopic methods as well as their combination (spectroelectrochemistry). Multi-cycle CV curves (scan rate 100 mV/s) were recorded within the range between −0.005 V and +0.695 V. Spectroelectrochemical characterization of PB/PPy coatings on optically transparent electrodes was performed with the use of a homemade miniature single-compartment three-electrode cell (including Ag/AgCl reference electrode separated by glassy frit from the working solution) constructed on the basis of conventional spectroscopic quartz cuvette (Hellma, optical length 10 mm) which may be inserted into the holder of the spectrophotometer Lightwave II (Biochrom). This device enables us to carry out electrochemical and spectroscopic measurements simultaneously, in particular to record absorbance at 720 nm for various oxidation states of the film depending on the electrode potential (Fig. 1). By means of the spectroelectrochemical method, one can study both electrochromic properties of composite films and the stability of their electrochromic switch.
Results and discussion
SEM images demonstrate homogeneity of film surfaces at the microscale, without cracks or defects (Fig. 2). TEM images (Fig. 3) and AFM results (not presented) show the structure of composite coatings at the submicrometer scale: PB crystals (size about 100 nm) are surrounded by PPy matrix. Microscopic data clearly indicate the influence of Py content in reaction mixture on morphology of PB/PPy films. Composites in which isolated PB crystals are distributed inside polymer matrix were deposited from solutions with high content of Ру (1 mM), whereas in films formed in solution with low content of Py (0.5 mM), one can see “agglomerates” of PB crystals with PPy globules and chains distributed between them.
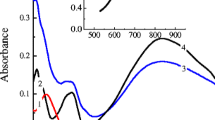
Figure 4 presents absorption spectra of PB/PPy composite film on the surface of optical transparent electrode corresponding to its PB and PW redox states (curves 1 and 2, respectively). Incomplete decoloration of the film in reduced state originates from the presence of PPy inside composite coating due to its yellowish coloring, even though this contribution of the PPy absorption is almost invisible since the composite film is very thin (~100–150 nm). Color contrast coefficient (CCC) of the film is defined as the ratio of the composite film absorptions in the PB (curve 1) and its reduced PW (curve 2) states at wavelength of 720 nm, i.e., at maximal absorption for the oxidized state. CCC values were estimated for films synthesized under various conditions (Table 1). The data in Table 1 indicate that the highest color contrast is observed for composite coatings deposited from the most dilute solutions via one-step redox procedure. This result can be explained by the smallest contribution of PPy into the film absorption in the reduced state, due to its lowest fraction in the composite for these systems. Color contrast coefficient however is not a stability index for electrochromic film by repeated redox switch.
Absorption spectra of РВ/РРу composite synthesized via chemical procedure (molar ratio 1:1:10) Absorbance was measured for two potential values: +0.545 V (“oxidized” state, PB) for curves 1 and 3 and −0.100 V (reduced state, PW) for curves 2 and 4. Spectra 1 and 2 correspond to the initial state of composite films. Spectra 3 and 4 were recorded after 10 % diminution of the current response (χ i = 90 %) in the course of the multi-CV treatment.
Another principal parameter of electrochromic films, stability of the electrochromic switch between PB and PW states, was estimated via recording the evolution (diminution) of either anodic or cathodic redox charge, Q redox, for the film in the course of its multi-CV treatment. Degradation degree of the film for cycle i, χ i , was calculated by means of the formula:
\( {\chi}_i=\frac{Q_{{\mathrm{redox}}_2}-{Q}_{{\mathrm{redox}}_i}}{Q_{{\mathrm{redox}}_2}}\kern0.5em \cdotp \kern0.5em 100\% \), where Q redox2 and Q redoxi are the redox charges for CV cycle 2 or i, respectively.
The results for the simultaneously measured current response and absorbance of the composite film at 720 nm for cycles 2 and i = 1600 are shown in Fig. 5. The amplitudes of both quantities diminish in the course of the multi-CV procedure (compare curves 1 and 2 as well as 3 and 4) which means a progressive degradation of the film.
Similar comparison between data for cycles 2 and i for the whole spectrum (Fig. 4) shows that this diminution of the film absorbance with the number of cycles takes place for all wavelengths in both “oxidized” (curves 1 and 3) and reduced (curves 2 and 4) states. For each state of the film, the variation of the whole spectrum with cycling only takes place in the range of the inorganic component absorption (PB or PW) while the PPy absorption in the range of 700–900 nm remains constant. It means that the degradation of the composite material is caused by the changes of the inorganic component, expectedly due to insufficient stability of its reduced form, PW, which is washed out of the film due to its “peptization.”
Dependence of degradation degree for various composite PB/PPy films in comparison with pure PB film on the number of CV cycles is presented in Fig. 6. It is clear that addition of Py in synthetic mixture leads to the increase of PB nanoparticles stability due to their distribution inside polymer matrix. As shown in Table 1, the degradation degree, χ i , of composite films which were deposited with the use of redox or electrochemical method under various synthesis conditions did not exceed 30 % even after several thousand potentiodynamic cycles. For pure PB films deposited via similar electrochemical procedure (without adding Py monomer), this parameter reached 50 % already within 100 cycles for 1:1:2 system (system PB-E-1:1) and 12 % for 1:1:10 system. On the contrary, for pure PPy film degradation degree is much lower, ~5 %, within 100 cycles (Fig. 7).
Degradation degree of pure PB film deposited from solution with FeIII(NO3)3:K3[FeIII(CN)6] molar ratio 1:1 (1) and composite PB/PPy films on the ITO–glass electrode deposited from solutions with FeIII(NO3)3:K3[FeIII(CN)6]:Py molar ratios 1:1:10 (2) and 1:1:2 (3) via electrochemical procedure and via chemical procedure from solutions with FeIII(NO3)3:K3[FeIII(CN)6]:Py molar ratios 1:1:5 (4), 1:1:10 (5), 1:1:1 (6), and 1:1:2 (7)
It was found out that good adhesion (the coating was not detached by rubbing or even ultrasonic treatment) was observed for films deposited via redox synthesis from mixed solutions of four different compositions (Table 1) or via electrochemical procedure from a solution of composition PB/PPy-E-1:1:10 (their parameters are given in Table 1). Synthesis conditions affect significantly the coloration intensity of composite films while all these coatings were very uniform as it is shown by their photos in Table 1.
Conclusion
Thus, one can conclude that our one-step deposition procedures (redox reaction in solution or double cathodic–anodic potential pulses) result in composite Prussian blue–polypyrrole films possessing both a high color contrast and a high stability of their electrochromic switch which exceeds strongly that of pure PB films. Such properties make these materials prospective for ECD applications. Composite films formed via redox synthesis procedure from solution where molar ratio Fe3+:[Fe(CN)6 3−]:Ру was 1:1:10 and 1:1:5 demonstrate the highest value of color contrast coefficient and the lowest degradation degree.
References
Mortimer RJ (1997) Electrochromic materials. Chem Soc Rev 26:147–156
Kawai M, Miyagi H, Ura M (1989) Pat US4801195. Nissan Motor Co
Ahmad S, Agnihotry SA, Deepa M (2014) Pat US8790537B2
Somani P, Radhakrishnan S (1998) Electrochromic response in polypyrrole sensitized by Prussian blue. Chem Phys Lett 292:218–222
Piroux F, Petit P, Andreau A (2010) Pat US20100027098A1. Saint Gobain Glass France
Heckner KH, Kraft A (2002) Pat WO2002040578B1. Gesimat
Andreau-Wiedenmaier A, Fanton X, Gillissen M, Giron JC, Joeris H, Letocart P, Messere R, Paffen KH (2008) Pat WO2008012461A3. Saint Gobain Glass France
Tung TS, Ho KC (2006) Cycling and at-rest stabilities of a complementary electrochromic device containing poly (3,4-ethylenedioxythiophene) and Prussian blue. Sol Energy Mater Sol Cells 90:521–537
Somani P, Mandale AB, Radhakrishnan S (2000) Study and development of conducting polymer-based electrochromic display devices. Acta Mater 48:2859–2871
Wang H, Guo C, Zhou S, Hu X, Hu Y, Li F, Miao Y (2012) One-step synthesis and self-organization of polypyrrole ultrathin films inlayed with Prussian blue nanoparticles induced by a drop of toluene solution on water surface. Thin Solid Films 520:2026–2031
DeLongchamp DM, Hammond PT (2004) High-contrast electrochromism and controllable dissolution of assembled Prussian blue/polymer nanocomposites. Adv Funct Mater 14(3):224–232
Zolotukhina EV, Vorotyntsev MA, Bezverkhyy I, Borisova AV, Karyakin AA, Zolotov YA (2012) Composite materials based on Prussian blue nanoparticles and polypyrrole for design of a highly stable sensor for hydrogen peroxide. Dokl Phys Chem 444(1):75–78
Talagaeva NV, Zolotukhina EV, Bezverkhyy I, Konev DV, Lacroute Y, Maksimova EY, Koryakin SL, Vorotyntsev MA (2015) Stability of Prussian blue–polypyrrole (PB/PPy) composite films synthesized via one-step redox-reaction procedure. J Solid State Electrochem 19:2701–2709
Zolotukhina EV, Bezverkhyy IS, Vorotyntsev MA (2014) One-stage periodical anodic-cathodic double pulse deposition of nanocomposite materials. Application to Prussian blue/polypyrrole film coated electrodes. Electrochim Acta 122:247–258
Acknowledgments
The study was performed with the support of the Ministry of Education and Science of Russian Federation (FTsP grant No. 2014-14-576-0056-058, contract 14.574.21.0004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talagaeva, N.V., Zolotukhina, E.V., Pisareva, P.A. et al. Electrochromic properties of Prussian blue–polypyrrole composite films in dependence on parameters of synthetic procedure. J Solid State Electrochem 20, 1235–1240 (2016). https://doi.org/10.1007/s10008-015-3116-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-3116-0