Abstract
Context
The reaction between Na and HF is a typical harpooning reaction which is of great interest due to its significance in understanding the elementary chemical reaction kinetics. This work aims to investigate the detailed reaction mechanisms of sodium with hydrogen fluoride and the adsorption of HF on the resultant NaF as well as the (NaF)4 tetramer. The results suggest that the reaction between Na and HF leads to the formation of sodium fluoride salt NaF and hydrogen gas. Na interacts with HF to form a complex HF···Na, and then the approaching of F atom of HF to Na results in a transition state H···F···Na. Accompanied by the broken of H-F bond, the bond forms between F and Na atoms as NaF, then the product NaF is yielded due to the removal of H atom. The resultant NaF can further form (NaF)4 tetramer. The interaction of NaF with HF leads to the complex NaF···HF; the form I as well as II of (NaF)4 can interact with HF to produce two complexes (i.e., (NaF)4(I-1)···HF, (NaF)4(I-2)···HF, (NaF)4(II-1)···HF and (NaF)4(II-2)···HF), but the form III of (NaF)4 can interact with HF to produce only one complex (NaF)4(III)···HF. These complexes were explored in terms of noncovalent interaction (NCI) and quantum theory of atoms in molecules (QTAIM) analyses. NCI analyses confirm the existences of attractive interactions in the complexes HF···Na, NaF···HF, (NaF)4(I-1)···HF, (NaF)4(I-2)···HF, (NaF)4(II-1)···HF and (NaF)4(II-2)···HF, and (NaF)4(III)···HF. QTAIM analyses suggest that the F···Na interaction forms in the HF···Na complex while the F···H hydrogen bonds form in NaF···HF, (NaF)4(I-1)···HF, (NaF)4(I-2)···HF, (NaF)4(II-1)···HF and (NaF)4(II-2)···HF, and (NaF)4(III)···HF complexes. Natural bond orbital (NBO) analyses were also applied to analyze the intermolecular donor-acceptor orbital interactions in these complexes. These results would provide valuable insight into the chemical reaction of Na and HF and the adsorption interaction between sodium fluoride salt and HF.
Methods
The calculations were carried out at the M06-L/6-311++G(2d,2p) level of theory which were performed using the Gaussian16 program. Intrinsic reaction coordinate (IRC) calculations were carried out at the same level of theory to confirm that the obtained transition state was true. The molecular surface electrostatic potential (MSEP) was employed to understand how the complex forms. Quantum theory of atoms in molecules (QTAIM) and noncovalent interaction (NCI) analysis was used to know the topology parameters at bond critical points (BCPs) and intermolecular interactions in the complex and intermediate. The topology parameters and the BCP plots were obtained by the Multiwfn software.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen fluoride (HF) and alkali metal M (M = alkali metal) have high reactivities which enable them to react with each other to form alkali metal fluoride salt MF and hydrogen gas (i.e., 2HF + 2M → 2MF +H2), and these reactions are of great interest due to their significance in understanding the elementary chemical reaction kinetics [1]. Especially, for the reaction between Na and HF, it is a typical harpooning reaction [2, 3] which has been extensively investigated by many experimental and theoretical work [1, 2, 4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. For instance, experimental studies focus on the characteristics of Na + HF reaction, including how the vibrational states of HF [5, 8, 9, 24] and HF(j) initial rotational excitations [6] affect the reaction. Düren and coworkers have done the scattering of Na from HF and suggested that the van der Waals wells exist and locate in the entrance channel of both ground and first excited potential energy surfaces [7,8,9]. A lot of theoretical work have been devoted to investigating the potential energy surface (PES) of Na + HF reaction system [1, 2, 4, 9, 11, 13, 16,17,18,19, 21,22,23, 25, 26, 29,30,31,32]. Different methods including semiempirical [19] and ab initio [2, 9, 16,17,18, 21, 30, 31] methods were used to explore the PES of the ground and excited electronic states of the Na + HF reaction. The investigations of ground state PES confirmed the existence of van der Waals well and late barrier etc.[1, 4, 10, 11, 13, 16, 22, 23, 25, 26, 30, 31] While the research on excited electronic state PES proved the harpooning process in the Na + HF reaction and the presence of a deep well in the first excited state.[18, 21, 30, 31]
The Na + HF reaction can lead to NaF monomer or larger cluster (NaF)n (in which n=4, 6, 9, 12, 15, and 18.),[33] and the resultant NaF monomer or (NaF)n tetramer might further adsorb HF. The interesting reaction processes from HF and Na to NaF and then NaHF2 or (NaF)n•HF attract our attention, and the present work aims at exploring the detailed reaction mechanisms and the further adsorption processes. As a representative cluster, the (NaF)4 tetramer can exist as cube (I), ladder (II), and ring (III) forms, [34,35,36] so it is selected in this study because the investigations of the adsorptions of HF on these three isomeric forms will be fascinating and thus provide insightful results. It can be expected that the Na + HF reaction might go through the processes including the interaction between Na and F atom of HF, the H-F bond breakage in the transition state H···F···Na, the Na-F bond formation in the intermediate NaF···H, and the generation of the final product NaF, while the NaF molecule can assemble into larger cluster (NaF)4, and both of them can adsorb HF via hydrogen bond. Thereby, in the present work, quantum chemical calculations were carried out; the intermolecular interactions, the transition states, and intermediates formed in the reaction processes will be discussed; the reaction mechanism and the further adsorption processes will be elaborated.
Computational methods
The structures of HF, Na, the complex formed via intermolecular interaction, transition state, intermediate and product, the (NaF)4•HF complexes were optimized at the M06-L [37, 38] /6-311++G(2d,2p) level of theory corrected with the Grimme’s dispersion (D3) [39]. Vibrational frequency calculations at the same level of theory were carried out to make sure that the optimized reactants, intermediates, and product have no imaginary frequencies while the transition state has only one imaginary frequency. Intrinsic reaction coordinate (IRC) [40] calculations were carried out at the same level of theory to confirm that the obtained transition state was true. The calculations were performed using the Gaussian16 program [41].
To understand how the complex forms, the molecular surface electrostatic potential (MSEP) [29, 42,43,44,45,46] was employed to analyze the molecular electronic property. To know the topology parameters at bond critical points (BCPs) and intermolecular interactions in the complex and intermediate quantum theory of atoms in molecules (QTAIM [47,48,49]) and noncovalent interaction (NCI [50, 51]), analysis was used. The topology parameters and the BCP plots were obtained by the Multiwfn software [52]. The relative Gibbs free energy (i.e., ΔG) is calculated as the difference between the Gibbs free energy of the complex (or the transition state or intermediate or product) and the sum of Gibbs free energies of the reactants. The interaction energy (i.e., ΔE) of the complex is calculated as the energy difference between the total energy of the complex or the intermediate and the sum of energies of its components.
The reaction between HF and Na at 298 K leading to the formation of NaF, the formation of (NaF)4, and the adsorption of HF on NaF as well as (NaF)4 was investigated (i.e., formulas 1, 2, 3 and 4 represent the generation of NaF, the adsorption of HF on NaF, the generation of (NaF)4, and the adsorption of HF on (NaF)4, respectively.):
Results and discussion
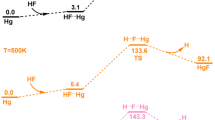
With regard to the reaction between HF and Na, the calculated results show that HF firstly interacts with Na to form a complex HF···Na, as shown in Fig. 1. The bond length and stretching vibrational frequency of HF bond, the intermolecular distance, and interaction energy for the complex HF···Na are outlined in Table 1. It can be seen that the HF bond length in the complex HF···Na is elongated compared to that of HF, and the stretching vibrational frequency of HF bond in the complex HF···Na decreases relative to that of HF. The intermolecular distance for F···Na in the complex HF···Na is 2.4133 Å (Fig. 1 and Table 1). The interaction energy for the complex HF···Na is −1.07 kcal/mol, meaning that it exists as an intermolecular interaction.
To understand how the complex HF···Na forms, the molecular surface electrostatic potential (MSEP) [29, 42,43,44,45] was employed to reveal the electronic properties of HF and Na, as depicted in Fig. 2. Evidently, the positive MSEP region is observed around Na atom which enables it to be an electron acceptor, while the negative MSEP region around the F atom of HF makes F atom donate electrons, so the F and Na atoms can interact with each other as F···Na interaction in the complex HF···Na.
Noncovalent interaction (NCI [50, 51]) analysis of the complex HF···Na is shown in Fig. 3a; it can be observed that a small green circle appears between F atom of HF and Na atom in the reduced density gradient (RDG) isosurface, indicating the existence of attractive interaction between them. Also, the intermolecular interaction in the complex HF···Na was assessed by QTAIM, the intermolecular BCP is depicted in Fig. 3b, and the topological parameters at the intermolecular BCP is summarized in Table 2. The appearance of intermolecular BCP between F atom of HF and Na atom in the complex HF···Na suggests the formation of F···Na interaction. The electron density (i.e., ρ) at the BCP of F···Na interaction is in the range of 0.01323 a.u., and its Laplacian (i.e., ∇2ρ) ranges from 0.07946 a.u. (Table 2). These values are within the ranges of the criteria for the existence of hydrogen bond (i.e., ρ and ∇2ρ should be within the ranges 0.002–0.035 and 0.024–0.139 a.u., respectively [53]), indicating that the F···Na interaction in the complex HF···Na is an attractive intermolecular interaction. The electronic energy density (i.e., H) with positive or negative value at the BCP means that the interaction is electrostatic or covalent dominant [54], respectively. It can be seen that the H value for F···Na interaction in the complex HF···Na is positive (Table 2), suggesting that the F···Na interaction is electrostatic dominant.
Natural bond orbital (NBO) [55, 56] analyses were applied to analyze the weak noncovalent interactions formed in the complex HF···Na, as depicted in Fig. 4. The intermolecular donor-acceptor orbital interaction (i.e., E(2)) between the bonding orbital of HF and the lone pair orbital of Na atom is 0.96 kcal/mol, while that between the lone pair orbitals of F and Na atoms is 3.58 kcal/mol, meaning the strong electron transfer from HF to Na atom.
As displayed in Fig. 5, the initial step in the formation the complex HF···Na needs to absorb an energy of 3.4 kcal/mol. Then, the conversion from the complex HF···Na to the transition state H···F···Na needs to overcome an energy of 9.5 kcal/mol, as can be seen from the Gibbs free energy profile in Fig. 5. The optimized structures of the transition state H···F···Na, intermediate NaF···H, and NaF are shown in Fig. 6. The H···F distance in the transition state H···F···Na is 1.3899 Å which is even longer than the H-F bond length (ca. 0.92 Å), suggesting that the H-F bond is broken. The F···Na distance in the transition state H···F···Na is 2.0509 Å which is longer than the Na-F bond length (ca. 1.93 Å). The approaching of F atom towards Na atom leads to the formation of intermediate NaF···H in which the NaF bond forms. After the removal of H atom, the final product NaF is yielded.
The resultant NaF can further form larger cluster [33], for instance, the (NaF)4 tetramer (Fig. 7) [35]. As outlined in Table 3, the generation of (NaF)4 tetramer including forms I, II, and III from NaF is exothermic. The resultant NaF as well as the (NaF)4 tetramer has the capability of adsorbing HF, as revealed by the MSEP results (Fig. 8) in which the F atom in NaF or (NaF)4 possessing the negative MSEP region can donate electrons to H atom of HF, so the interaction of NaF as well as the (NaF)4 tetramer with HF was further investigated.
As depicted in Fig. 9, the interaction of NaF with HF leads to the complex NaF···HF; the form I as well as II of (NaF)4 can interact with HF to produce two complexes (i.e., (NaF)4(I-1)···HF, (NaF)4(I-2)···HF, (NaF)4(II-1)···HF and (NaF)4(II-2)···HF), but the form III of (NaF)4 can interact with HF to produce only one complex (NaF)4(III)···HF. These processes are exothermic, as can be seen from the Gibbs free energy changes in Table 3. These complexes have strong interaction energies which can be seen from the ΔEint,1 values in Table 4. The interaction energy of (NaF)4(I-2)···HF (or (NaF)4(II-2)···HF) is much stronger than that of (NaF)4(I-1)···HF (or (NaF)4(II-1)···HF).
NCI analysis shows that the green circle appears between F atom of NaF (or (NaF)4(I-1), (NaF)4(I-2), (NaF)4(II-1), (NaF)4(II-2), (NaF)4(III)), and H atom of HF in the RDG isosurface (Fig. 10), suggesting that the attractive interaction forms between them. As displayed in Fig. 11, the intermolecular BCP between F atom of NaF (or (NaF)4(I-1), (NaF)4(I-2), (NaF)4(II-1), (NaF)4(II-2), (NaF)4(III)), and H atom of HF appears in the complex NaF···HF (or (NaF)4(I-1)···HF, (NaF)4(I-2)···HF, (NaF)4(II-1)···HF, (NaF)4(II-2)···HF, and (NaF)4(III)···HF), meaning the formation of F···H interaction. The H values for F···H interactions in the complexes NaF···HF, (NaF)4(I-1)···HF, (NaF)4(II-1)···HF, and (NaF)4(II-2)···HF are negative (Table 4), indicating that these F···H interactions are covalent dominant, while the F···H interactions in (NaF)4(I-2)···HF and (NaF)4(III)···HF are electrostatic dominant due to their positive H values.
The intermolecular donor-acceptor orbital interactions (i.e., E(2)) for the complexes NaF···HF, (NaF)4(I-1)···HF, (NaF)4(I-2)···HF, (NaF)4(II-1)···HF and (NaF)4(II-2)···HF, and (NaF)4(III)···HF are depicted in Fig. 12. It can be seen that the E(2) values for the intermolecular donor-acceptor orbital interactions between the lone pair orbital of F and antibonding orbital of HF are very large for these complexes, indicating the strong electron transfer from F atom to HF during the formation of F···HF hydrogen bond.
Conclusions
In this work, the reaction of sodium (Na) with hydrogen fluoride (HF) was theoretically investigated, and the detailed mechanism was explored. At first, Na interacts with HF to form a complex HF···Na, and then the approaching of F atom of HF to Na results in a transition state H···F···Na. Accompanied by the broken of H-F bond, the bond forms between F and Na atoms as NaF, then the product NaF is yielded due to the removal of H atom. The resultant NaF can further form (NaF)4 tetramer, and NaF as well as (NaF)4 tetramer can further adsorb HF to form a strong complex. These results would provide valuable insight into the chemical reaction of Na and HF and the adsorption interaction between sodium fluoride salt and HF.
References
Yan W, Tan RS, Lin SY (2023) New ab initio potential energy surface of NaFH (1A′) system and quantum dynamics studies for the Na + HF (v, j) / NaF + H reaction. RSC Adv 13:15506–15513
Chang XY, Ehlich R, Hudson AJ, Piecuch P, Polanyi JC (1997) Dynamics of harpooning studied by transition state spectroscopy Na...FH. Faraday Discuss 108:411–425
Herschbach DR (1966) Reactive scattering in molecular beams. Adv Chem Phys 10:319–393
Antunes AWS, Ferreira Da Cunha W, Silva G ME, Martins JB, Gargano R (2010) Dynamical properties and thermal rate coefficients for the Na + HF reaction using genetic algorithm. Int J Quantum Chem 110:1070–1079
Bartoszek FE, Blackwell BA, Polanyi JC, Sloan JJ (1981) Effect of changing reagent energy on reaction dynamics. XI. Dependence of Reaction Rate on Vibrational Excitation in Endothermic Reactions HX (vreag)+ Na→H+NaX (X≡ F, Cl). J Chem Phys 74:3400–3410
Blackwell BA, Polanyi JC, Sloan JJ (1978) Effect of changing reagent energy. IX. Dependence of Reaction Rate on Rotational Excitation in HX(J:ν) + Na → H + NaX (X = F, Cl). Chem Phys 30:299–306
Düren R, Lackschewitz U, Milosevic S (1988) Reactive scattering of sodium and hydrogen fluoride evolving on an electronically excited surface. Chem Phys 126:81–91
Düren R, Lackschewitz U, Milosevic S, Panknin H, Schirawski N (1988) Differential cross sections for reactive and non-reactive scattering of electronically excited Na from HF molecules. Chem Phys Lett 143:45–50
Düren R, Lackschewitz U, Milosevic S, Waldapfel HJ (1989) Differential scattering of Na(3P) from HF. reactive and non-reactive processes. J Chem Soc, Faraday Trans 2(85):1017–1025
Espinola Lopez LE, Gargano R, Mundim KC, Soares Neto JJ (2002) The Na + HF reactive probabilities calculations using two different potential energy surfaces. Chem Phys Lett 361:271–276
Ferreira Da Cunha W, Roncaratti LF, Gargano R, Silva GME (2006) Fitting potential energy surface of reactive systems via genetic algorithm. Int J Quantum Chem 106:2650–2657
Garashchuk S, Rassolov VA (2007) Semiclassical nonadiabatic dynamics of NaFH with quantum trajectories. Chem Phys Lett 446:395–400
Gargano R, Crocchianti S, Lagana A, Parker GA (1998) The quantum threshold behavior of the Na + HF Reaction. J Chem Phys 108:6266–6271
Jasper AW, Truhlar DG (2007) Non-Born-Oppenheimer molecular dynamics of Na···FH photodissociation. J Chem Phys 127:194306
Katz G, Zeiri Y, Kosloff R (2002) Three-dimensional quantum time-dependent study of the photodissociation dynamics of Na···FH/D. Chem Phys Lett 359:453–459
Lagana A, Alvariño JM, Hernandez ML, Palmieri P, Garcia E, Martinez T (1997) Ab initio calculations and dynamical tests of a potential energy surface for the Na + FH reaction. J Chem Phys 106:10222–10229
Paniagua M, Garcia De La Vega JM, Alvarez Collado JR, Sanz JC, Alvariño JM, Lagana A (1986) RHF potential energy surface for the collinear reaction of Na with HF. J Mol Struct 142:525–528
Sevin A, Hiberty PCL, J. M. (1987) Theoretical study of the ground- and excited-state reactivity of sodium and hydrogen fluoride. Comparison of SCF-CI and VB Treatments. J Am Chem Soc 109:1845–1852
Shapiro M, Zeiri Y (1979) Semiempirical potential surfaces for the alkali hydrogen-halide reactions. J Chem Phys 79:5264–5270
Spirko V, Piecuch P, Bludsky O (2000) Bound and quasibound states of the Na···FH Van der Waals Molecule. J Chem Phys 112:189–202
Topaler MS, Truhlar DG, Chang XY, Piecuch P, Polanyi JC (1998) Potential energy surfaces of NaFH. J Chem Phys 108:5349–5377
Vilela AFA, Soares Neto JJ, Mundim KC, Mundim KC, Gargano R (2002) Fitting potential energy surface for reactive scattering dynamics through generalized simulated annealing. Chem Phys Lett 359:420–427
Wang D, Shi G, Fu L, Yin R, Ji Y (2019) Accurate potential energy surfaces for the three lowest electronic states of N(2D)+H2(X1 ) scattering reaction. ACS Omega 4:12167–12174
Weiss PS, Mestdagh JM, Covinsky MH, Balko BA, Lee YT (1988) The reactions of ground and excited state sodium atoms with hydrogen halide molecules. Chem Phys 126:93–109
Yan W, Tan RS, Lin SY (2019) Quantum dynamics calculations of Na (32S, 32P) + HF → NaF + H reactions. J Phys Chem A 123:2601–2609
Yin R, Gao N, Cao J, Li Y, Wang D, Huang X (2020) Global accurate diabatic potential surfaces for the reaction H + Li2. RSC Adv 10:39226–39240
Yin R, Gao N, Zhang R, Wang D, Huang X (2020) Accurate potential energy surfaces for the excited state of CF2 molecule. Chem Phys 538:110906
Zeiri Y, Katz G, Kosloff R, Topaler MS, Truhlar DG, Polanyi JC (1999) Quantum mechanism in the photodissociation of NaFH complex: a challenge to semiclassical analysis. Chem Phys Lett 300:523–528
Espinosa E, Lecomte C, Ghermani NE, Devemy J, Rohmer MM, Bernard M et al (1996) Hydrogen bonds: first quantitative agreement between electrostatic potential calculations from experimental X−(X + N) and theoretical ab initio SCF models. J Am Chem Soc 118:2501–2502
Jasper AW, Hack MD, Chakraborty A, Truhlar DG, Piecuch P (2001) Photodissociation of LiFH and NaFH Van der Waals complexes: a semiclassical trajectory study. J Chem Phys 115:7945–7952
Topaler MS, Truhlar DG, Chang XY, Piecuch P, Polanyi JC (1998) The photoabsorption spectrum of Na···FH Van der Waals molecule: comparison of theory and experiment for a harpooning reaction studied by transition state spectroscopy. J Chem Phys 108:5378–5390
Yan W, Tan RS, Lin SY (2019) Quantum dynamics studies of the Na(3S)+HF and Na(3S/3P)+DF reactions: the effects of the initial rotational excitation and the isotopic substitution. Chem Phys Lett 730:378–383
Hargittai M (2000) Molecular structure of metal halides. Chem Rev 100:2233–2301
Aguado A, Ayuela A, Lopez JM, Alonso JA (1997) Structure and bonding in small neutral alkali halide clusters. Phys Rev B 56:15353
Bickelhaupt FM, Sola M, Guerra CF (2007) Covalent versus ionic bonding in alkalimetal fluoride oligomers. J Comput Chem 28:238–250
Lintuluoto M (2001) Theoretical study on the structure and energetics of alkali halide clusters. J Mol Struct (THEOCHEM) 540:177
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:194101
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Fukui K (1981) The path of chemical reactions-the IRC approach. Acc Chem Res 14:363–368
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2016) Gaussian 16, revision A.03Revision A.03 edn. Gaussian, Inc, Wallingford, CT
Dimitrova V, Ilieva S, Galabov B (2002) Electrostatic potential at atomic sites as a reactivity descriptor for hydrogen bonding. Complexes of Monosubstituted Acetylenes and Ammonia. J Phys Chem A 106:11801–11805
Duarte DJR, de las Vallejos MM, Peruchena NM (2010) Topological analysis of aromatic halogen/hydrogen bonds by electron charge density and electrostatic potentials. J Mol Model 16:737–748
Galabov B, Bobadova-Parvanova P (1999) Molecular electrostatic potential as reactivity index in hydrogen bonding: ab initio molecular orbital study of complexes of nitrile and carbonyl compounds with hydrogen fluoride. J Phys Chem A 103:6793–6799
Kenny PW (2009) Hydrogen bonding, electrostatic potential and molecular design. J Chem Inf Model 49:1234–1244
Politzer P, Murray JS, Concha MC (2007) Halogen bonding and the design of new materials: organic bromides, chlorides and perhaps even fluorides as donors. J Mol Model 13:643–650
Bader RFW (1985) Atoms in molecules. Acc Chem Res 18:9–15
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford, UK
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN et al (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Lipkowski P, Grabowski SJ, Robinson TL, Leszczynski J (2004) Properties of the C-H···H dihydrogen bond: an ab initio and topological analysis. J Phys Chem A 108:10865–10872
Arnold WD, Oldfied E (2000) The chemical nature of hydrogen bonding in proteins via NMR: J-Couplings, Chemical Shifts, and AIM Theory. J Am Chem Soc 122:12835–12841
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Funding
This research was supported by the Open and Cooperation Innovation Fund from Xi’an Modern Chemistry Research Institute and the Fundamental Research Funds for the Central Universities under Grant No. lzujbky-2021-sp43.
Author information
Authors and Affiliations
Contributions
QY, JY, and P-PZ: data analysis, writing—review and editing; H-RZ, P-YL, GG, YY, and WD: data analysis and discussion; P-YL and H-RZ: calculations and data collection
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 51 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Q., Yang, J., Zhang, HR. et al. Investigations of the reaction mechanism of sodium with hydrogen fluoride to form sodium fluoride and the adsorption of hydrogen fluoride on sodium fluoride monomer and tetramer. J Mol Model 30, 26 (2024). https://doi.org/10.1007/s00894-023-05821-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05821-z