Abstract
Purpose
The aim of the present study was to morphometrically analyze the mandibular canal through the mandibular ramus by cone beam computed tomography (CBCT) and to relate the findings to performing sagittal split ramus osteotomy.
Methods
CBCT of 200 patients were analyzed. Five parameters were measured at the axial scan, from the mandibular foramen to 21 mm below it (3-mm intervals). The canal was classified according to the position within the bone marrow space. Variations were evaluated according to age, sex, side, and number of mandibular teeth.
Results/conclusions
The following measurements increased gradually towards the most inferior level of measurement: the total thickness of the mandibular ramus through the center of the mandibular canal, the width of the bone marrow space (both buccal and lingual), and the narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical. The inner diameter of the mandibular canal slightly decreased to the same direction. Concerning the mandibular canal position within the bone marrow space, the percentage of the separate type increased towards the most inferior level of measurement, and the contact and fusion types decreased. Age, number of teeth, and sex had no significant influence on the total thickness of the mandibular ramus and on the narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sagittal split ramus osteotomy (SSRO) is one the most widely used orthognathic surgical procedure to correct jaw deformity. Due to the position and course of the mandibular canal, the inferior alveolar nerve (IAN) is at great risk of injury during SSRO [1]. It is suggested that the causes of neurosensory disturbance may involve traction on the IAN inside the ramus of the mandible during surgery, injury to the nerve when the ramus of the mandible is split or when the screw holes are drilled, and compression of the nerve by rigid fixation [2]. The incidence of immediate postoperative sensory impairment ranges from 49 to 100 % [3–5]. Some studies showed that neurosensory disturbance in the lower lip and mental skin may remain in 15 % [4] and 22.6 % [6] of the patients 1 year after surgery or even 39–66 % after 2 years [7–9]. Since this is an elective operation, the very real disabilities caused by damage to the IAN cannot be ignored [10].
Anatomic studies of the mandibular canal involving human cadaver mandibles have been performed [11, 12]. More recently, a few studies have examined the position and course of the mandibular canal in patients [1, 2], using computed tomographic (CT) images. Yamamoto et al. [2] described the contact made between the mandibular canal and the external cortical bone, while Tsuji et al. [1] investigated the position and course of the mandibular canal through the mandibular ramus.
Precise knowledge of the location of reference points in the oral and maxillofacial area provides important data in local anesthesia and in maxillofacial operations [13–18]. Knowledge of the anatomic location and course of the mandibular canal is imperative in order to reduce injuries to the inferior alveolar nerve during SSRO [1]. The aim of the present study was to evaluate the anatomical variability of the mandibular canal and its correlation with sex, age, and number of mandibular teeth using cone beam computed tomography (CBCT) scans, moreover, to relate the findings to the performance of SSRO.
Materials and methods
The present retrospective analysis included scans of a total of 200 patients of the database of Slice Diagnóstico Volumétrico por Imagem, in Belo Horizonte, Brazil. Only CBCT examinations from patients who accepted to participate were included in the study. The CBCT examinations with the presence of technical artifacts that hindered the evaluation of the mandibular foramen and canal were excluded.
CBCT scanning was performed with an i-CAT CBCT system (Imaging Sciences International, Hatfield, PA, USA). The scans were acquired using the i-CAT 3D imaging system (i-CAT Vision Software, Imaging Sciences International, Hatfield, PA, USA) and included the entire mandible. The following CBCT scan parameters were used for all patients: a tube voltage of 110 kV, 1 to 20 mA, emission of x-rays over an interval of 40 s, and an effective dose of 136 μSV.
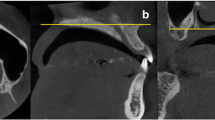
As the data were obtained bilaterally, 400 mandibular foramina and canals were analyzed. Axial sections of 0.6-mm thickness were obtained. In each patient, CBCT axial scans at 3-mm intervals were obtained beginning at the lowest point of the mandibular foramen to 21 mm below it (Fig. 1).
At each axial scan, the following parameters were measured (Fig. 2): (a) total thickness of the mandibular ramus through the center of the mandibular canal; (b) inner diameter of the mandibular canal, narrowest portion of the bone marrow space between the mandibular canal outer cortical and both the (c) lateral and (d) medial cortical bone of the ramus; and (e) narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical.
Axial scan diagram showing measurements. (a) Thickness of the mandible ramus passing through the center of mandibular canal; (b) inner diameter of the mandibular canal, width of the bone marrow space at the (c) buccal and (d) lingual sides; and the (e) narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical
Moreover, the mandibular canal was also morphologically classified into three types, according to the position within the bone marrow space and the relation with the inner surface of the buccal cortical bone (Fig. 3): (1) separate type with the bone marrow space visible, (2) contact type with the outer surface of the canal and inner surface of the buccal cortical bone in contact, and (3) fusion type with the outer cortical plate of the canal not evident.
Computed tomography axial scan examples showing the classification of the position of the mandibular canal within the bone: a separate type, bone marrow space evident; b contact type, outer surface of the canal and inner surface of buccal cortical bone in contact; and c fusion type, outer cortical plate of the canal not evident
The mean, standard deviation, minimum, and maximum for each of the measurements were assessed. Variations were evaluated according to gender and side (the predictor variables). The primary outcome variables were the morphometric measurements. The other variable was the patients’ age. Kolmogorov–Smirnov test was performed to evaluate the normal distribution. Levene test evaluated homoscedasticity. Paired t test and Wilcoxon test, when indicated, were performed to compare each of the morphometric variables between the left and right sides of the mandible. The performed tests for independent groups (sex) were Student’s t test or Mann–Whitney test, depending on the normality. Pearson correlation and linear regression were performed to verify the relationship between the patients’ age and the number of mandibular teeth and the following morphometric variables: total thickness of the mandibular ramus through the center of the mandibular canal and narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical. Spearman correlation was performed to check the relationship between the sex and the same two morphometric variables mentioned above. The degree of statistical significance was considered p < 0.05. All data were statistically analyzed using the Statistical Package for the Social Sciences (SPSS) version 20 software (SPSS Inc., Chicago, IL, USA).
The study was approved by the local Ethics Committee. The patients were contacted through a telephone call, and a signed informed and written consent form was obtained from each patient approving the use of their scans. The patients were not identifiable in any way, and a decoding list linking patient names and numbers was used and stored by the principal investigator, which was destroyed after completion of the study.
Results
The individuals (100 men, 100 women) had a mean age of 44.0 years (SD 15.3, range 13.3–88.0), at the time of the exam (men 45.0 ± 14.1, range 13.3–74.5; women 43.0 ± 16.5, range 16.4–88.0). The mean number of mandibular teeth was 12.30 ± 2.98 (range 0–16). Only 24 patients had less than nine teeth, of which four were completely edentulous.
Age of the patients was normally distributed in the male group (p = 0.602), but not in the female group (p = 0.010; Kolmogorov–Smirnov test). Thus, there were no statistically significant differences in the study variables concerning sex (the same number of males and females) and age (p = 0.065; Mann–Whitney test), which suggested that the final differences between the groups were not influenced by the initial characteristics, thus allowing the results to be compared.
Total thickness of the mandibular ramus through the center of the mandibular canal
The mean total thickness of the mandibular ramus through the center of the mandibular canal increased gradually towards the most inferior level of measurement (Table 1). There were no statistically significant differences between left and right sides at all levels. When the thickness of the mandible was compared between men and women, there was a statistically significant difference only at level −21 mm, at both right (p = 0.011) and left (p = 0.009) sides.
Inner diameter of the mandibular canal
The mean inner diameter of the mandibular canal showed a tendency to decrease as the canal followed its path towards the most inferior level of measurement (Table 2). There was a statistically significant difference between the mean values at the most superior and the most inferior levels of measurement (not shown in Table 2—most superior right side 2.74 ± 0.51, most inferior right side 2.42 ± 0.6, p < 0.001; most superior left side 2.65 ± 0.62, most inferior left side 2.22 ± 0.58; p < 0.001; paired-samples t test). In almost all levels, there was a statistically significant difference of the mean values between men and women, at both right and left sides.
Width of the bone marrow space at the buccal and lingual sides
The mean width of the bone marrow space showed a tendency to increase as the canal followed its path towards the most inferior level of measurement, at both buccal (Table 3) and lingual (Table 4) sides. In most of the levels, there was no statistically significant difference of the mean values between men and women, at both right and left sides, and also when both sides were compared.
Narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical
The mean narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical increased gradually towards the most inferior level of measurement (Table 5). There were no statistically significant difference between men and women, at both right and left sides. When the narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical was compared between left and right sides, there was a statistically significant difference only at levels −9 mm (p = 0.024) and −12 mm (p = 0.034).
Morphological classification of the mandibular canal position within the bone marrow space
The mandibular canal was morphologically classified into three types according to its position in the relation with the inner surface of the buccal cortical bone (Fig. 3): (1) separate, (2) contact, and (3) fusion types. In both sides, the percentage of the separate type increased towards the most inferior level of measurement, and the contact and fusion types decreased (Figs. 4a, 4b).
Correlation analyses (age, number of mandibular teeth, sex vs. morphometric variables)
The relationship between age and the total thickness of the mandibular ramus through the center of the mandibular canal was shown to be very weak for both sides and all levels, with the Pearson correlation coefficient varying from 0.017 to 0.196.
The relationship between age and the narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical was shown to be very weak or weak for both sides and all levels, with the Pearson correlation coefficient varying from 0.011 to 0.251.
The relationship between the number of mandibular teeth and the total thickness of the mandibular ramus through the center of the mandibular canal was shown to be very weak for both sides and all levels, with the Pearson correlation coefficient varying from 0.014 to 0.168.
The relationship between the number of mandibular teeth and the narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical was shown to be very weak for both sides and all levels, with the Pearson correlation coefficient varying from 0.001 to 0.120.
The relationship between sex and the total thickness of the mandibular ramus through the center of the mandibular canal was shown to be very weak or weak for both sides and all levels, with the Spearman correlation coefficient varying from 0.011 to 0.247.
The relationship between sex and the narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical was shown to be very weak for both sides and all levels, with the Spearman correlation coefficient varying from 0.006 to 0.125.
Discussion
The findings of the present are important to the oral and maxillofacial surgery because knowledge of the anatomic location and course of the mandibular canal is imperative in order to reduce injuries to the inferior alveolar neurovascular bundle while performing SSRO. In relation to a previous study on the same subject [1], the present study analyzed the variations according to age, sex, side (left/right), and number of mandibular teeth. Moreover, the correlations between two of the variables and age, sex, number of teeth, and side were also analyzed.
The results of the present study showed that even though there was no statistically significant difference of the total thickness of the mandibular ramus through the center of the mandibular canal between men and women, at both right and left sides, men usually showed a significantly larger inner diameter of the mandibular canal than women. As the differences of the inner diameter of the mandibular canal between sexes were at the level of tenths of a millimeter, this was not enough to significantly affect the differences in the total thickness of the mandibular ramus between sexes.
There was no correlation between the predictors: number of mandibular teeth or sex and two morphometric measurements (the total thickness of the mandibular ramus through the center of the mandibular canal and the narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical). A study on the morphological variation in dentate and edentulous mandibles [15] showed that the presence or absence of the teeth can significantly alter the mandibular shape and raises the intriguing possibility that edentulism may be associated with specific shape changes in the mandible. This study [15] also observed that the dental status has a higher influence on the mandibular anatomy than the difference in gender. However, of the 200 patients analyzed in the present study, only 24 patients had less than nine teeth, of which only four were edentulous.
Although Kaji et al. [6] did not find a statistically significant difference for neurosensory disturbance between patients of different age ranges (<19, 20–29, >30 years), it was observed by several studies that the patient’s age and injury to the nerve during surgery were positively correlated with postoperative neurosensory disturbance [5, 9, 19–23]. The reasons for this might be that the healing ability decreases with increasing age [24] and that more bone is usually removed owing to completely formed roots or increased bone mineralization [25]. Even though patients undergoing SSRO are generally young individuals, we decided to not exclude older patients in order to verify whether age has some influence on the mandibular canal position within the mandibular ramus. The results of the present study showed that there was no correlation between age and two measurements: the total thickness of the mandibular ramus through the center of the mandibular canal and the narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical. It would be pure speculation to suggest that the neurosensory disturbance may be more strongly related to age than to the distance from the mandibular ramus external cortical to the nerve itself because the present study only assessed CBCTs, and none of the patients really underwent SSRO.
Concerning the width of the bone marrow space at the buccal and lingual sides, there was, in the great majority of the measurement levels, no statistically significant difference of the mean values between men and women, at both right and left sides, and also when both sides were compared. It has been suggested that the nearer the mandibular canal is situated to the buccal cortical plate at the site of operation, the higher is the danger of causing nerve lesions at operation [4]. In the present study, the fusion type (the outer cortical plate of the canal is not evident, with no bone marrow between the inner surface of the buccal cortical bone and the mandibular canal) was more commonly observed closer to the mandibular foramen. This detail may have clinical implications. Even if a vertical cut is made at the safest site with careful splitting, the inferior alveolar neurovascular bundle may still be encountered or impaired in individuals with fusion-type mandibular canals [1]. As the mean width of the bone marrow space of the buccal side showed a tendency to increase as the canal followed its path towards the most inferior level of measurement, there is more available space for the saw at the inferior part of the mandibular ramus than at levels closer to the mandibular foramen, while performing the SSRO without directly touching the IAN. Once again, as none of the patients of the present study underwent SSRO, the higher or lesser probability to injury the IAN due to these anatomical characteristics is only speculative.
Still, it would be important to discuss some possible clinical implications of these anatomical characteristics of the mandible on the SSRO surgical technique, based on previous studies. Some studies analyzed the neurosensory disturbance in relation to the surgical technique. In the study of Yamamoto et al. [2], neurosensory disturbance was significantly more likely to be present 1 year after surgery when the width of the marrow space between the mandibular canal and the external cortical bone was 0.8 mm or less, and neurosensory disturbance remained on all ten sides on which a marrow space was absent. The authors concluded that separating the inferior alveolar nerve from the external cortical bone without injuring the IAN canal is difficult when a marrow space is absent. It has been stated that SSRO does not involve surgical risk in cases of wide and thick rami for a great majority of cases. In cases when the preoperative radiological and CT findings unequivocally point to thin rami, an indication of the splitting technique is open to question. In certain instances, another operative technique has to be used [2, 12]. Intraoral vertical ramus osteotomy (IVRO) and the inverted L ramus osteotomy (ILRO) are suggested to produce less neurologic damage during surgery than SSRO [8, 26]. However, IVRO and ILRO are more appropriate when a mandibular setback is planned, in cases of mandibular prognathism. In case of mandibular advancement, the IVRO technique does not provide osseous contact between the proximal and distal segments and thereby increases the probability of condylar luxation caused by the instability of the placement of the proximal segment [27]. The SSRO allows for the apposition of the broad cancellous bony surfaces [12]. Brusati et al. [10] and Simpson [28] recommended using a thin osteotome driven to the inferior border from a lateral cortical cut. The technique of splitting of the mandible without inserting the osteotome into the bone marrow space, as described by Wolford et al. [29] and Loh [30] was also proposed. It was also suggested that the posterior siting of the anterior vertical bone incision as in the original Obwegeser technique [31] should be used to avoid development of neurosensory disturbance. In the series of Yoshida et al. [4], however, neurosensory disturbance which showed no tendency to recovery was not encountered even in cases of thin rami. Therefore, the SSRO may not be absolutely contraindicated, even if the narrowest width of the buccal trabecular bone is minimal. Moreover, the overall impression of intraoperative nerve encounter and nerve manipulation is that nerve manipulation does not seem to have any major influence on nerve dysfunction after SSRO. These findings may indicate that something apart from the intraoperative nerve encounter may be at least partly responsible for the nerve damage [9].
Conclusions
The following measurements increased gradually towards the most inferior level of measurement: the total thickness of the mandibular ramus through the center of the mandibular canal, the width of the bone marrow space (both buccal and lingual), and the narrowest width from the mandibular canal inner cortical to the mandibular ramus external cortical. The inner diameter of the mandibular canal slightly decreased to the same direction. Concerning the mandibular canal position within the bone marrow space, the percentage of the separate type increased towards the most inferior level of measurement, and the contact and fusion types decreased. Age, number of teeth, and sex had no significant influence on the measurements.
References
Tsuji Y, Muto T, Kawakami J, Takeda S (2005) Computed tomographic analysis of the position and course of the mandibular canal: relevance to the sagittal split ramus osteotomy. Int J Oral Maxillofac Surg 34:243–246
Yamamoto R, Nakamura A, Ohno K, Michi KI (2002) Relationship of the mandibular canal to the lateral cortex of the mandibular ramus as a factor in the development of neurosensory disturbance after bilateral sagittal split osteotomy. J Oral Maxillofac Surg 60:490–495
Walter JM Jr, Gregg JM (1979) Analysis of postsurgical neurologic alteration in the trigeminal nerve. J Oral Surg 37:410–414
Yoshida T, Nagamine T, Kobayashi T, Michimi N, Nakajima T, Sasakura H, et al. (1989) Impairment of the inferior alveolar nerve after sagittal split osteotomy. J Craniomaxillofac Surg 17:271–277
Blomqvist JE, Alberius P, Isaksson S (1998) Sensibility following sagittal split osteotomy in the mandible: a prospective clinical study. Plast Reconstr Surg 102:325–333
Kaji M, Ohashi Y, Mutoh Y, Yagi M (1998) Study of late sensory paralysis in the lower lip after sagittal split osteotomy, part 1: investigation of factors on multivariate analysis. Niigata Dental Journal 28:1–6
Coghlan KM, Irvine GH (1986) Neurological damage after sagittal split osteotomy. Int J Oral Maxillofac Surg 15:369–371
Westermark A, Bystedt H, von Konow L (1998) Inferior alveolar nerve function after mandibular osteotomies. Br J Oral Maxillofac Surg 36:425–428
Westermark A, Bystedt H, von Konow L (1998) Inferior alveolar nerve function after sagittal split osteotomy of the mandible: correlation with degree of intraoperative nerve encounter and other variables in 496 operations. Br J Oral Maxillofac Surg 36:429–433
Brusati R, Fiamminghi L, Sesenna E, Gazzotti A (1981) Functional disturbances of the inferior alveolar nerve after sagittal osteotomy of the mandibular ramus: operating technique for prevention. J Maxillofac Surg 9:123–125
Rajchel J, Ellis E 3rd, Fonseca RJ (1986) The anatomical location of the mandibular canal: its relationship to the sagittal ramus osteotomy. Int J Adult Orthodon Orthognath Surg 1:37–47
Tamas F (1987) Position of the mandibular canal. Int J Oral Maxillofac Surg 16:65–69
Chrcanovic BR, Cavalcanti YS, Reher P (2009) Temporal miniplates in the frontozygomatic area—an anatomical study. Oral Maxillofac Surg 13:201–206
Chrcanovic BR, Custodio AL (2010) Anatomical variation in the position of the greater palatine foramen. J Oral Sci 52:109–113
Chrcanovic BR, Abreu MH, Custodio AL (2011) Morphological variation in dentate and edentulous human mandibles. Surg Radiol Anat 33:203–213
Chrcanovic BR, Abreu MH, Custodio AL (2011) A morphometric analysis of supraorbital and infraorbital foramina relative to surgical landmarks. Surg Radiol Anat 33:329–335
Chrcanovic BR, Custodio AL (2011) Optic, oculomotor, abducens, and facial nerve palsies after combined maxillary and mandibular osteotomy: case report. J Oral Maxillofac Surg 69:e234–e241
Chrcanovic BR, Freire-Maia B (2012) Risk factors and prevention of bad splits during sagittal split osteotomy. Oral Maxillofac Surg 16:19–27
MacIntosh RB (1981) Experience with the sagittal osteotomy of the mandibular ramus: a 13-year review. J Maxillofac Surg 9:151–165
Nishioka GJ, Zysset MK, Van Sickels JE (1987) Neurosensory disturbance with rigid fixation of the bilateral sagittal split osteotomy. J Oral Maxillofac Surg 45:20–26
Upton LG, Rajvanakarn M, Hayward JR (1987) Evaluation of the regenerative capacity of the inferior alveolar nerve following surgical trauma. J Oral Maxillofac Surg 45:212–216
Karas ND, Boyd SB, Sinn DP (1990) Recovery of neurosensory function following orthognathic surgery. J Oral Maxillofac Surg 48:124–134
August M, Marchena J, Donady J, Kaban L (1998) Neurosensory deficit and functional impairment after sagittal ramus osteotomy: a long-term follow-up study. J Oral Maxillofac Surg 56:1231–1235 discussion 1236
Valmaseda-Castellon E, Berini-Aytes L, Gay-Escoda C (2001) Inferior alveolar nerve damage after lower third molar surgical extraction: a prospective study of 1117 surgical extractions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92:377–383
Bruce RA, Frederickson GC, Small GS (1980) Age of patients and morbidity associated with mandibular third molar surgery. J Am Dent Assoc 101:240–245
Naples RJ, Van Sickels JE, Jones DL (1994) Long-term neurosensory deficits associated with bilateral sagittal split osteotomy versus inverted ‘L’ osteotomy. Oral Surg Oral Med Oral Pathol 77:318–321
Hara S, Mitsugi M, Kanno T, Tatemoto Y (2013) Clinical approach for mandibular advancement by intraoral vertical ramus osteotomy with endoscopically assisted intraoral fixation of an L-shaped compact lock plate. J Craniofac Surg 24:545–547
Simpson W (1981) Problems encountered in the sagittal split operation. Int J Oral Surg 10:81–86
Wolford LM, Bennett MA, Rafferty CG (1987) Modification of the mandibular ramus sagittal split osteotomy. Oral Surg Oral Med Oral Pathol 64:146–155
Loh FC (1992) Technical modification of the sagittal split mandibular ramus osteotomy. Oral Surg Oral Med Oral Pathol 74:723–726
Trauner R, Obwegeser H (1957) The surgical correction of mandibular prognathism and retrognathia with consideration of genioplasty. I. Surgical procedures to correct mandibular prognathism and reshaping of the chin. Oral Surg Oral Med Oral Pathol 10:677–689
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. All persons gave their informed consent prior to the inclusion of their CBCT examinations in the study. The study was approved by the local Ethics Committee.
Rights and permissions
About this article
Cite this article
Chrcanovic, B.R., de Carvalho Machado, V. & Gjelvold, B. A morphometric analysis of the mandibular canal by cone beam computed tomography and its relevance to the sagittal split ramus osteotomy. Oral Maxillofac Surg 20, 183–190 (2016). https://doi.org/10.1007/s10006-016-0550-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-016-0550-9