Abstract
Context
In this study, theoretical and experimental analysis of the electrical, optical, and structural properties of a wurtzite-like zinc oxide (ZnO) nanostructure has been done. To investigate how quantum confinement affects the optical characteristics, two distinct ZnO clusters in nanowire structures have been investigated. The [(ZnO)55(H2O)4] system’s HOMO–LUMO band gap (BG) was calculated to be 2.99 eV, which is quite close to the experimental measurement. It was found that the BG decreases with the increase in the number of atoms in the cluster in connection with the quantum confinement in nanoclusters. In addition, the lowest excitation energy in TD-DFT calculations of the identical system is in fairly good agreement with the experimental value with a difference of 0.1 eV. We conclude that the CAM-B3LYP functional has highly successful in reproducing the experimental data reported in the present study and previously reported experimental data.
Methods
The geometrical optimization of two different sizes of ZnO clusters ([(ZnO)25(H2O)4] and [ZnO)55(H2O)4]) was performed using the CAM-B3LYP functional with no symmetry constraints applied in the gas phase. LANL2DZ basis sets were used for the Zinc (Zn) atom and 6-31G* basis sets for the O and H atoms. To determine their optical and electronic properties, excited state calculations of the pre-optimized structures were performed using the Time-Dependent DFT (TD-DFT) method. Multiwfn, Gaussum 3.0, and GaussView 5.0 programs were used to visualize the results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ZnO is a semiconductor material having chemical and thermal stability and is excellent for short-wavelength optoelectronic applications due to its high exciton binding energy of 60 meV and direct band gap of 3.37 eV at ambient temperature. The high exciton binding energy of this compound gives it UV luminescence and efficient excitonic emission properties [1].
In addition, non-toxicity, good permeability and electrical conductivity, high electron mobility and low cost make ZnO one step ahead of other metal oxides [2]. Thanks to these properties, ZnO nanomaterials are suitable for producing advanced technology devices such as solar cells, photocatalysts, sensors, detectors and light-emitting diodes [3,4,5,6,7]. When analyzed morphologically, it is seen that ZnO materials can be in rod, wire, flower, leaf, arch and tube structures. Morphological properties, crystal structure and size of ZnO materials affect their electrical and optical properties [8,9,10,11,12,13].
ZnO semiconductor is a compound formed by the combination of sp3 covalent and ionic bonding of Zn and O atoms in group II-VI of the periodic table. ZnO can be found in wurtzite, zinc blende and rocksalt crystal structures and its density is 5.606 g/cm3. The wurtzite is the thermodynamically stable phase at room temperature, with hexagonal unit cells.
One Zn atom coordinates with four O atoms in the crystal structure to form a tetrahedral arrangement. This result in a crystal structure without a center of symmetry and ZnO has two polar surfaces facing each other, each ending with a single type of ion. The lack of symmetry in the crystal structure gives ZnO strong piezoelectric and pyroelectric properties and great electromechanical properties [14,15,16,17]. When the studies related to ZnO are examined [18], it found that the bandgap energies of ZnO and indium-doped ZnO samples synthesized by hydrothermal method decreased and that indium doping further increased this decrease and that the dopant concentration also decreased the photocurrent intensity. In another study [19], it was investigated the antibacterial and biocompatibility properties of synthesized ZnO and Ca-doped ZnO samples, and it was suggested that these materials have high aforementioned properties. Giannoudakos et al. [20] calculated the catalytic and adsorption properties of the un-doped, Au- and Pd-doped ZnO thin films using PC programs written in Fortran. In a similar study, El Mragui et al. [21] determined the photocatalytic and physicochemical properties of ZnO/TiO2 nanomaterials. Li et al. [22] showed that the visible emission of ZnO nanomils and ZnO@ZnS micromil composites can be tuned, which may have potential applications in luminescence, electronics and sensors. Fujinami et al. [23] evaluated the adsorption and binding mechanism between Zn2O2 cluster and crystalline polyimide substrate by semi-experimental molecular orbital calculations and concluded that reactions involving a chemical bond between a cluster of Zn atoms and a carbonyl O atom in the polyimide take place and that the adsorption processes are almost a thermal.
Kaygili et al. [24] experimentally synthesized, characterized and theoretically modeled (DFT) ZnO powders and compared the results with each other and with the results of other studies in the literature. As a result, they found that the electron excitation of ZnO is most likely to occur at the Γ-Γ point, where the energy gap between the valence and conduction bands has the lowest value. Sheela et al. [25] tried to determine the structural, morphological, optical and photocatalytic properties of the un-doped, Ni- and Co-doped ZnO samples produced by hydrothermal method. In their review article [26], suggested that ZnO-based polymer nanocomposites have dielectric, thermal, antibacterial, biomedical, catalytic and mechanical properties and that ZnO doping of these nanocomposites improves their thermal, electrical, piezoelectric, mechanical, electromagnetic, energy storage properties and environmental application potential. Baranov et al. [27] found a decrease in the melting temperature of ZnO samples produced under high pressure and they observed a single crystal growth occurred in these samples.
Raha and Ahmaruzzaman [28] suggested that ZnO nanoparticles can be used in the treatment of diabetes by lowering blood sugar and increasing insulin levels, as well as for anticancer and antibacterial purposes by causing high inhibition on cancerous cells and bacteria, and that ZnO nanoparticles are recognized as safe substances by the FDA. Chu et al. [29] investigated the use of ZnO samples with different forms (e.g., nanoparticles, nanowires, nanoneedles, nanorods and microtubes, etc.) as UV photodetectors and they exhibited different photoelectric properties such as response, on/off ratio and response time. Puspasari et al. [30] claimed that ZnO-based antibacterial coatings have great potential in biomedical and medical fields and have shown positive results in clinical applications.
Many DFT studies have been reported in the literature on the optical, electronic and structural parameters of wurtzite-like ZnX (X = O, Se, Te and S) systems [31,32,33,34]. In these studies, Local density approximation (LDA) and generalized gradient approximation (GGA) functionalism are generally used to determine the structural parameters. However, since these functional are insufficient for determining optical and electronic properties [35,36,37,38], the B3LYP hybrid functional is used to calculate optical and electronic properties such as UV–VIS, DOS and band gap.
Here, unlike the existing studies, since the deficiencies of B3LYP functional are known, further studies using more adequate functionals are needed. To this end, the structural, optical and electronic properties of wurtzite ZnO have been calculated using the CAM-B3LYP functional. CAM-B3LYP is an exchange–correlation functional [39], which is a combination of the hybrid properties of B3LYP and long-range correction. These functional gives results very close to experimental findings, especially for UV–VIS, DOS, PL and band gap calculations where long-range interactions are important [40,41,42].
Materials and methods
Synthesis and characterization of ZnO sample
The synthesis procedure of the ZnO sample is given as follows: 100 mL of 40 mmol of zinc acetate dihydrate (Sigma-Aldrich) solution was prepared by using ethanol (Sigma-Aldrich) as the solvent. This solution was stirred at room temperature for 1 h, and put in an oven at 50 °C for 10 days to dry. The as-obtained powder was heated in an electric furnace at 650 °C for 1.5 h.
A Rigaku RadB-DMAX II model diffractometer utilizing CuKα radiation was used to collect the X-ray diffraction (XRD) data. A LEO EVO 40xVP model scanning electron microscope (SEM) was used to investigate the morphology of the as-produced sample.
Computational details
Owing to the effects of quantum confinement in nanostructures, the optical properties (absorption and emission) are dependent on the structural size and an energetic upward shift in the onset of absorption and emission spectra is observed with decreasing structural size [43,44,45]. However, studies on ZnO nano-structures have shown that the main directions responsible for quantum confinement effects are the crystal axes [0001] and [1010] [46]. For this reason, in the present study, 2D ZnO cluster geometries of different sizes were formed starting from the bulk wurtzite structure to make a convenient comparison with the experimental data.
Similarly, in the past studies on Zn-based II-VI semiconductor nano-structures [47,48,49], an unrealistically high dipole moment in the c direction due to Zn- and O-terminated (0001) and (000) surfaces in non-centrosymmetric WZ structures is observed and this dipole moment makes the structure unstable [47, 48]. For this reason, in accordance with the literature [47, 50, 51], polar surfaces were saturated with dissociating water molecules. Zinc atoms on polar surfaces were saturated with OH− and oxygen atoms were saturated with H+ ions. Considering that protic solvents are used in the synthesis of semiconductor nanostructures, it should be emphasized that this saturation process is not just a calculation trick.
The geometrical optimization of ZnO clusters of two different sizes ([(ZnO)25(H2O)4] and [ZnO)55(H2O)4]) was performed using the CAM-B3LYP [39] functional as implemented in the GAUSSIAN 09 suite of programs [52] and Gaussian type orbits (GTO) basis sets. For simplicity, the calculations were performed in the gas phase and no symmetry constraints were applied. In the calculations, LANL2DZ (Los Alamos National Lab 2 Double Zeta) [53] for Zinc (Zn) atom and 6-31G* basis sets for O and H atoms were used. In addition, to determine the optical (UV) and electronic (band gap) properties of ZnO clusters, excited state calculations of the pre-optimized structures were performed in the gas phase by Time-Dependent DFT (TD-DFT) [54] method. Multiwfn [55], Gaussum 3.0 [56] and GaussView 5.0 [57] programs were used to visualize the data obtained from the calculations.
Findings and discussion
The XRD plot of the sample confirms the polycrystalline ZnO structure with the hexagonal crystal structure (JCPDS PDF No: 36–1451) (Fig. 1). No impurity phase was detected. Table 1 lists the observed and estimated values of the distance between two adjacent planes (d) and 2θ angles for each Miller index (hkl). Both the calculated and the observed values are in agreement.
To calculate the crystallinity percent (XC%) of ZnO, the following relation was used [58]
where ΣAC and ΣAA are the total areas under crystal and amorphous peaks, respectively. This value was estimated to be 95.7% in the present study.
The lattice parameters (a and c) and unit cell volume (V) were found to be a = 0.3251 nm, c = 0.5207 nm, and V = 0.0477 nm.3 by using Eqs. (2) and (3), respectively [59]
Both Scherrer and Williamson-Hall equations were used to calculate the crystallite size, respectively [24]
where β, λ and ε are the full width at half maximum, wavelength, and lattice strain, respectively. The DS value was found to be 32.51 nm, and the DWH and ε values estimated from the βcosθ vs. 4 sin θ graph shown in Fig. 2a were calculated to be 46.84 nm and 1.02 × 10–3, respectively. To calculate the stress (σ), the Eq. (6) was used:
To estimate Young's modulus (Y), the following relation was used
The slope of the βcosθ vs. 4sinθ/Y graph (Fig. 2b) gives the σ value. This value was computed to be 88.56 MPa.
To calculate the energy density (u), the following relation was used:
The u value was calculated from the βcosθ vs. 25/2sinθ Y−1/2 graph (Fig. 2c), and this value was found to be 52.90 kJ m−3 in the present work.
The bond length (L) between Zn and O, and atomic packing factor (APF) were calculated by using Eqs. (9) and (10), respectively [60]
The L value was estimated to be 1.9783 Å. For the hexagonal crystal structure, the APF% value is 74%. For the as-prepared ZnO sample, this value was found to be 75.46%.
The truncated hexagonal pyramid morphology is seen in the SEM images of the ZnO sample shown in Fig. 3. The average size of the experimental nanocluster has been determined as 5.598 µm, which was calculated by Imagej Software using the SEM image. The EDX result supports the formation of the ZnO structure.
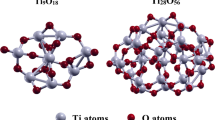
Geometry
After the optimization of the constrained [ZnO)25(H2O)4] and [ZnO)55(H2O)4] clusters, their c-axis lengths were calculated as 13.1 Å and 29.2 Å, respectively (Figs. 4 and 5). As a result of the calculations, the Zn-O bond length of the optimized [ZnO)55(H2O)4] cluster was calculated as 1.93 Å at the Zn-terminated (0001) surface and 1.90 Å at the O-terminated (000) surface. Considering that the bond length in bulk ZnO in the wurtzite structure is 1.96 Å, it is seen that there is a shortening of 1.5% in the Zn- terminated surface and 3.1% in the O- terminated surface. The bond length at the core of the nano-particle was found to be slightly higher than the experimental value of bulk ZnO. The Zn-O bond length in the interior of the nanoparticle was calculated as 1.99 Å, which is 1.5% longer than the experimental value. We have experimentally calculated this value as 1.9783 Å mentioned in the experimental section. Similarly, the O-Zn-O bond angle was calculated as 112.2° at the Zn-terminated surface and 118.8° at the O-terminated surface. Compared to this angle of 109.2 in bulk ZnO, the O-Zn-O angle increased by 2.7% and 8.8% in Zn- and O-terminated surfaces, respectively. The O-Zn-O angles in the inner part of the cluster were calculated as 104.7° and were found to be 4.5% smaller than the experimentally measured value.
Similarly, when geometrical parameters such as bond angle and bond length of [ZnO)25(H2O)4] cluster were analyzed, it was observed that these parameters were similar to [ZnO)55(H2O)4] cluster and there was no significant difference. When the geometrical parameters obtained as a result of the calculations are compared with bulk ZnO, it is seen that the values are in agreement with each other and there is no phase transition from wurtzite to rock salt during the optimization process.
Electronic structure and absorption spectra
In the energy calculations of [ZnO)55(H2O)4] cluster in vacuum, the HOMO energy level of this structure is -6.03 eV, LUMO energy level is -3.04 eV and HOMO-LUMO band gap is 2.99 eV. Similarly, the energy calculations of the [ZnO)25(H2O)4] cluster showed that the HOMO energy level was -6.68 eV, the LUMO energy level was -2.47 eV and the HOMO-LUMO band gap was 4.27 eV. As the length in the c direction decreases, the band gap increases due to quantum confinement effects. In the present study, the variation of the band gap with respect to the c direction is in parallel with the previously reported studies [46, 51]. However, it should be noted that the variation of the band gap with respect to the c direction does not differ much after a certain length (about 5 nm) [46, 51]. It is observed that the HOMOs and LUMOs calculated in both clusters are distributed throughout the system rather than on polar surfaces (Fig. 6). As can be seen from this distribution, the saturation of the polar surfaces with dissociating water molecules prevented the formation of surface localized trap states of atoms lacking coordination.
A more precise prediction of the excitation energies of confined nanostructures needs TD-DFT computations, despite the fact that Kohn-Sham (KS) molecular orbitals and their eigenvalues can provide a fair estimate for the bandgap of bulk II-VI semiconductor materials. TD-DFT calculations more accurately predict bandgap transitions by taking into account electron-hole interactions, exchange-correlation effects, and configurational mixing of single excitations. Figure 7 shows the TD-DFT spectra of [ZnO)55(H2O)4] and [ZnO)25(H2O)4] clusters for the 16 lowest electronic transitions. According to these spectra, the calculated lowest excitation energies are 3.13 eV (396 nm) for [ZnO)55(H2O)4] and 3.61 eV for [ZnO)25(H2O)4]. Considering that the experimental value determined by the Tauch plot in Fig. 7 is 3.25 eV, it is seen that the TD-DFT calculated band gap transitions for [ZnO)55(H2O)4] cluster are quite compatible with the experimental value.
Experimental and calculated absorption spectra of the ZnO nanostructures, obtained by a Gaussian convolution with FWHM = 0.333 eV, calculated taking into account the 16 lowest singlet − singlet electronic transitions. The inset show the Tauc plot of the optical transmittance spectra of the ZnO nanoparticles
To better comprehend the electronic structures of the nanoparticles, density of states (DOS) and partial density of states (PDOS) plots of [ZnO)25(H2O)4] and [ZnO)55(H2O)4] clusters were plotted as shown in Fig. 8. The PDOS analysis of the nanocluster is very important in terms of providing information about the contribution of atomic orbitals to the CB (conduction band) and VB (valence band). As can be seen from Fig. 8a and b, the VB edge is significantly affected by the change in the size of the clusters. On the other hand, there is no significant difference in the variation of CB edge energy with cluster size. However, it was found that the band gap decreases with the increase in the number of atoms in the clusters. As can be seen in previously reported studies [36, 50, 51, 61, 62], the systematic decrease in band gap with the increasing number of atoms in clusters is attributed to the quantum confinement effect in nanoclusters. The identification of the observed peaks may be done easily because the valence band structures of all the binary tetrahedrally coordinated semiconductors are comparable. Accordingly, following the notation suggested in the literature [63], the valence band features are labeled as Pı, Pıı and d. The peak labeled as Pı originates from the 2p orbitals of the O atom and is clearly visible in Fig. 8d, which shows the partial density of states analysis for the [ZnO)55(H2O)4] cluster.
Similarly, the Pıı and d peaks are associated with the 4s and 3d orbitals of the Zn atom, respectively, and this relationship is shown in Fig. 8d. The 4s and 4p orbitals of the Zn atom make up the majority of the low-lying empty orbitals in the conduction band. The relationship of the Pı, Pııı and d peaks with the orbitals in the present study was found to be in great agreement with the experimental and theoretical studies in the literature [50, 51].
If the PDOS graphs are interpreted on an atom basis (Fig. 8c), it is seen that the Zn atom makes the highest contribution to the d peak and conduction band peak, while the O atom makes the highest contribution to the Pı peak. When the DOS plots of [ZnO)25(H2O)4] and [ZnO)55(H2O)4] clusters are analyzed (Fig. 8a and b), it is seen that with the increase in the number of atoms in the clusters, a broadening occurs in the Pı, Pı and d peaks.
Conclusions
In this study, calculation of the structural, electronic and optical properties of [ZnO)25(H2O)4] and [ZnO)55(H2O)4] clusters has been carried out and the obtained results have been compared with both experimental data and available data in literature. In order to create a realistic ZnO nanoparticle model, the lowest optical transition energies of [ZnO)25(H2O)4] and [ZnO)55(H2O)4] clusters were calculated by DFT/TD-DFT methods. The simulations showed that the [(ZnO)55(H2O)4] cluster was a good model for attaining the structural, optical, and electrical features discovered experimentally. In UV–VIS calculations, it was confirmed that the band gap decreases with the increase the number of atoms in cluster. On the other hand, the DOS calculations showed that the HOMO energy level increase with the increase the number of atoms in the clusters and no significant change was found in the LUMO energy levels. In addition, it was found that the CAM-B3LYP functional highly successful in reproducing the experimental data reported in the present study and previously reported data. Furthermore, the lowest excitation energy in TD-DFT calculations of the identical system is in perfect agreement with the experimental value with a difference of 0.1 eV.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author onreasonable request.
References
Wei S, Lian J, Chen X, Jiang Q (2008) Effects of seed layer on the structure and property of zinc oxide thin films electrochemically deposited on ITO-coated glass. Appl Surf Sci 254:6605–6610. https://doi.org/10.1016/j.apsusc.2008.04.039
Baviskar PK, Nikam PR, Gargote SS et al (2013) Controlled synthesis of ZnO nanostructures with assorted morphologies via simple solution chemistry. J Alloys Compd 551:233–242. https://doi.org/10.1016/j.jallcom.2012.10.052
Meng Y, Lin Y, Lin Y (2014) Electrodeposition for the synthesis of ZnO nanorods modified by surface attachment with ZnO nanoparticles and their dye-sensitized solar cell applications. Ceram Int 40:1693–1698. https://doi.org/10.1016/j.ceramint.2013.07.065
Hsu C-H, Chen D-H (2011) Photoresponse and stability improvement of ZnO nanorod array thin film as a single layer of photoelectrode for photoelectrochemical water splitting. Int J Hydrogen Energy 36:15538–15547. https://doi.org/10.1016/j.ijhydene.2011.09.046
Bai S, Sun C, Guo T et al (2013) Low temperature electrochemical deposition of nanoporous ZnO thin films as novel NO2 sensors. Electrochim Acta 90:530–534. https://doi.org/10.1016/j.electacta.2012.12.060
Przeździecka E, Gościński K, Stachowicz M et al (2013) Spectrum selective UV detectors from an p-ZnO:As/n-GaN diodes grown by Molecular Beam Epitaxy. Sensors Actuators A Phys 195:27–31. https://doi.org/10.1016/j.sna.2013.02.021
Guo H, Zhou J, Lin Z (2008) ZnO nanorod light-emitting diodes fabricated by electrochemical approaches. Electrochem Commun 10:146–150. https://doi.org/10.1016/j.elecom.2007.11.010
Sharma SK, Rammohan A, Sharma A (2010) Templated one step electrodeposition of high aspect ratio n-type ZnO nanowire arrays. J Colloid Interface Sci 344:1–9. https://doi.org/10.1016/j.jcis.2009.12.026
Hsieh C-T, Yang S-Y, Lin J-Y (2010) Electrochemical deposition and superhydrophobic behavior of ZnO nanorod arrays. Thin Solid Films 518:4884–4889. https://doi.org/10.1016/j.tsf.2010.03.081
Cao B, Cai W, Li Y et al (2005) Ultraviolet-light-emitting ZnO nanosheets prepared by a chemical bath deposition method. Nanotechnology 16:1734–1738. https://doi.org/10.1088/0957-4484/16/9/054
Liang Y-C (2012) Growth and physical properties of three-dimensional flower-like zinc oxide microcrystals. Ceram Int 38:1697–1702. https://doi.org/10.1016/j.ceramint.2011.08.028
Abd-Ellah M, Moghimi N, Zhang L et al (2013) Effect of electrolyte conductivity on controlled electrochemical synthesis of zinc oxide nanotubes and nanorods. J Phys Chem C 117:6794–6799. https://doi.org/10.1021/jp312321t
Lin C, Lin H, Li J, Li X (2008) Electrodeposition preparation of ZnO nanobelt array films and application to dye-sensitized solar cells. J Alloys Compd 462:175–180. https://doi.org/10.1016/j.jallcom.2007.07.104
Ding Y, Wang ZL (2007) Profile imaging of reconstructed polar and non-polar surfaces of ZnO. Surf Sci 601:425–433. https://doi.org/10.1016/j.susc.2006.07.063
Özgür Ü, Alivov YI, Liu C et al (2005) A comprehensive review of ZnO materials and devices. J Appl Phys 98:041301. https://doi.org/10.1063/1.1992666
Wang ZL (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys Condens Matter 16:R829–R858. https://doi.org/10.1088/0953-8984/16/25/R01
Hahn Y-B (2011) Zinc oxide nanostructures and their applications. Korean J Chem Eng 28:1797–1813. https://doi.org/10.1007/s11814-011-0213-3
Pál E, Hornok V, Oszkó A, Dékány I (2009) Hydrothermal synthesis of prism-like and flower-like ZnO and indium-doped ZnO structures. Colloids Surf A Physicochem Eng Asp 340:1–9. https://doi.org/10.1016/j.colsurfa.2009.01.020
Umavathi S, AlSalhi MS, Devanesan S et al (2020) Synthesis and characterization of ZnO and Ca-ZnO nanoparticles for potential antibacterial activity and plant micronutrients. Surf Interfaces 21:100796. https://doi.org/10.1016/j.surfin.2020.100796
Giannoudakos A, Agelakopoulou T, Asteriadis I et al (2008) Development and characterization of ZnO, Au/ZnO and Pd/ZnO thin films through their adsorptive and catalytic properties. J Chromatogr A 1187:216–225. https://doi.org/10.1016/j.chroma.2008.01.082
El Mragui A, Daou I, Zegaoui O (2019) Influence of the preparation method and ZnO/(ZnO + TiO2) weight ratio on the physicochemical and photocatalytic properties of ZnO-TiO2 nanomaterials. Catal Today 321–322:41–51. https://doi.org/10.1016/j.cattod.2018.01.016
Li F, Bi W, Liu L et al (2009) Preparation and characterization of ZnO nanospindles and ZnO@ZnS core–shell microspindles. Colloids Surf A Physicochem Eng Asp 334:160–164. https://doi.org/10.1016/j.colsurfa.2008.10.016
Fujinami A, Ogata S, Shibutani Y, Yamamoto K (2006) Adsorption process of Zn 2 O 2 on a crystal polyimide surface. Model Simul Mater Sci Eng 14:S95–S103. https://doi.org/10.1088/0965-0393/14/5/S11
Kaygili O, Ercan I, Ates T et al (2018) An experimental and theoretical investigation of the structure of synthesized ZnO powder. Chem Phys 513:273–279. https://doi.org/10.1016/j.chemphys.2018.08.021
Arul Hency Sheela J, Lakshmanan S, Manikandan A, Arul Antony S (2018) Structural, Morphological and Optical Properties of ZnO, ZnO:Ni2+ and ZnO:Co2+ Nanostructures by Hydrothermal Process and Their Photocatalytic Activity. J Inorg Organomet Polym Mater 28:2388–2398. https://doi.org/10.1007/s10904-018-0909-5
Asture A, Rawat V, Srivastava C, Vaya D (2022) Investigation of properties and applications of ZnO polymer nanocomposites. Polym Bull. https://doi.org/10.1007/s00289-022-04243-w
Baranov AN, Sokolov PS, Solozhenko VL (2022) ZnO under pressure: from nanoparticles to single crystals. Crystals 12:744. https://doi.org/10.3390/cryst12050744
Raha S, Ahmaruzzaman M (2022) ZnO nanostructured materials and their potential applications: progress, challenges and perspectives. Nanoscale Adv 4:1868–1925. https://doi.org/10.1039/D1NA00880C
Chu L, Xu C, Zeng W et al (2022) Fabrication and application of different nanostructured ZnO in ultraviolet photodetectors: a review. IEEE Sens J 22:7451–7462. https://doi.org/10.1109/JSEN.2022.3158650
Puspasari V, Ridhova A, Hermawan A et al (2022) ZnO-based antimicrobial coatings for biomedical applications. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-022-02733-9
Yadav SK, Sadowski T, Ramprasad R (2010) Density functional theory study of ZnX (X = O, S, Se, Te) under uniaxial strain. Phys Rev B 81:144120. https://doi.org/10.1103/PhysRevB.81.144120
Ren D, Xiang B, Gao Y et al (2017) Ab initio study of lattice instabilities of zinc chalcogenides ZnX (X=O, S, Se, Te) induced by ultrafast intense laser irradiation. AIP Adv 7:095021. https://doi.org/10.1063/1.4999445
Saha S, Pal S, Sarkar P et al (2012) A complete set of self-consistent charge density-functional tight-binding parametrization of zinc chalcogenides (ZnX; X=O, S, Se, and Te). J Comput Chem. https://doi.org/10.1002/jcc.22945
Saha S, Sarkar S, Pal S, Sarkar P (2013) Ligand mediated tuning of the electronic energy levels of ZnO nanoparticles. RSC Adv 3:532–539. https://doi.org/10.1039/C2RA22429A
Kohan AF, Ceder G, Morgan D, Van de Walle CG (2000) First-principles study of native point defects in ZnO. Phys Rev B 61:15019–15027. https://doi.org/10.1103/PhysRevB.61.15019
Lany S, Zunger A (2005) Anion vacancies as a source of persistent photoconductivity in II-VI and chalcopyrite semiconductors. Phys Rev B 72:035215. https://doi.org/10.1103/PhysRevB.72.035215
Janotti A, Van de Walle CG (2007) Native point defects in ZnO. Phys Rev B 76:165202. https://doi.org/10.1103/PhysRevB.76.165202
Shen X, Allen PB, Muckerman JT et al (2007) Wire versus tube: stability of small one-dimensional ZnO nanostructures. Nano Lett 7:2267–2271. https://doi.org/10.1021/nl070788k
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57. https://doi.org/10.1016/j.cplett.2004.06.011
Jacquemin D, Le BT, Adamo C, Ciofini I (2012) What is the “best” atomic charge model to describe through-space charge-transfer excitations? Phys Chem Chem Phys 14:5383. https://doi.org/10.1039/c2cp40261k
Charaf-Eddin A, Planchat A, Mennucci B et al (2013) Choosing a functional for computing absorption and fluorescence band shapes with TD-DFT. J Chem Theory Comput 9:2749–2760. https://doi.org/10.1021/ct4000795
Ciofini I, Le Bahers T, Adamo C et al (2012) Through-space charge transfer in rod-like molecules: lessons from theory. J Phys Chem C 116:11946–11955. https://doi.org/10.1021/jp3030667
Monticone S, Tufeu R, Kanaev AV (1998) Complex nature of the UV and visible fluorescence of colloidal ZnO nanoparticles. J Phys Chem B 102:2854–2862. https://doi.org/10.1021/jp973425p
van Dijken A, Meulenkamp EA, Vanmaekelbergh D, Meijerink A (2000) Identification of the transition responsible for the visible emission in ZnO using quantum size effects. J Lumin 90:123–128. https://doi.org/10.1016/S0022-2313(99)00599-2
Kahn ML, Cardinal T, Bousquet B et al (2006) Optical properties of zinc oxide nanoparticles and nanorods synthesized using an organometallic method. ChemPhysChem 7:2392–2397. https://doi.org/10.1002/cphc.200600184
De Angelis F, Armelao L (2011) Optical properties of ZnO nanostructures: a hybrid DFT/TDDFT investigation. Phys Chem Chem Phys 13:467–475. https://doi.org/10.1039/C0CP01234C
Meyer B (2004) First-principles study of the polar O-terminated ZnO surface in thermodynamic equilibrium with oxygen and hydrogen. Phys Rev B 69:045416. https://doi.org/10.1103/PhysRevB.69.045416
Li C, Guo W, Kong Y, Gao H (2007) First-principles study on ZnO nanoclusters with hexagonal prism structures. Appl Phys Lett 90:223102. https://doi.org/10.1063/1.2743934
Wander A, Harrison NM (2001) The stability of polar oxide surfaces: The interaction of H2O with ZnO(0001) and ZnO(0001̄). J Chem Phys 115:2312–2316. https://doi.org/10.1063/1.1384030
Azpiroz JM, Mosconi E, De AF (2011) Modeling ZnS and ZnO nanostructures: structural, electronic, and optical properties. J Phys Chem C 115:25219–25226. https://doi.org/10.1021/jp2083709
Azpiroz JM, Infante I, Lopez X et al (2012) A first-principles study of II–VI (II = Zn; VI = O, S, Se, Te) semiconductor nanostructures. J Mater Chem 22:21453. https://doi.org/10.1039/c2jm33744d
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, Revision B.01. Gaussian 09, Revis. B.01, Gaussian, Inc., Wallingford CT
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–310. https://doi.org/10.1063/1.448975
Runge E, Gross EKU (1984) Density-functional theory for time-dependent systems. Phys Rev Lett 52:997–1000. https://doi.org/10.1103/PhysRevLett.52.997
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
O’boyle NM, Tenderholt AL, Langner KM (2008) cclib: A library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845. https://doi.org/10.1002/jcc.20823
Nielsen AB, Holder AJ (2009) Gauss view 5.0, user’s reference. GAUSSIAN Inc, Pittsburgh
Okur HE, Bulut N, Ates T, Kaygili O (2019) Structural and optical characterization of Sm-doped ZnO nanoparticles. Bull Mater Sci 42:199. https://doi.org/10.1007/s12034-019-1877-2
Cullity BD (1978) Elements of X-ray diffraction, 2nd edn. Addison-Wesley Publ Co, Read, 100-105,277-279. https://doi.org/10.1119/1.1934486
Ibrahim EH, Albulym O, Kaygili O et al (2019) Green synthesis, structural, in vitro and in vivo bioactivity properties of ZnO nanoparticles for biomedical applications. J Biomater Tissue Eng 9:731–738. https://doi.org/10.1166/jbt.2019.2046
Reiss P, Protière M, Li L (2009) Core/shell semiconductor nanocrystals. Small 5:154–168. https://doi.org/10.1002/smll.200800841
Schrier J, Demchenko DO, Lin-Wang AAP (2007) Optical properties of ZnO/ZnS and ZnO/ZnTe heterostructures for photovoltaic applications. Nano Lett 7:2377–2382. https://doi.org/10.1021/nl071027k
Ley L, Pollak RA, McFeely FR et al (1974) Total valence-band densities of states of III-V and II-VI compounds from x-ray photoemission spectroscopy. Phys Rev B 9:600–621. https://doi.org/10.1103/PhysRevB.9.600
Acknowledgements
This publication is based upon the work of COST Action CA21101 “COSY”.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.O. and N.B.; methodology, C.O and O.K.; validation, C.O., N.B. and S.K.; formal analysis, N.B. and P.S.Z., investigation, C.O. and O.K,; resources, N.B and O.K.; data curation, C.O, S.K. and N.B.; writing—original draft preparation, C.O., O.K. and N.B,; writing—review and editing, P.S.Z. and N.B.; visualization, C.O and O.K.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Orek, C., Keser, S., Kaygili, O. et al. Structures and optical properties of zinc oxide nanoclusters: a combined experimental and theoretical approach. J Mol Model 29, 227 (2023). https://doi.org/10.1007/s00894-023-05641-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05641-1