Abstract
Follicular lymphoid hyperplasia is a very rare though benign reactive process of an unknown pathogenesis that may resemble a follicular lymphoma, clinically and histologically. Oral reactive follicular hyperplasia (RFH) has been described on the hard or soft palate and at the base of the tongue. We describe here the first case of RFH presenting as an aggressive tumor on the right posterior side of the maxilla in a 24-year-old male patient. The lesion had a clinical evolution of 18 months and was noticed after the surgical extraction of the right third molar, although we cannot assume a cause-effect relation with that surgical event whatsoever. His medical history was unremarkable. Following an incisional biopsy, histological examination revealed lymphoid follicles comprised by germinal centers surrounded by well-defined mantle zones. The germinal centers were positive for Bcl-6, CD10, CD20, CD21, CD23, CD79a, and Ki-67, while negative for Bcl-2, CD2, CD3, CD5, and CD138. The mantle and interfollicular zones were positive for Bcl-2, CD2, CD3, CD5, CD20, and CD138. Both areas were diffusively positive for kappa and lambda, showing polyclonality. The patient underwent a vigorous curettage of the lesion with no reoccurrences at 36 months of follow-up. This case report demonstrates that morphologic and immunohistochemical analyses are crucial to differentiate RFH from follicular lymphoma, leading to proper management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive follicular hyperplasia (RFH) is a reactive lymphoid proliferation to several unknown antigenic stimulus and resembles clinically and histologically the follicular lymphoma [1, 2]. Both lesions can present as bilateral or unilateral growths, which can be firm or soft, and generally exhibit an intact surface mucosa [2].
Oral RFH is a very rare and benign entity [3] that has been described on the palate [3,4,5] or at the base of the tongue [6]. RFH lesions affect predominantly older female patients, ranging from 38 to 79 years, with a mean evolution of 9 months [1]. On the other hand, non-Hodgkin lymphoma (NHL) is the most usual lymphoma of the paranasal sinuses and oral cavity [7, 8]. It frequently consists of a B cell lymphoma (most commonly a diffuse large B cell lymphoma) and mainly affects men from 23 to 94 years old [8, 9].
This case report describes the first case of a male patient presenting an oral RFH as an aggressive tumor on the right posterior side of the maxilla, followed by its immunohistochemical profile and successful management, which comprised the curettage of the lesion.
Case report
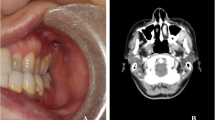
A 24-year-old man was referred for evaluation of a slightly symptomatic lesion composed by a noteworthy diffuse swelling located on the right side of the maxilla. The lesion was noticed after the surgical extraction of the right third molar, although we cannot assume a cause-effect relation with that surgical event whatsoever, being followed by a clinical evolution of 18 months. There were no extraoral alterations. The patient also denied any drug therapy, and his medical history was unremarkable. The intraoral examination revealed a firm swelling covered by normal mucosa, extending from the right lateral incisor to the right second molar (Fig. 1). The alveolar mucosa near the extracted right third molar was completely healed. Radiographically, a radiolucent lesion of irregular contours led to the resorption of the posterior wall and floor of the right maxillary sinus. The tomographic exam revealed an irregular radiotransparent lesion, causing bone destruction and invasion of the right maxillary sinus, adjacent to the apical portions of first and second molars (Fig. 2a–d).
Cone beam computed tomographic scans. a Planar parabolic curve manually adjusted along the dental arch to generate panoramic view. b Panoramic view showing irregular radiotransparent lesion causing bone destruction and invasion of the right maxillary sinus and adjacent to the apical portions of first and second molars. c Coronal view; note the alveolar bone destruction and the partial involvement of the inferior wall of the right maxillary sinus. d Sagittal view confirming the destructive aspect of the lesion
The clinical differential diagnosis was effectively based on the appearance and localization of the intraoral elevated mass, as well as the infiltrating and resorbing behavior of the lesion stated by its tomographic manifestation. In that way, several lesions could have been included in the differential diagnosis of this present case; we thus highlighted the possibility of the following malignant neoplasms: squamous cell carcinoma (SCC) of the maxillary sinus, sinonasal undifferentiated carcinoma, salivary gland neoplasms, and lymphoma.
After local anesthesia, an incisional biopsy was performed and the histopathologic study revealed a follicular lymphoid hyperplasia intermingled by fibrous connective tissue (interfollicular zone). The follicles were comprised by germinal centers (GCs) surrounded by well-defined mantle zones; both the GC and the mantle zones contained atypical lymphocytes, and the mantle zones still presented macrophages and occasional mitoses. Overlying the lymphocytic lesion, an acantotic epithelium and the lamina propria of the oral mucosa could be perceived (Fig. 3).
The immunostaining patterns of the evaluated proteins were as follows: Bcl-2 was consistently negative within the GC and positive within the mantle and interfollicular zones (Fig. 4a). Bcl-6, on the other hand, was weakly positive within the GC (Fig. 4b). CD3 was diffusively positive within the interfollicular zone (Fig. 4d), while CD2 and CD5 were widely positive (Fig. 4c, e, respectively). CD20 was strongly positive within the GC, mantle, and interfollicular zones (Fig. 4f). CD21, CD23, and CD79a stained the GC (Figs. 4g, h and 5b, respectively), whereas CD138 was positive within the interfollicular zone (Fig. 5c). CD10 revealed weak positivity within the GC (Fig. 5a). Synaptophysin was negative (Fig. 5d). Although cyclin D1 was negative (Fig. 5e), Ki-67 was strongly positive (Fig. 5f). The immunohistochemical studies unveiled polyclonality, which was illustrated by diffuse positivity for kappa and lambda light chains (Fig. 5g, h).
Thus, based on the clinical, histopathologic, and immunohistochemical findings, the final diagnosis was defined as RFH.
Following diagnostic confirmation and under general anesthesia, the patient underwent a vigorous curettage of the lesion. After 36 months of follow-up, the patient showed complete healing of the region and no reoccurrences until the present moment (Figs. 6 and 7).
Cone beam computed tomographic scans at the 36-month follow-up. a Planar parabolic curve manually adjusted along the dental arch to generate panoramic view. b Panoramic view showing complete bone healing with no signs of recurrence. c Coronal view. d Sagittal view confirming the absence of recurrence
Discussion
The etiology of RFH is unknown; nonetheless, this lesion can be a reactive lymphoid proliferation induced by some unspecified antigenic stimulation [2], mainly when the lesion develops in children and young adults, showing the follicular pattern characterized by proliferation of B cells, as an outcome of a humoral immune reaction stimulated by some antigens [10]. The RFH reported herein showed CD20-positive B cells in the germinal centers and, thus, could be related to any antigenic stimulus from the tooth extraction. In addition, as the patient was referred to our clinic after having the exodontia, we cannot affirm if the lesion was somehow inducted by the tooth extraction and/or appeared afterwards or if the lesion was already incipient and even doomed the respective molar.
Regarding the clinical presentation of RFH, it occurs more frequently in older patients (mean age of 62 years) and it also presents a tendency to be more common in women [4]; both of these aspects were not observed in the present case, because the patient was a young male. Still more abnormally, RFH usually grows on one side of the palate [1, 3, 4], while the present report is illustrated by a considerable swelling on the right side of the maxilla. Such presentation is truly unique and has never been described; the only reported case that originated from a different site other than the hard or soft palate comprised a swelling at the base of the tongue [6]. In that way, the unprecedented clinical appearance of the lesion did not permit us to include RFH as a clinical hypothesis; furthermore, RFH is remarkably infrequent (only 19 cases reported so far) [3].
As extracted from the pertinent literature, RFH apparently lacks bone involvement [2], missing intraosseous radiolucency, or periodontal ligament space widening when following periapical and occlusal radiographs [3]. Indeed, out of seven reports that deliberately chose to depict the radiographic appearance of their cases, none exhibited bone involvement [1,2,3,4,5,6, 11]. Nevertheless, with such a shortened amount of reported RFH cases, it is difficult to predict its radiographic appearance, besides the fact that some of those reports do not discriminate the radiographic expression of their respective lesions [12, 13]. Moreover, the lesion we report here exhibited an otherwise more aggressive radiolucent and radiotransparent manifestation that inclined our differential diagnoses towards malignant entities; in that way, RFH’s radiographic appearance may vary. The surgeon should be alert by considering clinical and radiographic characteristics that may suggest malignant lesions, and an incisional biopsy should be performed. Once the histopathological aspects of the lesion lead to a lymphoid origin, the immunohistochemical panel is fundamental to establish the correct diagnosis [6].
Out of all the clinical diagnostic possibilities raised, the risk of a lymphoma was definitely what concerned us the most. Indeed, a diagnostic challenge exists when differentiating RFH from follicular lymphoma (FL), although both lesions present very distinct prognoses [13]. Once mimicking a malignant neoplasia, RFH may confuse clinicians and has brought attention to studies aiming to discern both entities by assessing their histological and immunophenotypic features [10, 13,14,15].
Nevertheless, such histological and immunohistochemical distinction is commonly difficult to be performed [13]. Bcl-2, for instance, is the most useful biomarker employed for such differentiation, being constantly negative in RFH [16, 17]; however, 10–15% of FL test negative for Bcl-2 as well [18,19,20], and actually high-grade FL are consistently not positive for this protein [19]. On the other hand, the immunohistochemical detection of monotypic light chains is effective in low grade as well as in grade 3 FL [12], while RFH discloses polyclonality by showing both kappa and lambda positivities [1,2,3, 5, 6, 21].
Moreover, an additional differentiation between FL and RFH seems to be the employment of antibodies directed against IgM: a recent paper shows how IgM is infrequently and weekly observed in germinal center B cells of reactive lymphoid tissues, while it is consistently found in a high percentage of FL cases [13].
The histopathological findings of the current case did not leave any doubt about the diagnosis. The morphological manifestation of the lesion comprised the proliferation of many follicles that varied in shape and size and contained lymphocytes and a well-defined mantle zone [1,2,3,4, 6, 11]; the mantle zone was characteristically positive for the antiapoptotic protein Bcl-2, while the follicle centers were negative for the same protein [3]. And as mentioned, the diagnosis of RFH is consistently based on polyclonality, which was markedly evidenced in our case [3, 6, 11].
The singular immunohistochemical staining pattern of Bcl-2, as well as kappa and lambda light chains, already represents a strong basis for diagnosing RFH [6]. Nevertheless, we did utilize several other biomarkers that revealed consonant results when compared to previously reported cases, such as the B cell positivity for CD20 [1, 3] and CD79 in lymphoid follicles [1] and CD3 and CD5 positivity in the interfollicular zone [1, 3] and germinal centers [3], but not in the mantle zone [1, 3]. Follicles are also reported to be positive for the dendritic cell marker CD21 [1]; germinal centers of the present case were positive for this antibody.
Lymphoid follicles are also positively reactive to CD10 and Bcl-6 [1]; more specifically, Bcl-6 was strongly manifested in the mantle and interfollicular cells of 1 RFH case [3] and in the germinal centers of 25 other RFHs [13]. Suitably, our case revealed positivity for Bcl-6 in the germinal centers of the lesion. CD10 also stains the germinal centers of RFH [3, 13], blandly in our case. Moreover, our case was negative for the proliferation marker cyclin D1 and strongly positive for Ki-67; actually, cyclin D1 may reveal scattered positivity within a few cells [3], while Ki-67 staining is linked to higher proliferation indexes (more than 60%) in RFHs [13].
We also checked the immunohistochemical profile of different proteins not previously studied in RFH (CD2, CD23, CD138). As for CD2, a T cell and NK cell marker, there was diffuse positivity; CD23 is a B cell marker that can be used to visualize the follicular dendritic cell meshwork in FL and is positive in 24% of such malignant lesions [22]. Our case was positive for CD23 in the germinal centers of the follicles. CD138, on the other hand, stains plasmocytes and can be used to sort FL that present plasmacytic differentiation [23]; our results revealed CD138 positivity in the interfollicular zones of the lesion.
Other lesions could have been included in the differential diagnosis of this present case; we thus highlighted the possibility of the following malignant neoplasms: squamous cell carcinoma (SCC) of the maxillary sinus, sinonasal undifferentiated carcinoma, and salivary gland neoplasms. Nasal and paranasal sinus cavity carcinomas account for 3% of those that are localized in the head and neck region, being 60% of all sinonasal neoplasms that arise from the maxillary sinus, 20 to 30% from the oral cavity [7]. The SCC of the maxillary sinus predominantly affects white male individuals ranging between 55 and 65 years old [24], which is not in agreement with the case here reported. Radiographically, the SCC of the maxillary sinus presents a predominantly destructive growth [9], which is fairly similar to that of the present case.
Another malignant neoplasia that may develop in this region is the very hostile sinonasal undifferentiated carcinoma; such lesion has actually been diagnosed in people that belong from a wide range of ages, from the third to the ninth decades [25]. Interestingly, the sinonasal undifferentiated carcinoma leads to short-lasting symptoms (weeks to months), and the radiographic appearance usually shows a large and locally destructive mass that can invade skull and orbit [26, 27]. In that way, we considered this entity as a plausible hypothesis due to its radiographic similarity, although the average age was not analogous to the current case.
Salivary gland neoplasms of the sinonasal tract can be cited in the differential diagnosis. They are uncommon, and the majority is malignant [28]; among those, the adenoid cystic carcinoma and the mucoepidermoid carcinoma are the most frequent and are generally insidious, leading to nasal obstruction, epistaxis, and facial pain [29]. Although these lesions may lead to palatine mucosa expansion, facial tumefaction, and teeth mobility, they are usually not detected in panoramic radiographies; actually, they frequently and massively invade the adjacent bone before any radiographic evidence of bone destruction [7].
Following the diagnosis, a vigorous curettage of the lesion was performed and is in agreement with the majority of previously reported RFH cases that recommend simple surgical excision [2, 4,5,6, 11, 21]. Surgical treatment is indeed efficient [3]; correspondingly, our case is being followed for over 36 months with no reincidence. Still, one reported case was efficaciously treated with once-weekly injections of corticosteroids (total dose of 160 mg) and showed complete remission after four injections [3].
The prognosis of a reactive inflammatory lesion is completely distinct from that of malignant neoplasms. RFH presents a slow growth and a recurrence rate of 16.7% [3], possibly leading to complete resolution after treatment. On the other hand, oral NHL presents the 5-year overall survival rate for localized disease ranging from 50 to 80% [7]; regarding FL, 20% of patients show progression of the disease after chemoimmunotherapy and present poor prognosis [30].
References
Jham BC, Binmadi NO, Scheper MA, Zhao XF, Koterwas GE, Kashyap A et al (2009) Follicular lymphoid hyperplasia of the palate: case report and literature review. J Craniomaxillofac Surg 37:79–82. https://doi.org/10.1016/j.jcms.2008.11.007
Wright JM, Dunsworth AR (1983) Follicular lymphoid hyperplasia of the hard palate: a benign lymphoproliferative process. Oral Surg Oral Med Oral Pathol 55:162–168
Anjomshoaa I, Bulford LA, Dym H, Woo SB (2013) Florid follicular lymphoid hyperplasia of the hard palatal mucosa managed with intralesional steroids: a case report and review of the literature. J Oral Maxillofac Surg 71:1202–1208. https://doi.org/10.1016/j.joms.2013.01.015
Kolokotronis A, Dimitrakopoulos I, Asimaki A (2003) Follicular lymphoid hyperplasia of the palate: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 96:172–175. https://doi.org/10.1016/S1079210403000957
Mopsik ER, Adrian JC, Klein LE (1992) Follicular lymphoid hyperplasia of the hard palate: report of a case. J Oral Maxillofac Surg 50:538–540
Menasce LP, Shanks JH, Banerjee SS, Harris M (2001) Follicular lymphoid hyperplasia of the hard palate and oral mucosa: report of three cases and a review of the literature. Histopathology 39:353–358
Barnes L, Eveson JW, Reichart P, Sidransky D (2005) Pathology and genetics head and neck tumours. IARC Press, Lyon
Philipone E, Bhagat G, Alobeid B (2015) Oral cavity lymphoid neoplasms. A fifteen-year single institution review. N Y State Dent J 81:44–47
Kato H, Kanematsu M, Watanabe H, Kawaguchi S, Mizuta K, Aoki M (2015) Differentiation of extranodal non-Hodgkins lymphoma from squamous cell carcinoma of the maxillary sinus: a multimodality imaging approach. Spring 4:228. https://doi.org/10.1186/s40064-015-0974-y
Good DJ, Gascoyne RD (2009) Atypical lymphoid hyperplasia mimicking lymphoma. Hematol Oncol Clin North Am 23:729–745. https://doi.org/10.1016/j.hoc.2009.04.005
Davila MA, Thompson SH (1988) Reactive lymphoid hyperplasia of the hard palate. J Oral Maxillofac Surg 46:1103–1105
Weiss LM, Loera S, Bacchi CE (2010) Immunoglobulin light chain immunohistochemistry revisited, with emphasis on reactive follicular hyperplasia versus follicular lymphoma. Appl Immunohistochem Mol Morphol 18:199–205. https://doi.org/10.1097/PAI.0b013e3181c59a81
Zheng Y, Zhou X, Xie J, Zhu H, Zhang S, Zhang Y et al (2014) IgM expression in paraffin sections distinguishes follicular lymphoma from reactive follicular hyperplasia. Int J Clin Exp Pathol 7:3264–3271
Nathwani BN, Winberg CD, Diamond LW, Bearman RM, Kim H (1981) Morphologic criteria for the differentiation of follicular lymphoma from florid reactive follicular hyperplasia: a study of 80 cases. Cancer 48:1794–1806
Weiss LM, O’Malley D (2013) Benign lymphadenopathies. Mod Pathol 26(Suppl 1):S88–S96. https://doi.org/10.1038/modpathol.2012.176
qsujimoto Y, Cossman J, Jaffe E, Croce CM (1985) Involvement of the bcl-2 gene in human follicular lymphoma. Science 228:1440–1443
Zutter M, Hockenbery D, Silverman GA, Korsmeyer SJ (1991) Immunolocalization of the Bcl-2 protein within hematopoietic neoplasms. Blood 78:1062–1068
Gelb AB, Rouse RV, Dorfman RF, Warnke RA (1994) Detection of immunophenotypic abnormalities in paraffin-embedded B-lineage non-Hodgkin’s lymphomas. Am J Clin Pathol 102:825–834
Lai R, Arber DA, Chang KL, Wilson CS, Weiss LM (1998) Frequency of bcl-2 expression in non-Hodgkin’s lymphoma: a study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod Pathol 11:864–869
Utz GL, Swerdlow SH (1993) Distinction of follicular hyperplasia from follicular lymphoma in B5-fixed tissues: comparison of MT2 and bcl-2 antibodies. Hum Pathol 24:1155–1158
Napier SS, Newlands C (1990) Benign lymphoid hyperplasia of the palate: report of two cases and immunohistochemical profile. J Oral Pathol Med 19:221–225
Duarte IX, Domeny-Duarte P, Wludarski SC, Natkunam Y, Bacchi CE (2013) Follicular lymphoma in young adults: a clinicopathological and molecular study of 200 patients. Mod Pathol 26:1183–1196. https://doi.org/10.1038/modpathol.2013.50
Gradowski JF, Jaffe ES, Warnke RA, Pittaluga S, Surti U, Gole LA et al (2010) Follicular lymphomas with plasmacytic differentiation include two subtypes. Mod Pathol 23:71–79. https://doi.org/10.1038/modpathol.2009.146
Santos MR, Servato JP, Cardoso SV, de Faria PR, Eisenberg AL, Dias FL et al (2014) Squamous cell carcinoma at maxillary sinus: clinicopathologic data in a single Brazilian institution with review of literature. Int J Clin Exp Pathol 7:8823–8832
SY S, Bell D, Hanna EY (2014) Esthesioneuroblastoma, neuroendocrine carcinoma, and sinonasal undifferentiated carcinoma: differentiation in diagnosis and treatment. Int Arch Otorhinolaryngol 18:S149–S156. https://doi.org/10.1055/s-0034-1390014
Kim BS, Vongtama R, Juillard G (2004) Sinonasal undifferentiated carcinoma: case series and literature review. Am J Otolaryngol 25:162–166
Ejaz A, Wenig BM (2005) Sinonasal undifferentiated carcinoma: clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis. Adv Anat Pathol 12:134–143
Wolfish EB, Nelson BL, Thompson LD (2012) Sinonasal tract mucoepidermoid carcinoma: a clinicopathologic and immunophenotypic study of 19 cases combined with a comprehensive review of the literature. Head Neck Pathol 6:191–207. https://doi.org/10.1007/s12105-011-0320-9
Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L (2010) Major and minor salivary gland tumors. Crit Rev Oncol Hematol 74:134–148. https://doi.org/10.1016/j.critrevonc.2009.10.004
Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR et al (2015) Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the national LymphoCare study. J Clin Oncol 33:2516–2522. https://doi.org/10.1200/JCO.2014.59.7534
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from the patient.
Rights and permissions
About this article
Cite this article
Hanemann, J.A.C., de Carli, M.L., Dendena, E.R. et al. Rare case report of an aggressive follicular lymphoid hyperplasia in maxilla. Oral Maxillofac Surg 21, 475–481 (2017). https://doi.org/10.1007/s10006-017-0661-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-017-0661-y