Abstract
Context
High-energy density materials (HEDMs) have emerged as a research focus due to their advantageous ultra-high detonation performance and better sensitivity. The primary aim of this study revolves around crafting HEDMs that strike a delicate balance between exceptional performance and minimal sensitivity. Density functional theory (DFT) was utilized to evaluate the geometric structures, energies, densities, energy properties, and sensitivities of 39 designed derivatives. The theoretical density (ρ) and heat of formation (HOF) were used to estimate the detonation velocity (D) and pressure (P) of the title compounds. Our study shows that the introduction of fluorine-containing substituents or fluorine-free substituents into the CHOFN backbone or the CHON backbone can significantly enhance the detonation performance of derivatives. Derivative B1 exhibits the better overall performance, including superior density, detonation performance, and sensitivity (P = 58.89 GPa, D = 8.02 km/s, ρ = 1.93 g/cm3, and characteristic height H50 = 34.6 cm). Our molecular design strategy contributes to the development of more novel HEDMs with excellent detonation performance and stability. It also marks a significant step towards a material engineering era guided by theory-based rational design.
Methods
GaussView 6.0 was used for construction of molecular system coordinates, and Gaussian 16 was used to obtain optimal structures, energies, and volumes of all compounds at the B3LYP/6-31+G(d,p) level of theory. It was characterized to be the local energy minimum on the potential energy surface without imaginary frequencies at the same theory level. Molecular weight, isosurface area, and overall variance were obtained using the Multiwfn 3.3. The detonation properties of the materials were analyzed using the C-J thermodynamic detonation theory. Our broad analysis facilitated an extensive assessment of these properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energetic materials have been recognized for their ability to store large amounts of chemical energy and release it rapidly upon detonation. These materials play a crucial role in both military forces and civil construction, including grenades, mortars, warheads, and rocket propellants [1]. The invention of black powders in 220 BC marked the origin of energetic materials. This mixture of flammable charcoal and sulfur with the oxidizer potassium is considered one of the four greatest inventions in China and has set the foundation for the development of energetic materials [2]. Over time, pioneering scientists have developed various types of energetic materials, including nitroglycerin, 2,4,6-trinitrotoluene (TNT) [3], cyclotrimethylenetrinitroamine (RDX) [4], octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) [5], hexanitrohexaazaisowurtzitane (CL-20) [6], octanitrocubane (ONC) [7], and dinitroanisole (DNAN) [8]. However, the applications of these materials are by their relative disadvantages, such as TNT’s toxicity and environmental concerns, as well as the low detonation performance of DNAN. Although HMX and CL-20 have been recognized as high-energy density materials (HEDMs), their instability has also restricted their applications.
In response to the growing demand for new and improved energetic materials, scholars are actively engaged in the design and synthesis of HEDMs with enhanced stability and minimized hazardous waste production upon detonation. The past two decades have witnessed significant advances in both computational and experimental chemistry of HEDMs with superior heat of formation (HOF), nitrogen content, and detonation properties compared to conventional explosives [9,10,11]. Striking an ideal balance between excellent detonation performance, high thermal stability, and acceptable mechanical sensitivity remains a major challenge. Heterocyclic systems have emerged as a promising area for the design and synthesis of novel HEDMs, gradually becoming a hot spot in this field [12]. Nitrogen-rich heterocyclic backbones have proven to be effective for enhancing HOF, density (ρ), oxygen balance (OB100), and overall performance. Nitrogen-containing energetic materials feature N–N bonds and C–N bonds, which contribute to positive HOF. It is widely recognized that nitrogen-rich energetic materials often exhibit remarkable insensitivity toward electrostatic discharge, friction, and impact [13,14,15]. As the carbon and hydrogen content decreases, the density of the energetic material increases, leading to improved oxygen balance and higher gas (N2) production per gram of material than conventional explosives. This study focused on investigating a series of compounds characterized by a significant nitrogen content.
Incorporating fluorine into organic compounds has significant effects on their chemical, physical, and biological properties. Firstly, due to the similarity in atomic radius between F and H or hydroxyl, fluorine can easily replace these groups in a molecule, resulting in little change in the overall molecular volume. Moreover, the C–F bond length is shorter, and its bond dissociation energy is greater than that of C–H bonds. Secondly, fluorine has a simple structure, good target ability, and small volume. Upon explosion, it forms HF, releasing a significant amount of heat energy, which is highly favorable for both detonation velocity (D) and detonation pressure (P) [16, 17], as we have calculated. Although CHOFN-containing energetic materials may pose ecological concerns due to the formation of HF, they could also act as excellent biocides [18].
Given their promising properties, CHOFN-containing energetic materials have become a preferred research focus and are considered as promising candidates [19,20,21,22,23]. Incorporating fluorine-containing substituents or a fluorinated backbone often leads to excellent performance compared to conventional explosives such as RDX and HMX [24,25,26]. In support of the development of a new family of HEDMs, we designed 39 novel derivatives based on three parent compounds:
-
1.
2-nitro-5,7-bis(trifluoromethyl)-[1,2,4]triazolo[1,5-a]pyrimidine
-
2.
5-methyl-2-nitro-7-trifluoromethyl-[1,2,4]triazolo[1,5-a]pyrimidine
-
3.
5,7-dimethyl-2-nitro-[1,2,4]triazolo[1,5-a]pyrimidine
CHOFN-containing energetic materials can be classified into two types: those with fluorine-containing substituents or conventional substituents, incorporated into CHON compounds or CHOFN compounds. Such substituent groups include –C(NO2)3, –NO2, –CN, –NHNO2, –N3, –NC, –NH2, –ONO2, –NF2, –CF(NO2)2, –N(NO2)2, –N(NH2NO2), and–N(NO2ONO2) in line with the bridging strategies proposed by Tsyshevsky et al. [27], which utilize a fragment-based combinatorial approach to design new heterocyclic HEDMs. The detailed structures of each derivative and its substituents are illustrated in Fig. 1.
Given the poor measurement accuracy of kinetic data and the rapid decomposition rates of explosives, it is challenging to establish structure-activity relationships from experimental data alone. To address this issue, accurate theoretical calculations based on quantum chemistry are essential for achieving a balance between performance and safety [28]. In this study, we calculated the energy, density, and sensitivity of these derivatives and provided a comprehensive analysis. Our research design is expected to contribute to the expansion of the HEDM family with excellent properties.
Computation methods
Two types of CHOFN-containing energetic materials were investigated in this study using the Gaussian 16 program at the B3LYP/6-31+G(d,p) level of theory, with a focus on performing vibrational frequency analysis and structural optimization [29]. The default convergence criteria of the program were used for the optimization process, without any symmetry constraints. All optimized structures were confirmed to be true local energy minima on potential energy surfaces, with no imaginary frequencies. HOF values of the derivatives were calculated via DFT calculation and isodesmic reactions [30]. To minimize the calculation error of HOF, we employed the bond separation reaction (BSR) rule to design isodesmic reactions, in which the bond numbers remained constant. Isodesmic reactions were chosen because they have similar electronic environments between reactants and products, allowing for the cancellation of electron correction energy errors and greatly reducing the overall calculation error of HOF [31].
The isodesmic reaction (Fig. 2) operates through a mechanism whereby the conjugated bond within the heterocyclic skeleton remains unaltered, while the larger molecules undergo conversion into smaller molecules where R is
–C(NO2)3, –NO2, –CN, –NHNO2, –N3, –NC, –NH2, –ONO2, –NF2, –CF(NO2)2, –N(NO2)2, –N(NH2NO2), –N(NO2ONO2) (Fig. 1).
The heat of reaction (ΔH298) at 298 K was calculated and confirmed using the equation in the reference [32]. The HOF in solid state could be predicted using the following equation:
The sublimation enthalpy (ΔHsub) was evaluated using Eq. (2), suggested by Rice and Politzer et al [33, 34]:
where SA is the area of the isosurface of 0.001 e/Bohr3 electron density of a molecule calculated using a self-compiled program.
In order to gain a thorough understanding of the safety properties of certain compounds, an investigation was conducted into their shock sensitivity. An essential parameter for predicting the safety and shock sensitivity of HEDMs during storage or use is the characteristic height (to − 1.75%). To estimate the H50 value for all novel derivatives, a reliable method proposed by Pospíšil et al. was employed [35]:
The theoretical density was obtained by Eq. (4), which was proposed by Politzer et al. [36]:
where M is the molecular weight (g/mol), and Vm is the volume of the 0.001 a.u. (electrons/bohr3) contour of electronic density calculated using Monte Carlo integration. The coefficients α, β, and γ were taken from reference [37].
One of the fundamental objectives in the field of energetic materials is to predict D and P of novel HEDMs before their synthesis. The Chapman-Jouguet (C-J) thermodynamic detonation theory has conventionally been utilized to investigate the detonation of CHOFN explosives. It is universally acknowledged that the abovementioned thermodynamic detonation theories yield satisfactory concordance between the measured and calculated values of the detonation pressure for any CHOFN ideal or less ideal explosives.
For the CHOFN explosives, the C-J method was used to explore the detonation performance [38]:
where D is the detonation velocity (km/s), P is the detonation pressure (Gpa), M̅ is the average molecular weight of the gaseous product (g/mol), and α is the number of moles of detonation gaseous product per gram of explosive. Detonation heat (Q) (cal/g) is the heat of detonation which can be calculated in Eq. (7) and the basic component of detonation pressure and detonation velocity:
The quantity ρQmax represents the maximum heat of detonation per unit volume of the compound, and it plays a crucial role in predicting the compound’s sensitivity. It was suggested by Politzer et al [39]:
ρ0 was obtained from Eq. (9) above, where g/cm3 is the unit of molecule density.
In the case of explosives containing CaHbOcFdNe, the OB100 was accomplished through the use of Eqs. (10) and (11), which also provides a means for predicting the impact sensitivities of the explosives:
In the case of each CHOFN or CHON compound, all N atoms are assumed to convert into N2. Additionally, a subset of the O atoms preferentially reacts with H atoms, resulting in the formation of H2O and CO rather than CO2 with C atoms. If the number of O atoms is excessive for the complete oxidation of H and C atoms, the redundant O atoms transform into O2. However, if the number of atoms is not enough to satisfy full oxidation of H and C atoms, any remaining H atoms become H2, and the C atoms exist as solid-state C. Depending on the composition of CHOFN explosives, the primary detonation products may include CO, CO2, H2O, N2, HF, and solid carbon, along with minor quantities of H2, NH3, O2, NO, and other chemical species.
Equation (12) was employed to calculate the content of specific decomposition products. The detonation performance of HEDMs is contingent on characteristics such as α, M̅, Q, and others, which may be regulated by controlling the decomposition pathways of the compounds to achieve a dependable correlation for computing both ideal and non-ideal explosives. The quantities of diverse products rely on various parameters of the detonation process and other equilibrium effects.
Results and discussion
The compounds under investigation were divided into three distinct groups: Group A, Group B, and Group C. Group A was obtained from 2-nitro-5,7-bis(trifluoromethyl)-[1,2,4]triazolo[1,5-a]pyrimidine; meanwhile, a sequence of 5-methyl-2-nitro-7-trifluoromethyl-[1,2,4]triazolo[1,5-a]pyrimidine-based energetic derivatives and 5,7-dimethyl-2-nitro-[1,2,4]triazolo[1,5-a]pyrimidine-based derivatives were designed, which were named Group B and Group C, respectively.
HOF
Heats of formation (HOF) is viewed as a crucial gauge of the “energy content” of energetic materials. Due to its impact on detonation velocity and detonation pressure, the accurate prediction of HOF is pivotal in estimating the overall properties of HEDMs. Nevertheless, obtaining HOF through experimental means is both dangerous and challenging. Therefore, theoretical research and HOF prediction hold particular significance.
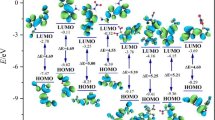
In the case of CHOFN or CHON compounds, the isodesmic reaction that was designed can be utilized. Prior research has revealed that this approach yields theoretical HOF values that correspond satisfactorily with the most precise experimental values [30, 40]. Table 1 shows the calculated HOFg, ΔHsub, and HOFs for the derivatives under study. It is apparent that the value of HOFs experiences a substantial increase with an increase in the number of nitrogen atoms in the substituent groups. This finding indicates that the –N3, –NC, –CN, and –NO2 groups play a crucial role in conferring energetic properties to the azole skeleton present in CHOFN compounds or CHON compounds. The existence of a double heterocycle and numerous N–N bonds contributes to achieving superior propellant performance by providing higher HOFs. Fluorinated energetic materials contain F atoms that boast high levels of chemical activity; thus, utilizing fluorinated energetic materials in the field of energetic materials may make a significant contribution to advancing combustion reactions [41].
Notably, not all derivatives exhibit higher HOF values than their parent compound, potentially due to an increased fluorine content [22, 23]. The majority of HOF values for Group A and Group B are negative, while those of Group C are positive. Calculation of HOF in a quantitative manner has established that the distinctiveness of fluorinated energetic materials compared to conventional energetic materials may be dependent on the extent of fluorination.
Furthermore, the majority of substituent groups contribute to HOF values. For instance, the addition of groups such as –N3 is conducive to higher HOFs, while the incorporation of groups such as –CF(NO2)2 does not improve HOFs. When the –N3 group is added to 2-nitro-[1,2,4]triazolo[1,5-a]pyrimidine, the resulting compound exhibits the highest HOF value (A5: − 488.36 kJ/mol, B5: 38.69 kJ/mol). The HOF value of C5 is also higher. This finding suggests that the –N3 group is among the most energetic functional groups and plays a vital role in augmenting HOF values. Conversely, when the substituent group shifts from –N3 to –CF(NO2)2, the HOF value becomes the smallest in the corresponding series of derivatives, indicating that different substituents exert varied effects on HOFs.
Additionally, for isomers containing the same atoms (derivatives A3 and A6, derivatives B3 and B6) but differing substituent groups (–CN, –NC), the HOF values of derivatives A6 and B6 are higher than those of derivatives A3 and B3. This finding implies that the –NC substituent group is more effective at enhancing HOFs. The order of contribution of substituent groups to HOF is as follows: –N3 > –NC > –CN > –N(NO2)2 > –N(NH2NO2) > –C(NO2)3 > –N(NO2ONO2) > –NHNO2 ≈ –NF2 > –NO2 > –NH2 > –ONO2 > –CF(NO2)2
Explosive performance
The addition of elemental fluorine has captured the attention of chemists due to a variety of performance is usually as much as doubled, such as D and P [16]. Density is an important parameter of energetic materials and a key factor to determine detonation performance. Table 2 shows the calculated densities of derivatives, most of which are excellent. Densities of many derivatives surpass 2.00 g/cm3, satisfying the necessary criteria for advanced energetic materials.
Moreover, the incorporation of F-containing substituents can significantly enhance detonation performance by exerting a notable influence on density [44]. As observed in Table 2, the addition of substituent groups such as –NF2 and –C(NO2)3 leads to an increase in density, which promotes the enhancement of explosive performance. Conversely, adding the –CN group results in a decrease in density.
The majority of the compounds analyzed exhibit superior performance when compared to their parent compounds. Interestingly, the derivatives A1, A10, A11, A13, B1, B2, B8, B10, B11, B12, B13, and C2 display notably higher detonation pressures than HMX (39.0 GPa) [42]. Additionally, the detonation pressures of derivatives A8, A12, B4, B8, and C1 exceed that of RDX (34.0 GPa) [42]. Derivative B1 boasts excellent detonation pressure, superior density, low sensitivities (ρ = 1.93 g/cm3, P = 58.89 GPa, and H50 = 34.6 cm, ρQmax=1.78 kcal/cm3) but detonation velocity (D = 8.02 km/s) is unsatisfactory. Some exceptional properties of derivatives highlight the benefits of our proposed design strategy, and some bad properties motivate us to further improve them.
When analyzing the –NO2 group, it is worth noting that it constitutes one of the most versatile and commonly employed functional groups, featuring robust electron-attracting capabilities. In this study, the detonation performance of the derivatives exhibits a substantial increase as the number of –NO2 group increases. The number of –NO2 groups contributed to the detonation performance, but exerted a less effect on HOF. Furthermore, we have established that the –NH2 group not only reduces HOF but also diminishes detonation performance.
These results suggest that the detonation velocity and pressure of HEDMs are more sensitive to substituent groups. Our analysis reveals that different substituent groups exert varied effects on explosive performance of HEDMs. The order of contribution of substituent groups to D, P, and Q is as follows: –C(NO2)3 > –N(NO2ONO2) > –N(NO2)2 > –CF(NO2)2 > –N(NH2NO2) > –ONO2 > –NO2 > –NHNO2 > –N3 > –NH2 ≈ –NC ≈ –CN ≈ –NF2.
Figure 3 illustrates a clear correlation between the product distribution of the HEDMs and their global performance. For example, the detonation properties (D = 7.83 km/s, P = 55.08 GPa) of derivative A1 surpass those of some conventional explosives. The generation of a substantial volume of N2 gas is highly favorable for enhancing the D, P, and Q of the HEDMs.
An increase in the proportion of F in the products leads to a decrease in the hydrogen content of the compound. The minimal hydrogen content ensures complete oxidation of the carbon element, thereby contributing to high detonation properties observed in several H-deficient compounds studied.
Our findings on the correlation between product distribution of the HEDMs and their detonation performance are consistent with the observations made in previous sections on the preferred molecular backbones and functional substituent groups of HEDMs. Specifically, high N and F content contribute significantly to various detonation properties when designing high-performing HEDMs.
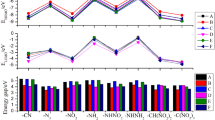
To derive the plausible physicochemical characteristics of high-performing HEDMs, the performance parameters (D, P, H50, and OB100) are plotted as a function of material densities for all the HEDMs studied, as depicted in Fig. 4. Our findings reveal that the performance parameters (D, P, H50, and OB100) of the HEDMs exhibit dependence on their material densities.
Correlation between the material density of the 39 HEDMs and their (a) detonation velocity, (b) detonation pressure, and (c) characteristic height, and (d) oxygen balance and the favorable derivatives. TNT’s D: 6.95 km/s, P: 22.35 GPa were set as the references, as marked by the dashed lines. For characteristic height and oxygen balance, the reference was set to be H50: 29.0 cm, OB100: − 25% to 0
Of particular importance, our findings reveal that OB100 (− 100% to 0) linearly increases with material densities, further supporting the notion that HEDMs typically display optimal performance as the value of OB100 approaches zero, indicating full oxidant utilization. Notably, the selection of excellent derivatives hinges on the following conditions being met: ρ > 1.60 g/cm3 and detonation performance exceeding that of TNT [42].
Impact sensitivity (H 50, ρQ max, and OB100)
When assessing stability, H50 and OB100 are considered the most crucial parameters.
H50 has been predicted for all molecules in this study. As illustrated in Table 3, all molecules exhibit potential shock sensitivity, with H50 values ranging from 20.4 to 49.7 cm. Notably, the H50 value is inversely proportional to sensitivity and is closely linked to the properties of the HEDMs. Most derivatives feature higher H50 values than RDX (26 cm) and HMX (29 cm) [45], with the H50 of all derivatives exceeding that of CL-20 (12 cm) [46]. The derivatives of series of C have excellent and concentrated H50 values (45.5–49.7cm), proving that the sensitivity of our designed derivatives is satisfactory. For obtaining low-impact sensitivity, a large heat of detonation is undesirable.
Calculated values of RDX and HMX were taken from Talawar, M. B. et al. [45, 47].
For obtaining excellent detonation velocity and detonation pressure, a large heat of detonation is also unnecessary, because moderate detonation heat energy can achieve higher detonation performance, but also increases the possibility of forming a lower sensitivity [48]. Fortunately, the majority of our designed derivatives possess a low detonation heat (see Table S2). The calculations of ρQmax and H50 produced consistent results, indicating that energetic materials we designed may exhibit lower sensitivity and provide a safety assurance.
Oxygen balance serves as an indicator of the degree to which HEDMs can be oxidized, as summarized in Table 3. OB100, a parameter frequently evaluated by scholars, is seldom close to zero, as observed for RDX (− 21.61%) and HMX (− 21.61%) [47]. Furthermore, derivative C1 exhibits an oxygen balance value of − 1.75%. When the oxygen balance approaches zero, derivatives typically exhibit robust detonation performance due to full oxidant utilization. Thus, achieving a near-zero oxygen balance represents an effective strategy for balancing detonation performance and sensitivity. Derivatives C1-C13 have oxygen balance values of − 8.65 to − 1.75%, consistent with the oxygen balance at about zero strategy. Moreover, near-zero oxygen balance allows for significant increases in the proportion of environmentally friendly molecular nitrogen and water in the product.
Conclusions
In the present study, we have designed and optimized a series of novel 2-nitro-[1,2,4]triazolo[1,5-a]pyrimidineis derivatives by introducing various substituent groups at the B3LYP/6-31+G(d,p) level. Our findings reveal that the addition of fluorine elements either to the backbone or as substituent groups can significantly enhance explosive performance, oxygen balance, and material densities of HEDMs. Based on our results, some valuable conclusions can be drawn, which are outlined below:
Previous studies have highlighted that one of the key features of excellent CHON energetic materials is high and positive HOF [49]. However, unlike conventional CHON energetic materials, the addition of fluorine can lead to negative HOF values. Moreover, different substituents exert varied effects on HOF. The order of contribution of substituent groups to HOF was determined as follows: –N3 > –NC > –CN > –N(NO2)2 > –N(NH2NO2) > –C(NO2)3 > –N(NO2ONO2) > –NHNO2 ≈ –NF2 > –NO2 > –NH2 > –ONO2 > –CF(NO2)2.
Most derivatives have relatively satisfactory H50 values, indicating lower sensitivity. This finding contributes not only to our understanding of the structural features and stabilities of energetic materials but also greatly advances the application of fluorinated energetic materials in the field of explosives. Most derivatives require both low-sensitivity and excellent explosive properties. The predicted detonation velocities and pressures highlight the nitroso group as an effective substituent group for enhancing detonation performance. While these derivatives may not exhibit remarkable detonation velocities, the incorporation of fluorine offers several advantages. These derivatives effectively fulfill certain explosive characteristics while maintaining low sensitivity, thus achieving a balanced condition. This insight also serves as inspiration for us to further investigate the potential of novel fluorine-containing HEDMs. Finally, the contribution of substituent groups to D, P, and Q was ranked as follows: –C(NO2)3 > –N(NO2ONO2) > –N(NO2)2 > –CF(NO2)2 > –N(NH2NO2) > –ONO2 > –NO2 > –NHNO2 > –N3 > –NH2 ≈ –NC ≈ –CN ≈ –NF2.
The performance of derivatives from Group A, Group B, and Group C suggests that these derivatives have the potential to be good explosives and expand the HEDMs family. Introducing substituent groups into the CHOFN backbone resulted in better performance than introducing fluorine-containing substituent groups into the CHON backbone. It is likely that this consistency is related to the fluorine content of the derivative products.
Data availability
The datasets analyzed during the current study are included in this paper and its supplementary information file.
References
Klapötke TM, Krumm B, Rest SF, Reynders M, Scharf R (2013) (2-Fluoro-2,2-dinitroethyl)-2,2,2-trinitroethylnitramine: a possible high-energy dense oxidizer. Eur J Inorg Chem 2013:5871–5878. https://doi.org/10.1002/ejic.201300923
Yin P and Shreeve J n M (2017) in Adv Heterocycl Chem, eds. E F V Scriven and C A Ramsden, Elsevier, New York,pp 89-131.
Wilbrand J (1863) Notiz über Trinitrotoluol. Justus Liebigs Ann Chem 128:178–179. https://doi.org/10.1002/jlac.18631280206
Keshavarz MH, Klapötke TM (2018) in Sensitivity, physical and thermodynamic properties. De Gruyter, Berlin, pp 119–121
Wang PC, Xu YG, Lin QH, Lu M (2018) Recent advances in the syntheses and properties of polynitrogen pentazolate anion cyclo-N5− and its derivatives. Chem Soc Rev 47:7522–7538. https://doi.org/10.1039/C8CS00372F
Carr M J, Perera S D, Jelínek T, Kilner C A, Clegg W, Štíbr B and Kennedy J D (2006) Macropolyhedral boron-containing cluster chemistry. Cluster opening and B-frame rearrangement in the reaction of B16H20 with [{(IrCl2(η5-C5Me5)}2]. Synchrotron X-ray structures of [(η5-C5Me5)2Ir2B16H17Cl] and [(η5-C5Me5)2Ir2B16H15Cl]. Dalton Trans, DOI: https://doi.org/10.1039/B611734A5221-5224.
Zhang MX, Eaton PE, Gilardi R (2000) Hepta-and octanitrocubanes. Angew Chem Int Ed 39:401–404. https://doi.org/10.1002/(SICI)1521-3773(20000117)39:2<401::AID-ANIE401>3.0.CO;2-P
(2017) 2,4-Dinitroanisole (DNAN) (2014) Toxicol Ind Health 34:2-7. https://doi.org/10.1177/0748233717719690
Xu Z, Cheng GB, Yang HW, Ju XH, Yin P, Zhang JH, Shreeve JN (2017) A facile and versatile synthesis of energetic furazan-functionalized 5-nitroimino-1,2,4-triazoles. Angew Chem Int Ed 56:5877–5881. https://doi.org/10.1002/anie.201701659
Lai Q, Pei L, Fei T, Yin P, Pang SP, Shreeve JN (2022) Size-matched hydrogen bonded hydroxylammonium frameworks for regulation of energetic materials. Nat Commun 13:6937. https://doi.org/10.1038/s41467-022-34686-8
Huang W, Tang YX, Imler GH, Parrish DA, Shreeve JN (2020) Nitrogen-rich tetrazolo[1,5-b]pyridazine: promising building block for advanced energetic materials. J Am Chem Soc 142:3652–3657. https://doi.org/10.1021/jacs.0c00161
Yin P, Zhang QH, Shreeve JN (2016) Dancing with energetic nitrogen atoms: versatile N-functionalization strategies for N-heterocyclic frameworks in high energy density materials. Acc Chem Res 49:4–16. https://doi.org/10.1021/acs.accounts.5b00477
Qu Y, Babailov SP (2018) Azo-linked high-nitrogen energetic materials. J Mater Chem A 6:1915–1940. https://doi.org/10.1039/C7TA09593G
Maan A, Ghule VD, Dharavath S (2021) Tetranitro-diazinodiazines as high energy materials: computational investigation of structural aspects of fused heterocyclic backbone and isomerism. Struct Chem 32:2175–2181. https://doi.org/10.1007/s11224-021-01791-1
Fu J, Wang B, Chen Y, Li Y, Tan X, Wang B, Ye B (2021) Computational analysis the relationships of energy and mechanical properties with sensitivity for FOX-7 based PBXs via MD simulation. R Soc Open Sci 8:200345. https://doi.org/10.1098/rsos.200345
Fei T, Du Y, Pang SP (2018) Theoretical design and prediction of properties for dinitromethyl, fluorodinitromethyl, and (difluoroamino)dinitromethyl derivatives of triazole and tetrazole. RSC Adv 8:10215–10227. https://doi.org/10.1039/C8RA00699G
Zhai DD, Ma CM, Ma P, Pan Y, Hao LN, Liu XQ, Jiang JC (2021) Theoretical insight into different energetic groups on the performance of energetic materials featuring RDX ring. Fuel 294:120497. https://doi.org/10.1016/j.fuel.2021.120497
Klapötke TM (2018) Energetic Materials Encyclopedia. De Gruyter, Berlin, Boston
Tang YX, Gao HX, Imler GH, Parrish DA, Shreeve JN (2016) Energetic dinitromethyl group functionalized azofurazan and its azofurazanates. RSC Adv 6:91477–91482. https://doi.org/10.1039/C6RA22007J
Christe KO, Wilson WW, Bélanger-Chabot G, Haiges R, Boatz JA, Rahm M, Prakash GKS, Saal T, Hopfinger M (2015) Synthesis and characterization of fluorodinitroamine, FN(NO2)2. Angew Chem Int Ed 54:1316–1320. https://doi.org/10.1002/anie.201410507
Huang S, Tian JJ, Qi XJ, Wang KC, Zhang QH (2017) Synthesis of gem- dinitromethylated and fluorodinitromethylated derivatives of 5,5′-dinitro-bis-1,2,4-triazole as promising high-energy-density materials. Chemistry – A. Eur J 23:12787–12794. https://doi.org/10.1002/chem.201702451
Kettner MA, Karaghiosoff K, Klapötke TM, Sućeska M, Wunder S (2014) 3,3′-bi(1,2,4-oxadiazoles) featuring the fluorodinitromethyl and trinitromethyl groups. chemistry – a. Eur J 20:7622–7631. https://doi.org/10.1002/chem.201402291
Kettner MA, Klapötke TM, Witkowski TG, von Hundling F (2015) Synthesis, characterisation and crystal structures of two bi-oxadiazole derivatives featuring the trifluoromethyl group. Chem– A Eur J 21:4238–4241. https://doi.org/10.1002/chem.201406436
Zhang JL, Liu YZ, Zhou J, Bi FQ, Wang BZ (2019) Effect of fluoro substituents on polynitroarylenes: design, synthesis and theoretical studies of fluorinated nitrotoluenes. ChemPlusChem 84:92–97. https://doi.org/10.1002/cplu.201800523
Chavez DE, Parrish DA, Mitchell L (2016) Energetic trinitro- and fluorodinitroethyl ethers of 1,2,4,5-tetrazines. Angew Chem Int Ed 55:8666–8669. https://doi.org/10.1002/anie.201604115
Guo ZH, Yu Q, Chen YC, Liu J, Li T, Peng YH, Yi WB (2023) Fluorine-containing functional group-based energetic materials. Chem Rec. https://doi.org/10.1002/tcr.202300108
Tsyshevsky R, Pagoria P, Zhang M, Racoveanu A, Parrish DA, Smirnov AS, Kuklja MM (2017) Comprehensive end-to-end design of novel high energy density materials: I. Synthesis and characterization of oxadiazole based heterocycles. J Phys Chem C 121:23853–23864. https://doi.org/10.1021/acs.jpcc.7b07584
Owens FJ (1999) Molecular orbital calculation of decomposition pathways of nitrocubanes and nitroazacubanes. J Mol Struct :THEOCHEM 460:137–140. https://doi.org/10.1016/S0166-1280(98)00312-1
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Petersson G A, Nakatsuji H, Li X, Caricato M, Marenich A V, Bloino J, Janesko B G, Gomperts R, Mennucci B, Hratchian H P, Ortiz J V, Izmaylov A F, Sonnenberg J L, Williams, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski V G, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr. J A, Peralta J E, Ogliaro F, Bearpark M J, Heyd J J, Brothers E N, Kudin K N, Staroverov V N, Keith T A, Kobayashi R, Normand J, Raghavachari K, Rendell A P, Burant J C, Iyengar S S, Tomasi J, Cossi M, Millam J M, Klene M, Adamo C, Cammi R, Ochterski J W, Martin R L, Morokuma K, Farkas O, Foresman J B and Fox D J (2016) Gaussian 16 Rev. A.03
Hehre WJ, Ditchfield R, Radom L, Pople JA (1970) Molecular orbital theory of the electronic structure of organic compounds. V. Molecular theory of bond separation. J Am Chem Soc 92:4796–4801. https://doi.org/10.1021/ja00719a006
Wiberg K B (1986) in J Comput Chem, John Wiley & Sons, Ltd, New York.
Zhao G, Kumar D, Hu L and Shreeve J n M (2019) A safe and scaled-up route to inert ammonia oxide hydroxylammonium azide (H7N5O2), hydrazinium azide (H5N5), and ammonium azide (H4N4). ACS Appl Energy Mater 2:6919-6923. https://doi.org/10.1021/acsaem.9b01447
Politzer P, Murray JS, Edward Grice M, Desalvo M, Miller E (1997) Calculation of heats of sublimation and solid phase heats of formation. Mol Phys 91:923–928. https://doi.org/10.1080/002689797171030
Rice BM, Pai SV, Hare J (1999) Predicting heats of formation of energetic materials using quantum mechanical calculations. Combust Flame 118:445–458. https://doi.org/10.1016/S0010-2180(99)00008-5
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) A possible crystal volume factor in the impact sensitivities of some energetic compounds. J Mol Model 16:895–901. https://doi.org/10.1007/s00894-009-0587-x
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107:2095–2101. https://doi.org/10.1080/00268970903156306
Politzer P, Murray JS (2002) The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor Chem Acc 108:134–142. https://doi.org/10.1007/s00214-002-0363-9
Keshavarz MH, Pouretedal HR (2004) An empirical method for predicting detonation pressure of CHNOFCl explosives. Thermochim Acta 414:203–208. https://doi.org/10.1016/j.tca.2003.11.019
Politzer P, Murray JS (2015) Some molecular/crystalline factors that affect the sensitivities of energetic materials: molecular surface electrostatic potentials, lattice free space and maximum heat of detonation per unit volume. J Mol Model 21:25. https://doi.org/10.1007/s00894-015-2578-4
Dorofeeva OV, Ryzhova ON (2016) Enthalpy of formation and O–H bond dissociation enthalpy of phenol: inconsistency between theory and experiment. J Phys Chem A 120:2471–2479. https://doi.org/10.1021/acs.jpca.6b02233
Meng S, Fu X, Jiang L, Shi L, Wang X, Liu X, Wang J (2022) Theoretical calculations and experiments on the thermal properties of fluorinated graphene and its effects on the thermal decomposition of nitrate esters. Nanomaterials 12:621. https://doi.org/10.3390/nano12040621
Politzer P and Murray J S (2011) in Cent Eur J Energetic Mater, Inst Industrial Organic Chemistry, Poland,pp 209-220.
Chinnam AK, Staples RJ, Shreeve JN (2021) Synthesis and energetic properties of trifluoromethyl-substituted 2-nitro-[1,2,4]triazolo[1,5-a]pyrimidine derivatives. J Fluor Chem 245. https://doi.org/10.1016/j.jfluchem.2021.109743
Zohari N, Abrishami F, Ebrahimikia M (2016) Investigation of the effect of various substituents on the density of tetrazolium nitrate salts as green energetic materials. Z Anorg Allg Chem 642:749–760. https://doi.org/10.1002/zaac.201600161
Guo CY, Zhang HB, Wang XC, Liu XF, Sun J (2013) Study on a novel energetic cocrystal of TNT/TNB. J Mater Sci 48:1351–1357. https://doi.org/10.1007/s10853-012-6881-5
Shen C, Wang P, Lu M (2015) Molecular design and property prediction for a series of novel dicyclic cyclotrimethylene trinitramines (RDX) derivatized as high energy density materialS. J Phys Chem A 119:8250–8255. https://doi.org/10.1021/acs.jpca.5b04969
Talawar MB, Sivabalan R, Mukundan T, Muthurajan H, Sikder AK, Gandhe BR, Rao AS (2009) Environmentally compatible next generation green energetic materials (GEMs). J Hazard Mater 161:589–607. https://doi.org/10.1016/j.jhazmat.2008.04.011
Politzer P, Murray JS (2015) Impact sensitivity and the maximum heat of detonation. J Mol Model 21:262. https://doi.org/10.1007/s00894-015-2793-z
Mehta N, Oyler K, Cheng G, Shah A, Marin J, Yee K (2014) Primary explosives. Z Anorg Allg Chem 640:1309–1313
Funding
This work was financially supported by the Foundation Project of Tangshan Normal University (2021B37), Tangshan Talent Funding Project (A202110004), the Tangshan Normal University Scientific Research Fund Project (2020A14), and Construction and innovation of practice teaching system of “Cross-platform Composite ability” (2020JG06).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by J.Y., T.B., J.G., M.L., and Y.W.; data collection was performed by J.Y., T.B., Z.Z., X.D., and Y.W.; and data analysis were performed by J.Y. and T.B. The first draft of the manuscript was written by J.Y., resources and inspiration were originally from J.Y., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 519 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Bai, T., Guan, J. et al. Novel fluorine-containing energetic materials: how potential are they? A computational study of detonation performance. J Mol Model 29, 228 (2023). https://doi.org/10.1007/s00894-023-05618-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05618-0