Abstract
In this article, the CL-20/HMX cocrystal model was established and its based polymer bonded explosives (PBXs) were designed. The static performances, including mechanical properties, stability and detonation performance of CL-20/HMX cocrystal model and PBXs models, were predicted by molecular dynamics (MD) method. The mechanical parameters, binding energy, and detonation parameters of PBXs models were calculated and compared with that of pure CL-20/HMX cocrystal model. The influence of polymer binders on performances of CL-20/HMX cocrystal explosive was evaluated. Results show that the polymer binders make the engineering moduli (tensile modulus, shear modulus, and bulk modulus) of PBXs declined and Cauchy pressure increased, meaning that the polymer binder can obviously improve mechanical properties of CL-20/HMX cocrystal explosive, and the PBXs model with fluorine rubber (F2311) has the best mechanical properties. In different PBXs models, the binding energy between CL-20, HMX molecules and F2311 is higher than other polymer binders, indicating that the CL-20/HMX/F2311 model is more stable. The PBXs models have lower value of crystal density and detonation parameters compared with pure CL-20/HMX cocrystal and the energetic performance of PBXs is weakened. The PBXs model with fluorine resin (F2314) has the highest energetic performance and it is higher than pure HMX. Therefore, the CL-20/HMX/F2311 and CL-20/HMX/F2314 models have more favorable comprehensive properties, proving that F2311 and F2314 are more preferable and promising to design CL-20/HMX cocrystal based PBXs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer bonder explosive (PBX) is mainly composed of different kinds of components, including high energy explosives, polymer binders, plus with a small amount of phlegmatizer and plasticizer. Compared with raw component of energetic materials, the mechanical properties (such as rigidity, plastic property and ductility) of PBX can be obviously improved with the influence of polymer binders. Besides, PBX can also maintain high energy density and relatively low mechanical sensitivity. Therefore, on the one hand, many different kinds of PBXs have been designed and prepared to improve properties of raw explosives, such as octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX), 2,4,6,8,10,12-hexanitro- 2,4,6,8,10,12-hexaazaisowurtzitane (CL-20), hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and so on. On the other hand, PBX has been extensively applied in warhead ammunitions and rocket propellants for a long time [1,2,3].
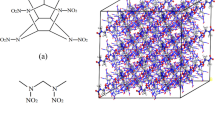
CL-20 (in Fig. 1a) is regarded as the most famous nitramine explosive with caged molecule structure and it has higher crystal density (ρ = 2.01 g/cm3 ~ 2.03 g/cm3), detonation pressure (p = 42GPa ~ 43GPa), detonation velocity (D = 9.5 km/s ~ 9.6 km/s) than most conventional energetic materials, so CL-20 is very promising in high energy density materials (HEDMs) field [4, 5]. HMX (in Fig. 1b) is also a typical nitramine explosive with high energetic performance (ρ = 1.894 g/cm3, p = 39.4GPa, D = 9.0 km/s, OB = -21.62%). Besides, HMX also has favorable thermal stability and has been applied widely since its first synthesis [6, 7]. In 2012, Bolton [8] successfully prepared a novel CL-20/HMX cocrystal explosive (molar ratio in 2:1) for the first time and tested its properties, including detonation performance, crystal structure, crystal polymorph, and mechanical sensitivity. Results showed that the energy density of CL-20/HMX cocrystal was slightly lowered than pure CL-20, and the impact sensitivity was near HMX. For energetic materials, HMX also exhibits relatively high mechanical sensitivity (drop height H50 = 32 cm) [9], i.e., the CL-20/HMX cocrystal still has high mechanical sensitivity. Owing to the fact that the polymer binders in PBXs can effectively improve performance of energetic materials without weakening its energy density sharply, the CL-20/HMX cocrystal based PBXs is designed to ameliorate the comprehensive properties of CL-20/HMX cocrystal explosive.
At present, to predict the structures and properties of energetic materials and its based PBXs, researchers usually used different methods, such as molecular dynamics (MD) method, molecular mechanics (MM) method, and quantum mechanics (QM) method. Up to now, MD, MM and QM method have been successfully applied for different kinds of energetic materials a long time [1,2,3, 10,11,12,13,14,15,16,17,18]. Compared with MM and QM method, MD method can accurately and quickly predict the physicochemical properties of energetic materials from molecule level. Therefore, MD method has become one of the most important methods in recent years for energetic compounds and its based PBXs.
In this article, the pure CL-20/HMX cocrystal model was established and its based different PBXs models were designed. The properties of CL-20/HMX cocrystal and PBXs models were predicted by MD method. The effects of polymer binders on performance of CL-20/HMX cocrystal explosive were evaluated and discussed.
Models and methods
CL-20/HMX cocrystal models
The CL-20/HMX cocrystal explosive is formed with molar ratio of 2:1 and it belongs to monoclinic crystal structure and P21/c space group [8]. The lattice parameters of cocrystal model are a = 16.3455(12)Å, b = 9.9361(5)Å, c = 12.1419(7)Å, α = 90.00°, β = 99.233(7)°, γ = 90.00° [8]. The CL-20/HMX cocrystal model is established based on its crystal structure and lattice parameters and is shown in Fig. 2c. Then, the primitive cocrystal model is expanded to 12 (2 × 3 × 2) supercells, including 48 CL-20 molecules and 24 HMX molecules in total (in Fig. 2d). To compare the performance of pure CL-20/HMX cocrystal model with that of its based PBXs models, the raw CL-20/HMX cocrystal model is marked as Model-I.
PBXs models
The CL-20/HMX cocrystal based PBXs are composed of CL-20/HMX cocrystal explosive and polymer binders. There are six different kinds of polymer binders in total, hydroxyl terminated polybutadiene (HTPB), polyvinylidene difluoride (PVDF), polychlorotrifluoroethylene (PCTFE), polytetrafluoroethylene (PTFE), fluorine rubber (F2311) and fluorine resin (F2314). Among these polymer binders, F2311 and F2314 are consisted of vinylidenedifluoride and chlorotrifluoroethylene with component ratio (or molecule ratio) of 1:1 and 1:4, respectively. For HTPB, the head atom and tail atom in polymer chains are saturated with hydroxyl (-OH group). For PVDF, PCTFE, PTFE, F2311 and F2314, the head atom and tail atom are saturated with hydrogen (H) atom or fluorine (F) atom according to its type. In practice, the mass content (or mass percentage) of polymer binders in PBXs is about 4 ~ 5%; therefore, the total number of polymer chains is determined based on this principle (Fig. 3).
The models of polymer binders were built by amorphous cell module in Materials Studio Package (Version 7.0).
To select the most suitable polymer binder in PBXs models, different kinds of polymer chains were built. Then, the polymer binders were simulated with 2 ns by using the MD method. The COMPASS force field was selected, the temperature was set as 295 K (in solid state), and the vdW and electrostatic interaction energies were truncated with the cutoff distance of 9.5 Å. After this process, the optimized polymer chain was put into an amorphous unit cell, which made it present a real state. Then, the amorphous unit cell was minimized to equilibrium the polymer chain. Finally, the polymer binder conformation which had the least value of energy would be chosen to build the PBX model.
Based on the CL-20/HMX cocrystal supercell model (Model-I), the primitive model was cleaved along c axis into three major crystal surfaces, (1 0 0), (0 1 0) and (0 0 1), respectively. Then, the cleaved three surfaces were rebuilt into crystals with vacuum layer height of 20 Å. The polymer binders which was in the most stable conformation were put into the vacuum layer parallel to the three cleaved (1 0 0), (0 1 0) and (0 0 1) surfaces respectively and the primitive PBXs models were obtained. Next, the original PBXs models would be compressed and optimized by molecular mechanics (MM) method adequately along with the c direction, namely minimizations were initially performed for 10,000 iterations to equilibrate the PBXs models and the simulation boxes of PBXs models were compressed slightly (0.3%) along the c direction. Afterward, another 10,000 iterations of minimizations were carried out to reach the equilibrium state and the boxes would be compressed further along the c direction. This process would be repeated step by step until the crystal densities approach to the theoretical values (ρ = 1.875 g/cm3, 1.873 g/cm3, 1.894 g/cm3, 1.888 g/cm3, 1.903 g/cm3, 1.912 g/cm3, respectively). Then, another MD simulation was applied to optimize the crystal structure of PBX model and make it reach the equilibrium state. For example, the different PBXs models under equilibrium state on (0 1 0) cleaved surface are presented in Fig. 4. The six different PBXs models are labeled as Model-II, Model-III, Model-IV, Model-V, Model-VI, and Model-VII, respectively.
Calculation conditions and details
In this article, the crystal structure of CL-20/HMX cocrystal and its based PBXs were optimized and the properties were predicted by MD method. All the MD simulation was performed under the NVT ensemble (constant number of crystal volume, atoms, and temperature) with periodic boundary conditions and the temperature was set as 295 K. The MD simulation data were obtained through COMPASS force field [19, 20], because this force field was appropriate for energetic materials, especially suitable for nitramine energetic materials, such as CL-20, HMX, CL-20/HMX cocrystal model and the associated PBXs [21,22,23,24]. The thermostat is chosen as Andersen [25] and the barostat is chosen as Parrinello [26]. To accurately figure out the non-bond interactions, the atom-based iteration method [27] was selected to calculate the van der Waals (vdW) interactions, and the Ewald method [28] was applied to calculate the electrostatic interaction energies. Both of the vdW and electrostatic interaction energies were truncated with the distance of 9.5 Å. The time step in MD simulation was set as 1 fs and the total simulation time was 2 ns (2 × 106 fs). In the first 1 ns MD simulation process, the CL-20/HMX cocrystal model and PBXs models were under equilibration runs to optimize the crystal structure and make the model reach the equilibrium state. Next, in the second 1 ns MD simulation process, another production runs with 1 ns was performed based on the equilibrium state to calculate the correlated parameters and collect data for making analysis of static coefficients and properties.
Results analysis and discussion
Choice of force field
In MD simulation, to accurately figure out the parameters and predict the properties of CL-20/HMX cocrystal and its based PBXs models, it is required that the force field must be suitable for CL-20/HMX cocrystal model. To test the accuracy and determine the most suitable force field, the primitive CL-20/HMX cocrystal model was optimized with different force fields, including PCFF force field [29], Universal force field [30], Dreiding force field [31], and COMPASS force field [19, 20]. The theoretical predicted lattice parameters of CL-20/HMX cocrystal explosive with different force fields are listed in Table 1.
As presented in Table 1, the predicted crystallographic parameters and crystal density acquired from COMPASS force field are more consistent with experimental results, corresponding to a = 16.3509 Å, b = 9.9394 Å, c = 12.1460 Å, α = 90.00°, β = 99.27°, γ = 90.00°, respectively, meaning that these parameters with COMPASS force field are more accurate than that with other three force fields. Based on the data in Table 1, it can be concluded that COMPASS force field is more applicable for CL-20/HMX cocrystal model than PCFF, Universal and Dreiding force fields. Besides, it also implies that the selection of COMPASS force field in MD simulation is reasonable and reliable.
Equilibrium state of PBXs models
In MD simulation, the CL-20/HMX cocrystal and its based PBXs models would be optimized within 1 ns process. During this process, the crystal structure will be optimized and the energy will be minimized. For example, the temperature curve and energy curve of CL-20/HMX/F2311 PBX model (Model-VI) is shown in Fig. 5.
It is clearly shown in Fig. 5 that both of the temperature curve and energy curve fluctuates within ± 5% after 0.4 ns, implying that the temperature and energy in PBXs model has reached the equilibrium state. Based on the temperature curve and energy curve, it can be concluded that the CL-20/HMX/F2311 PBX model (Model-VI) has reached the equilibrium state after MD simulation with 1 ns. For other PBXs models, the equilibrium state is also judged by the temperature curve and energy curve.
Mechanical properties
Mechanical properties of energetic materials are mainly evaluated by the correlated mechanical parameters, such as K, G, E, γ and (C12-C44), where K is named as bulk modulus, G is defined as shear modulus, E is tensile modulus (also called Young’s modulus), γ is Poisson’s ratio, (C12-C44) is called Cauchy pressure. Among these five mechanical parameters, the three engineering moduli (K, G, and E) are mainly related with the rigidity, hardness, yield stress, and rupture strength of materials. In other words, materials with high positive value of K, G, and E will also have high yield stress, rigidity or hardness [32]. The symbol γ is defined as the ratio of transverse strain to longitudinal strain and it can reflect the elastic property of materials. Cauchy pressure is an important parameter to judge the ductility or brittleness of materials [33]. If the value of Cauchy pressure is positive, it may imply that the material exhibits plastic property and has desirable ductility. On the contrary, negative value of Cauchy pressure means that the material exhibits brittle property and has undesirable ductility.
When subjected to external loading (including compression, or stretching), the elastic stress (σ) and elastic strain (ε) in materials can be described by the Hooke’s law [34, 35] as following:
where, C = [Cij] (i, j = 1, 2, ···, 6) is called elastic coefficients matrix, Cij is elastic coefficients and Cij = Cji. The elastic coefficients (Cij) can be obtained from the second 1 ns MD simulation by analyzing the equilibrium configuration of PBXs models.
Shear modulus (GR) and bulk modulus (KR) can be obtained by the Reuss-mean method [36] as that:
where, the subscript R is the symbol of Reuss, the parameter Sij (i, j = 1, 2, ···, 6) is called the stiffness coefficient, and the stiffness matrix S = [Sij] is equal to the inverse matrix of elastic coefficients matrix, i.e., S = C−1.
The three engineering moduli (K, G and E) and Poisson’s ratio (γ) is related together as following:
Therefore, Poisson’s ratio (γ) and tensile modulus (E) is obtained as that:
It is presented in Fig. 6 that the raw CL-20/HMX cocrystal model (Model-I) has relatively higher value of K, G, and E than the PBXs models (Model-II ~ Model-VII), but it also has the lowest value of Cauchy pressure (C12-C44). The decline of three engineering moduli (K, G, and E) illustrates that the rigidity, rupture strength, and hardness of PBXs models are decreased than raw CL-20/HMX cocrystal model, while the increase of Cauchy pressure means that the ductility and plastic property is enhanced. Therefore, the variation of mechanical parameters clearly states that the mechanical properties of PBXs can be effectively improved by adding polymer binders into CL-20/HMX cocrystal explosive. Among the six different kinds of polymer binders, the CL-20/HMX/F2311 (Model-VI) and CL-20/HMX/F2314 (Model-VII) has lower value of engineering moduli and higher value of Cauchy pressure than other PBXs models, especially the CL-20/HMX/F2311 model, thus meaning that the CL-20/HMX/F2311 and CL-20/HMX/F2314 PBXs models has more desirable mechanical properties. What’s more, the data in Fig. 6 also states that F2311 and F2314 may be more effective and advantageous in tuning mechanical properties of CL-20/HMX cocrystal explosive.
Stability
Stability of PBX is mainly characterized by the binding energy between energetic materials component and polymer binder. PBX with higher positive value of binding energy will mean that the intermolecular interaction force between energetic material component and polymer binder is stronger, and the stability is better. Besides, the binding energy of PBXs can also reflect the compatibility between energetic component and polymers, and higher value of binding energy will imply that the compatibility is better. On the contrary, lower value of binding energy in PBX models will correspond to worse stability and weaker intermolecular compatibility.
In CL-20/HMX cocrystal based PBXs models, binding energy can be calculated as that:
where, Eb is defined as the binding energy of PBXs (kJ/mol), Einter is the intermolecular interaction force between CL-20/HMX cocrystal explosive and polymers (kJ/mol), Etotal is defined as the total energy of PBX model when it is under equilibrium state (kJ/mol), ECL-20/HMX is called the energy of CL-20/HMX cocrystal explosive when the polymer is removed from PBX model (kJ/mol), Epoly is the total energy of polymer chains with all the CL-20 and HMX molecules removed from the PBX model (kJ/mol).
As illustrated in Fig. 7, the binding energy of CL-20/HMX cocrystal based PBXs on the three cleaved surfaces varies as that (0 0 1) > (1 0 0) > (0 1 0), meaning that the intermolecular interaction energy of CL-20/HMX cocrystal explosive and polymer binders on (0 0 1) surface is the highest and this crystal surface is more stable, next is the (1 0 0) surface, while the (0 1 0) surface has the weakest stability. Among the different PBXs models, Model-II (CL-20/HMX/HTPB) has the lowest value of binding energy, meaning that the interaction energy between CL-20/HMX cocrystal explosive and polymer binder HTPB is the weakest. Besides, the lowest value of binding energy also indicates that the stability and compatibility of CL-20/HMX/HTPB is the weakest. Oppositely, Model-VI (CL-20/HMX/F2311) has the highest value of binding energy, indicating that this PBX model has the most desirable stability, next is Model-VII (CL-20/HMX/F2314). Based on the value of binding energy, it can be concluded that among the different polymer binders, the interaction energy between CL-20/HMX cocrystal explosive and fluorine polymer F2311 is stronger, which may further imply that F2311 is more suitable and attractive to be the polymer binder for CL-20/HMX cocrystal based PBXs.
Detonation performance
Detonation performance is a vital guideline to reflect energy density of energetic materials and it is commonly depicted by detonation parameters, including crystal density, detonation pressure, detonation velocity and etc. In this article, the detonation parameters of CL-20/HMX cocrystal and its based PBXs are calculated by the nitrogen equivalent coefficient (NEC) method [37]. The NEC method takes numerous factors which may affect detonation properties of energetic materials into account, including detonation products, chemical bonds and chemical groups contained in explosive molecule. In previous studies [38,39,40,41], the NEC method has been applied to predict detonation properties of energetic materials and the results show that this method can accurately predict detonation properties of explosives.
According to the NEC theory [37], detonation velocity (D) and detonation pressure (p) is illustrated as follows:
where, D is detonation velocity (m/s), p is detonation pressure (GPa), ρ is the mass density of explosive (g/cm3) and the mass density can be obtained from the MD simulation results under equilibrium state, \(\sum {N}_{\text{ch}}\) is the total value of nitrogen equivalent coefficient for explosives, Mr is the molar weight of explosive, pi is the total number of detonation product, Npi is the nitrogen equivalent coefficient for the ith detonation product, BK is the total number of chemical bonds existed in explosive molecules, such as C-H bond, C-N bond, N–N bond, NBK is the nitrogen equivalent coefficient of the Kth chemical bond, Gj is the total number of chemical groups contained in explosive molecules, such as C-NO2 group, N-NO2 group, NGj is the nitrogen equivalent coefficient for the jth chemical group.
From Eq. (8), it can be concluded that the total number of nitrogen equivalent coefficient is determined by three factors, i.e., the detonation product, chemical bonds and chemical groups contained in explosive molecules. For PBX models, it is composed of C, H, O, N, F and Cl elements, assume that the molecular formula of PBX is CaHbOcNdFeClf, the detonation equation of PBXs is illustrated as that:
The crystal density and detonation parameters of CL-20/HMX cocrystal explosive and its based PBXs are presented in Table 2.
As clearly shown in Table 2, it can be concluded that raw CL-20/HMX cocrystal model (Model-I) exhibits higher crystal density and detonation parameters than its based PBXs models (Model-II ~ Model-VII), corresponding to 1.998 g/cm3, 9389 m/s, and 43.08 GPa, respectively, which is lower than pure CL-20 (Model-VIII), but obviously higher than pure HMX (Model-IX), and these parameters are in good agreement with the results reported by Bolton [8]. The reason for the decline of crystal density and detonation parameters in PBXs models is that the polymer binder has lower energy density. For the different PBXs models, crystal density is within 1.871 ~ 1.910 g/cm3, detonation velocity is within 8692 ~ 9075 m/s, detonation pressure is within 37.53 ~ 39.85 GPa. Among the different PBXs, CL-20/HMX/PVDF (Model-III) has the lowest crystal density (1.871 g/cm3), detonation velocity (8692 m/s), and detonation pressure (37.53 GPa). On the contrary, CL-20/HMX/F2314 (Model-VII) has the highest energetic parameters (ρ = 1.910 g/cm3, D = 9075 m/s, p = 39.85 GPa).
Compared with HMX (Model-IX), it can be concluded that the crystal density and/or detonation parameters of CL-20/HMX/HTPB (Model-II), CL-20/HMX/PVDF (Model-III), CL-20/HMX/PCTFE (Model-IV), CL-20/HMX/PTFE (Model-V), and CL-20/HMX/F2311 (Model-VI) is lower than HMX, meaning that the energy density of these PBXs is lower than HMX, while CL-20/HMX/F2314 (Model-VII) has higher density, higher detonation velocity and higher detonation pressure than HMX, indicating that CL-20/HMX/F2314 has higher energetic performance than HMX. Consequently, CL-20/HMX/F2314 maintains high energy density and can be regarded as a novel high energy explosive.
Conclusions
In this article, the CL-20/HMX cocrystal explosive and its based different PBXs models were established. MD method was applied to predict the properties of CL-20/HMX cocrystal model and PBXs models. The effect of polymer binders on properties of CL-20/HMX cocrystal explosive was estimated. The main conclusions were summarized as follows:
-
(1)
Compared with raw CL-20/HMX cocrystal model, the PBXs models have lower value of engineering moduli and higher value of Cauchy pressure, meaning that the PBXs models own better mechanical properties than pure CL-20/HMX cocrystal model and the CL-20/HMX/F2311 model has the most desirable mechanical properties.
-
(2)
The value of binding energy for CL-20/HMX/F2311 model is higher, indicating that the intermolecular interaction strength is stronger, the CL-20/HMX/F2311 model is relatively more stable and the compatibility between CL-20/HMX cocrystal explosive and fluorine polymer F2311 is better.
-
(3)
The crystal density and detonation parameters of PBXs are lower than pure CL-20 and CL-20/HMX cocrystal explosive, implying that the energy density of PBXs is weakened, the CL-20/HMX/F2314 PBX has the highest crystal density and detonation parameters, and the energy density is equivalent to HMX.
In a word, the CL-20/HMX/F2311 model has the best mechanical properties and stability, while the CL-20/HMX/F2314 model has the highest energetic performance. Therefore, CL-20/HMX/F2311 and CL-20/HMX/F2314 has relatively better comprehensive properties, which may indicate that F2311 and F2314 are more preferable to be applied in PBXs to tune properties for CL-20/HMX cocrystal explosive.
Data availability
All data generated or analyzed during this study are included in this article.
Code availability
Not applicable.
Abbreviations
- CL-20:
-
2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane
- F2311 :
-
Fluorine rubber
- F2314 :
-
Fluorine resin
- HEDMs:
-
High energy density materials
- HMX:
-
Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
- HTPB:
-
Hydroxyl terminated polybutadiene
- PBX:
-
Polymer bonded explosive
- PCTFE:
-
Polychlorotrifluoroethylene
- PTFE:
-
Polytetrafluoroethylene
- PVDF:
-
Polyvinylidene difluoride
- RDX:
-
Hexahydro-1,3,5-trinitro-1,3,5-triazine
References
Xu XJ, Xiao HM, Xiao JJ, Zhu W, Huang H, Li JS (2006) Molecular dynamics simulations for pure ε-CL-20 and ε-CL-20-based PBXs. J Phys Chem B 110:7203–7207
Xiao JJ, Ma XF, Zhu W, Huang YC, Xiao HM (2007) Molecular dynamics simulations of polymer-bonded explosives (PBXs): modeling, mechanical properties and their dependence on temperatures and concentrations of binders. Propellants Explos Pyrotech 32:355–359
Qiu L, Xiao HM (2009) Molecular dynamics study of binding energies, mechanical properties, and detonation performances of bicyclo-HMX-based PBXs. J Hazard Mater 164:329–336
Nielsen AT, Chafin AP, Christian SL, Moore DW, Nadler MP, Nissan RA, Vanderah DJ, Gilardi RD, George CF, Flippen-Anderson JL (1998) Synthesis of polyazapolycyclic caged polynitramines. Tetrahedron 54:11793–11812
Agrawal JP (2005) Some new high energy materials and their formulations for specialized applications. Propellants Explos Pyrotech 30:316–328
Simpson RL, Urtiew PA, Ornellas DL, Moody GL, Scribner KJ, Hoffman DM (1997) CL-20 performance exceeds that of HMX and its sensitivity is moderate. Propellants Explos Pyrotech 22:249–255
Sikder AK, Sikder N (2004) A review of advanced high performance, insensitive and thermal stable energetic materials emerging for military and space applications. J Hazard Mater A 112:1–15
Bolton O, Simke LR, Pagoria PF, Matzger AJ (2012) High power explosive with good sensitivity: a 2:1 cocrystal of CL-20:HMX. Cryst Growth Des 12:4311–4314
Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP (2008) Advances in science and technology of modern energetic materials: an overview. J Hazard Mater 151:289–305
Xie HM, Zhu WH (2020) Thermal decomposition mechanisms of the energetic benzotrifuroxan:1,3,3-trinitroazetidine cocrystal using ab initio molecular dynamics simulations. J Chin Chem Soc 67:218–226
Xiao JJ, Zhao L, Zhu W, Chen J, Ji GF, Zhao F, Wu Q, Xiao HM (2012) Molecular dynamics study on the relationships of modeling, structural and energy properties with sensitivity for RDX-based PBXs. Sci China Chem 55:2587–2594
Abou-Rachid H, Lussier LS, Ringuette S (2008) On the correlation between miscibility and solubility properties of energetic plasticizers/polymer blends: modeling and simulation studies. Propellants Explos Pyrotech 33:301–310
Cui HL, Ji GF, Chen XR, Wei DQ (2013) Mesoscopic simulation of aggregate behaviour of polymers in β-HMX-based PBXs. Chin J Chem Phys 26:462–466
Liu ZC, Zhu WH, Ji GF, Song KF, Xiao HM (2017) Decomposition mechanisms of α-octahydro-l,3,5,7-tetranitro-l,3,5,7-tetrazocine nanoparticles at high temperatures. J Phys Chem C 121:7728–7740
Liu ZC, Zhu WH, Xiao HM (2016) Surface-induced energetics, electronic structure, and vibrational properties of β-HMX nanoparticles: a computational study. J Phys Chem C 120:27182–27191
Ren CX, Liu H, Li XX, Guo L (2020) Decomposition mechanism scenarios of CL-20 co-crystals revealed by ReaxFF molecular dynamics: similarities and differences. Phys Chem Chem Phys 22:2827–2840
Mathieu D, Borges I (2022) Molecular dynamics simulation of hot spot formation and chemical reactions. Theor Comput Chem 22:255–289
Mathieu D (2022) Molecular modeling of the sensitivities of energetic materials. Theor Comput Chem 22:2–471
Sun H, Ren PJ, Fried R (1998) The COMPASS force field: parameterization and validation for phosphazenes. Comput Theor Polym Sci 8:229–246
Michael JM, Sun H, Rigby D (2004) Development and validation of COMPASS force field parameters for molecules with aliphatic azide chains. J Comput Chem 25:61–71
Xu XJ, Xiao JJ, Huang H, Li JS, Xiao HM (2010) Molecular dynamic simulations on the structures and properties of ε-CL-20(0 0 1)/F2314 PBX. J Hazard Mater 175:423–428
Xiao JJ, Wang WR, Chen J, Ji GF, Zhu W, Xiao HM (2012) Study on the relations of sensitivity with energy properties for HMX and HMX-based PBXs by molecular dynamics simulation. Physica B 407:3504–3509
Sun T, Xiao JJ, Liu Q, Zhao F, Xiao HM (2014) Comparative study on structure, energetic and mechanical properties of a ε-CL-20/HMX cocrystal and its composite with molecular dynamics simulation. J Mater Chem A 2:13898–13904
Tao J, Wang XF, Zhao SX, Diao XQ, Wang CL, Han ZX (2016) Molecular dynamics simulation of CL-20/HMX cocrystal and blends. Chin J Energ Mater 24:324–330
Andersen HC (1980) Molecular dynamics simulations at constant pressure and/or temperature. J Chem Phys 72:2384–2393
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190
Allen MP, Tildesley DJ (1987) Computer simulation of liquids. Oxford University Press, Oxford
Ewald PP (1921) Evaluation of optical and electrostatic lattice potentials. Ann Phys 64:253–287
Sun H (1994) Force field for computation of conformational energies, structures, and vibrational frequencies of aromatic polyesters. J Comput Chem 15:752–768
Casewit CJ, Colwell KS, Rappé AK (1992) Application of a universal force field to organic molecules. J Am Chem Soc 114:10035–10046
Mayo SL, Olafson BD, Goddard WA III (1990) Dreiding: a generic force field for molecular simulations. J Phys Chem B 94:8897–8909
Pugh SF (1954) Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos Mag 45:823–843
Pettifor DG (1992) Theoretical predictions of structure and related properties of intermetallics. Mater Sci Technol 8:345–349
Weiner JH (1983) Statistical mechanics of elasticity. John Wiley, New York
Wu JL (1993) Mechanics of elasticity. Tongji University Press, Shanghai
Watt JP, Davies GF, O’Connell RJ (1976) The elastic properties of composite materials. Rev Geophys Space Phys 14:541–563
Guo YX, Zhang HS (1983) Nitrogen equivalent coefficient and revised nitrogen equivalent coefficient equations for calculating detonation properties of explosives: detonation velocity of explosives. Explos Shock Waves 3:57–65
Hang GY, Wang JT, Wang T, Shen HM, Yu WL (2022) Theoretical investigations on stability, sensitivity, energetic performance, and mechanical properties of CL-20/TNAD cocrystal explosive by molecular dynamics method. J Mol Model 28:58
Hang GY, Yu WL, Wang T, Wang JT (2019) Theoretical investigations into effects of adulteration crystal defect on properties of HMX by molecular dynamics method. Theor Chem Acc 138:33
Miao S, Zhang L, Wang T, Wang YL, Hang GY, Mei ZS (2018) Molecular dynamics study on effects of RDX dopants on properties of HMX. Chin J Energ Mater 26:828–834
Miao S, Wang T, Wang YL, Hang GY, Qi CB, Lu CB (2019) Theoretical calculation of the effect of crystal defects on properties of HMX-based PBX. Chin J Energ Mater 27:636–643
Tao J, Wang XF, Zhao SX, Han ZX, Li WH, Wang CL, Huang YF, Fang W (2017) Theoretical calculation of the random interaction and co-crystal interaction of CL-20/HMX. Chin J Explos Propellants 40:50–55
Funding
This research was supported by Young Talent Fund of University Association for Science and Technology in Shaanxi, China (grant number 20200604).
Author information
Authors and Affiliations
Contributions
Gui-yun HANG: Investigation, methodology, and writing-original draft.
Tao WANG: Investigation, and software.
Jin-tao WANG: Conceptualization, and data curation.
Wen-li YU: Visualization, and validation.
Hui-ming SHEN: Modeling, and simulation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hang, Gy., Wang, T., Wang, Jt. et al. Theoretical research on performances of CL-20/HMX cocrystal explosive and its based polymer bonded explosives (PBXs) by molecular dynamics method. J Mol Model 28, 385 (2022). https://doi.org/10.1007/s00894-022-05380-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05380-9