Abstract
To research and estimate the effects of molar ratios on structures, stabilities, mechanical properties, and detonation properties of CL-20/HMX cocrystal explosive, the CL-20/HMX cocrystal explosive models with different molar ratios were established in Materials Studio (MS). The crystal parameters, structures, stabilities, mechanical properties, and some detonation parameters of different cocrystal explosives were obtained and compared. The molecular dynamics (MD) simulation results illustrate that the molar ratios of CL-20/HMX have a direct influence on the comprehensive performance of cocrystal explosive. The hardness and rigidity of the 1:1 cocrystal explosive was the poorest, while the plastic property and ductibility were the best, thus implying that the explosive has the best mechanical properties. Besides, it has the highest binding energy, so the stability and compatibility is the best. The cocrystal explosive has better detonation performance than HMX. In a word, the 1:1 cocrystal explosive is worth more attention and further research. This paper could offer some theoretical instructions and technological support, which could help in the design of the CL-20 cocrystal explosive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In modern wars, insensitive and high energy-density explosive was very promising, while there existed a contradiction between energy density and safety for traditional energetic materials, which limited its development and application [1, 2]. To improve the energy density and decrease the sensitivity of energetic materials, researchers have carried on many theoretical and experimental investigations. During the development process, cocrystallization [3, 4] aroused researchers’ attention, because cocrystallization had many advantages. For example, cocrystallization could be used as an effective method to decrease the sensitivity, improve the safety. At the same time, it could also enhance the stability, alter the mechanical properties.
The definition of cocrystallization could be that many different molecules could be put together in one supercell at a certain molar ratio and the intermolecular forces might be formed among these molecules. Many researchers had pointed out that cocrystallization might formed by the intermolecular forces and these forces were usually very weak and these intermolecular interactions could be classified into hydrogen bonds, electrostatic interactions, and van der Waals (vdW) forces [5, 6]. Cocrystallization could be used as an effective method to alter the oxygen balance (OB), molecular structure, density, mechanical properties, sensitivity, detonation performance, and thermal stability of explosives. Consequently, many investigations have been conducted on cocrystallization [7–26].
2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (HNIW) was the most promising high energy-density material and it had been investigated for a long time since its first synthesization. HNIW was also called CL-20 and it had a great deal of advantages and merits. Up to now, the investigations on CL-20 were still going on and we might predict that more and more achievements about CL-20 would be acquired in the near future. Compared to commonly used explosives, such as HMX, RDX, TATB and TNT, CL-20 had the most power [27, 28] and highest energy density, which made it outstanding. Besides, CL-20 also had excellent oxygen balance. The number of total polymorphs for CL-20 was four, namely, CL-20 could be classified into α-CL-20, β-CL-20, γ-CL-20, and ε-CL-20. Among them, more attention had been paid to ε-CL-20, because both of the density and detonation parameters of ε-CL-20 were the highest, therefore, it was the most promising and excellent polymorph. However, CL-20 was very sensitive to external stimulus, such as heat and impact. Influenced by this, it was very likely that CL-20 would explode or decompose when subjected to an external stimulus, which would have a negative effect on safety and limit further its wide application. Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) was a typical representation of the high-energy explosives and it had been used extensively in many areas for its great capabilities. Besides, HMX had favorable detonation performance and mechanical properties. According to Refs. [29, 30], we knew that the number of polymorph for HMX was also four, and β-HMX was more stable than the other three polymorphs (α-, γ-, δ-) at standard conditions. Both CL-20 and HMX had -NO2 bonds and hydrogen atoms, if CL-20 cocrystallized with HMX, the hydrogen bonds might be formed between -NO2 bonds and hydrogen atoms, namely, H · · · O bonds or H · · · N bonds might exist in cocrystal model. Influenced by this, the molecule structure in the cocrystal explosive might be altered, and the stability could be improved. What’s more, HMX was less sensitive than CL-20, and consequently, the sensitivity of CL-20 might be decreased and safety could be enhanced. While in the cocrystal explosive, the molecular ratios of CL-20 and HMX might have a direct influence on the comprehensive performances, such as mechanical properties, binding energy, stability, sensitivity, oxygen balance, molecular structure, crystal parameters, detonation property, etc. Therefore, it was very significant to investigate the stabilities, mechanical properties and detonation performance of cocrystal explosives with different molar ratios.
In this article, the crystal models of CL-20/HMX cocrystal explosives with different molar ratios were established in Materials Studio (MS) [31] and the mechanical properties, crystal parameters, stability, and detonation performance of cocrystal explosives were investigated by molecular dynamics (MD) simulation method. The main aim was to research the influence of molar ratios on properties of CL-20/HMX cocrystal explosives. The results could offer some theoretical instructions and technological support, which could help in the design of the CL-20 cocrystal explosive.
Methods
Single molecular model
The molecular formula of CL-20 was C6H6O12N12 and it was C4H8O8N8 of HMX. In this paper, we chose ε-CL-20 and β-HMX to establish the cocrystal model and investigate its properties, because both of these two polymorphs had typical characteristics, prominent advantages, and had been investigated widely.
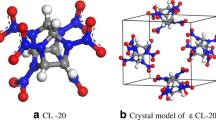
To build the crystal model, the space group and crystal parameters should be obtained. ε-CL-20 (as shown in Fig. 1) had a monoclinic P21/A structure and the crystal parameters was a = 1.3696 nm, b = 1.2554 nm, c = 0.8833 nm, α = γ = 90°, β = 111.18° and four molecules were included in a single crystal cell [32]. For β-HMX (as shown in Fig. 2), the crystal space group was P21/c and 2 HMX molecules were included in a single cell. The crystal parameters of β-HMX was a = 0.6540 nm, b = 1.1050 nm, c = 0.8700 nm, while the values of three angles, α, β, and γ were 90, 124.3, and 90°, respectively [29]. The molecule structure of CL-20 was shown in Fig. 1, and HMX structure was shown in Fig. 2.
Cocrystal explosive model
In this article, the molar ratios of CL-20 and HMX (CL-20:HMX) was 10:1, 9:1, 8:1, 7:1, 6:1, 5:1, 4:1, 3:1, 2:1, 1:1, respectively, so there were ten kinds of different cocrystal explosives in total. The crystal models of CL-20/HMX cocrystal explosives were established by random substituting method, namely, HMX molecules were used to replace the CL-20 molecules.
The correlated parameters of different cocrystal explosives (including molar ratio of CL-20/HMX, mass percent of CL-20, supercell models, number of CL-20 molecules, number of HMX molecules, total number of atoms) are illustrated in Table 1.
Take the cocrystal explosive with molar ratio of 9:1 as an example, primary simulation cells containing 80 molecules were established in MS for ε-CL-20, corresponding to 20 (5 × 2 × 2) unit cells. Then, 8 CL-20 molecules were substituted by HMX molecules randomly and the initial crystal model was obtained. A total of 2816 atoms were included in the crystal model.
After establishing the crystal model, MD simulations would be performed in Discover and Forcite modules to optimize the energy and structure of the primary model. Through optimization, the crystal parameters of CL-20/HMX explosive were a = 7.8666 nm, b = 2.4699 nm, c = 1.8732 nm, α = 92.06°, β = 115.12°, γ = 87.95°, and the density was 2.025 g/cm3. The crystal model of CL-20/HMX cocrystal explosive is shown in Fig. 3.
Calculation conditions
This paper mainly investigated the effects of molar ratios on comprehensive properties of CL-20/HMX cocrystal explosives. The comprehensive properties mainly concluded mechanical properties, structures, intermolecular interactions, stability, and detonation performance. MD simulations were conducted on in the NVT ensemble, namely, the total number of molecules, volume, and temperature were constant. COMPASS force field [33–35] was chosen to calculate the energy and other parameters. The temperature control method was chosen as Andersen, for electrostatic, Ewald was used as the summation methods and it was atom-based for vdW. The temperature was 295 K, the step size was 1 fs and the MD simulation process would last for 2 × 105 fs. In the first 1 × 105 fs simulation process, simulations would be conducted in the Discover modules and it was in this process that the temperature and energy of cocrystal model would be in equilibrium state. Next, another 1 × 105 fs MD simulations would be carried on in the Forcite modules to calculate and analyze the energies and static properties. One frame was saved per 1000 steps, and in total 100 frames were saved to make analysis of static properties, energies, and some other parameters of cocrystal explosives.
Results and discussion
Equilibrium of cocrystal model
Only when the cocrystal model had been in the equilibrium state could we analyze and calculate the static properties and energy of cocrystal explosive. While the equilibrium state of cocrystal system was required that both of the temperature and energy be in the equilibrium state. In other words, we could use the temperature and energy to estimate the state of cocrystal system and judge whether the cocrystal model had reached the equilibrium state. Generally speaking, when the temperature and energy fluctuated within 5 ∼ 10%, it could be concluded that the system had reached the equilibrium state.
For example, when the molar ratio was 3:1, during the MD simulation process, the temperature curve was shown in Fig. 4, while the energy curve was shown in Fig. 5.
In Fig. 4, the temperature curve illustrated that the temperature increased at the beginning and then fluctuated at a certain range and the system equilibrated in less than 5 × 104 fs. At last, the temperature fluctuated within ±15 K, while the energy curve (shown in Fig. 5) implied that both the potential energy and nonbond energy fluctuated within ±5 ∼ 10%. Based on these two curves, it could be concluded that the system had reached an equilibrium state. For other conditions, the equilibrium state of the system was based on these two principles.
Mechanical properties
For a material, the mechanical properties could be depicted and related by stress (σ) and strain (ε). To describe the relation between σ and ε, we could use Hooke’s law [36, 37] and the concrete form could be depicted as follows:
In Eq. (1), C ij (i, j = 1, 2, …, 6) was the elastic parameters and the total number of elastic parameters was 36. The elastic matrix was symmetric, namely, C ij = C ji, so there were 21 independent coefficients in total.
The mechanical properties for a material mainly concluded E, K, G, ν and C 12-C 44, where E represented the tensile modulus, K referred the bulk modulus, G was the shear modulus, ν represented the Poisson’s ratio, and C 12-C 44 was called Cauchy pressure. E, K, G could be used as a criterion to estimate the hardness, rigidity, stiffness, and plastic property of a material. Besides, the K value could be applied to assess the rupture strength. The value of C 12-C 44 could help us judge the ductibility or brittleness. If the value of Cauchy pressure was positive, it would mean that the material was ductile; otherwise, if it was negative, the material was brittle. All the mechanical properties had a correlation with two Lamé coefficients λ and μ.
The mechanical properties (E, K, G, ν) could be figured out according to the following formulas:
When the system reached the equilibrium state, calculations would be conducted under the Forcite modules in MS and we could get the mechanical properties, binding energy, and some other correlated parameters of cocrystal explosives. The elastic parameters and mechanical properties of the different cocrystal explosives are shown in Table 2 and Fig. 6.
What could be concluded from Table 2 and Fig. 6 was that the molar ratio of CL-20/HMX had a direct influence on elastic coefficients and mechanical properties (E, K, G, ν, C 12-C 44) of cocrystal explosive. The values of three engineering moduli (E, K, G) changed in the order of 1:0 > 10:1 > 9:1 > 8:1 > 7:1 > 6:1 > 5:1 > 4:1 > 3:1 > 2:1 > 1:1. For raw ε-CL-20, the value of E was 17.166 GPa, G was 6.955 GPa, and it was 10.758 GPa for K. The values of these three moduli were very high, thus implying that the stiffness or rigidity of CL-20 was good, while the value of Cauchy pressure (C 12-C 44) was negative, which indicated that the plastic property and ductibility was poor, and it had a negative effect on mechanical properties. Owing to the addition of HMX, the elastic coefficients and mechanical moduli declined gradually, namely, the rigidity of the explosive was weakened. The decline of K meant that the rupture strength became weak. In other words, when the cocrystal explosive was under the external loading, it would distort more easily. A decrease of engineering moduli also implied that the cocrystal explosive was more easily to be processed in practice when necessary. However, the values of Cauchy pressure (C 12-C 44) were in the order of 1:1 > 2:1 > 3:1 > 4:1 > 5:1 > 6:1 > 7:1 > 8:1 > 9:1 > 10:1 > 0:1 > 1:0. The changing tendency of Cauchy pressure indicated that the ductibility of cocrystal explosive improved. What’s more, the MD simulation results explained that in the cocrystal explosives, the mechanical properties could be improved efficiently. For the different CL-20/HMX cocrystal explosives, when the value of molar ratio (CL-20:HMX) was 1:1, the values of E, K, and G were the least, while the Cauchy pressure was the highest, so the ductibility was the best. Consequently, the 1:1 cocrystal model had the best mechanical properties.
Binding energy
Binding energy (E bind) was an important criterion for evaluating the miscibility and compatibility of the different components. Besides, it could also directly reflect the intermolecular interaction energy (E inter). Previous studies [38–40] have shown that binding energy can be used to assess the stability and compatibility of an explosive and the higher the value of binding energy, the more stable the explosive, and the better the compatibility.
For the CL-20/HMX cocrystal explosive, binding energy referred to the intermolecular interaction energy between CL-20 and HMX molecules. Binding energy could be determined as follows:
where E b is the binding energy, E total is the total energy of cocrystal explosive at equilibrium state, E inter is the intermolecular interaction energy, E CL-20 and E HMX are the total energies of isolated CL-20 and HMX, E b * is defined as the binding energy after correction, N i is the number of molecules in cocrystal explosive, and N 0 is the number of molecules for the 1:1 cocrystal model. In this article, the number of CL-20 and HMX molecules in 1:1 cocrystal model is 16, so 32 molecules were included in the cocrystal explosive, namely, N 0 = 32.
Based on the MD simulation results, we can determine the binding energy of CL-20/HMX cocrystal explosive with different molar ratios; the results are shown in Table 3 and Fig. 7.
From Table 3 and Fig. 7, we can clearly conclude that the molar ratios had a direct influence on the binding energy of cocrystal explosive. With the decrease of CL-20 mass percent in cocrystal explosive, the binding energy between CL-20 and HMX molecules exhibited an increasing changing tendency. In other words, the binding energy changed in the order of 1:1 > 2:1 > 3:1 > 4:1 > 5:1 > 6:1 > 7:1 > 8:1 > 9:1 > 10:1. For the explosive with the molar ratio of 10:1, the binding energy is 315.76 kJ/mol, while it is 402.88 kJ/mol when the molar ratio is 1:1. The increase of binding energy indicated that the compatibility and the stability of cocrystal model improved. Besides, the results of binding energy also implied that when the molar ratio was 1:1, the cocrystal explosive formed more easily, thus illustrating that CL-20 preferred cocrystallizing with HMX in this condition. What’s more, compared with other cocrystal models, the stability of the 1:1 cocrystal explosive was the best.
Considering the mechanical properties and binding energy of the different cocrystal explosives, it could be obviously seen that CL-20 preferred cocrystallizing with HMX at 1:1 molar ratio, and in this condition, the cocrystal model had the best mechanical properties, highest binding energy, and the cocrystal was more stable. Consequently, it was quite promising and deserved more attention and further investigation.
Detonation performance
The detonation parameters for an explosive mainly included detonation heat (Q), detonation pressure (P), detonation temperature (T B ), detonation volume (V 0) and detonation velocity (D). Among them, Q, P, and D were the three most important detonation parameters. These detonation parameters could be used to estimate the energy density and detonation performance of an explosive. Besides, detonation parameters had a direct influence on the property of the weapons and it could also be used to judge the power and damage efficiency of ammunitions. Detonation parameters had a certain correlation with many factors, such as density (ρ), oxygen balance (OB), molecule structures, external conditions, and so on. The detonation parameters could be acquired by experimental tests, theoretical analysis, and empirical equations.
For a certain explosive composed only of C-H-O-N elements with the molecular formula of C a H b O c N d , the oxygen balance (OB) can be determined as follows:
where, M r is the molecular mass of the explosive.
Detonation velocity (D) can be obtained as follows [41]:
In Eq. (10), we can see that the detonation velocity (D) has a direct relationship to the coefficient F, where F is defined as the detonation factor in Eq. (9). F is determined by the molecule structure, explosive category, explosive state, and other factors. The unit of detonation velocity is km/s. In Eq. (9), the correlated parameters, including A, nB, nC, nD, nE, and G are concretely explained in Ref. [7]. To better understand the definitions, we can consult Ref. [7] to get the useful information.
Detonation pressure (P) is illustrated as follows [42]:
In Eq. (11), P, ρ, D refers to the detonation pressure, density, and detonation velocity of the explosive, respectively. The unit for P, ρ, D is GPa, g/cm3, and km/s, individually.
Detonation heat (Q) can be determined according to the following equation [43, 44]:
where Q is the detonation heat of explosive and the unit is kJ/kg, ω i is the mass percent of ith component in explosive, and Q i is the detonation heat of ith component with the unit of kJ/kg.
Based upon the empirical equations, the detonation parameters of cocrystal explosive were obtained, the density of explosive was directly derived from the MD simulation results. Table 4 gives the detonation parameters (ρ, OB, D, P, Q) of the different cocrystal explosives.
What can be concluded from Table 4 is that the molar ratios of CL-20/HMX had a direct influence on density, oxygen balance, and detonation parameters of cocrystal explosives. With a decrease of CL-20 in the cocrystal explosive, the density and oxygen balance decreased gradually, and the detonation parameters also exhibited a declined variation tendency. For raw ε-CL-20 (molar ratio of 1:0), the density was 2.035 g/cm3, oxygen balance was −10.96%, and detonation velocity, detonation pressure, and detonation heat was 9.50 km/s, 46.60 GPa, 6230 kJ/kg, respectively. For β-HMX (molar ratio of 0:1), the density was 1.894 g/cm3, OB was −21.62%, detonation parameters (D, P, Q) were 9.05 km/s, 39.45 GPa, and 6190 kJ/kg, respectively, while for the cocrystal explosive with the molar ratio of 1:1, the density was 1.978 g/cm3, oxygen balance was −15.26%, the values of detonation parameters (D, P, and Q) were 9.35 km/s, 43.60 GPa, and 6214 kJ/kg, individually. Based on these results, we could clearly see that compared with ε-CL-20, the decreasing extent of detonation parameters (D, P, Q) for the cocrystal explosive with molar ratio of 1:1 was 1.58, 6.44, and 0.26%, respectively. However, the values of these detonation parameters were still higher than that of HMX, thus illustrating that cocrystal explosive had better detonation performance than HMX. Therefore, the CL-20/HMX cocrystal explosive was very promising and it was very likely that the cocrystal explosive might become a new candidate for high energy-density compounds in the future.
Conclusions
In this paper, the cocrystal models of CL-20/HMX explosives with different molar ratios were established in MS and the comprehensive properties (structures, mechanical properties, binding energy, and detonation property) for the cocrystal explosives were derived and compared. Besides, the effects of molar ratios on the comprehensive properties were investigated and estimated. The major findings and results were generalized as follows:
-
(1)
In cocrystal explosive, the values of E, K, and G declined, while the C 12-C 44 increased, namely, the mechanical properties could be improved owing to the adding of HMX. For the cocrystal explosive with molar ratio of 1:1, the values of E, K, and G were the least, while the Cauchy pressure was the highest, thus implying that the cocrystal explosive with the molar ratio of 1:1 had the best mechanical properties.
-
(2)
For the different cocrystal explosives, the sequence of binding energy was 1:1 > 2:1 > 3:1 > 4:1 > 5:1 > 6:1 > 7:1 > 8:1 > 9:1 > 10:1, which indicated that the intermolecular interaction energy for the cocrystal explosive with the molar ratio of 1:1 was the strongest and it was more stable, and the compatibility between CL-20 and HMX molecules was better. Besides, it also meant that CL-20 preferred cocrystallizing with HMX in this condition.
-
(3)
Compared with raw ε-CL-20, the density, oxygen balance, and detonation parameters (D, P, and Q) of cocrystal explosive decreased gradually, while the values of detonation parameters were still higher than that of HMX, so the cocrystal explosive had an excellent detonation performance.
In a word, the 1:1 cocrystal explosive had the best mechanical properties, highest binding energy, and excellent detonation performance, therefore, it was worth experimental tests. The CL-20/HMX cocrystal explosive might become a new candidate for high energy-density compounds in the future. This paper could offer some theoretical instructions and technological support, which could help in the design of CL-20 cocrystal explosives.
References
Agrawal JP (1998) Recent trends in high-energy materials. Prog Energy Combust Sci 24:1–30
Sikder AK, Sikder N (2004) A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J Hazard Mater 112:1–15
Lara OF, Espinosa PG (2007) Cocrystals definitions. Supramol Chem 19:553–557
Shan N, Zaworotko MJ (2008) The role of cocrystals in pharmaceutical science. Drug Discov Today 13:440–446
Clarke SM, Friscic T, Jones W (2011) Observation of a two-dimensional halogen-bonded cocrystal at sub-monolayer coverage using synchrotron X-ray diffraction. Chem Commun 47:2526–2528
Alhalaweh A, George S, Bostrom D (2010) 1:1 and 2:1 urea-succinic acid cocrystals: structural diversity, solution chemistry, and thermodynamic stability. Cryst Growth Des 11:4847–4855
Xu HF, Duan XH, Li HZ, Pei CH (2015) A novel high-energetic and good-sensitive cocrystal composed of CL-20 and TATB by a rapid solvent/non-solvent method. RSC Adv 5:95764–95770
Guo DZ, An Q, Goddard WA, Zybin SV, Huang FL (2014) Compressive shear reactive molecular dynamics studies indicating that cocrystals of TNT/CL-20 decrease sensitivity. J Phys Chem C 118:30202–30208
Guo CY, Zhang HB, Wang XC, Xu JJ, Liu Y, Liu XF, Huang H, Sun J (2013) Crystal structure and explosive performance of a new CL-20/caprolactam cocrystal. J Mol Struct 1048:267–273
Liu K, Zhang G, Luan JY, Chen ZQ, Su PF, Shu YJ (2016) Crystal structure, spectrum character and explosive property of a new cocrystal CL-20/DNT. J Mol Struct 110:91–96
Wu JT, Zhang JG, Li T, Li ZM, Zhang TL (2015) A novel cocrystal explosive NTO/TZTN with good comprehensive properties. RSC Adv 5:28354–28359
Bolton O, Simke LR, Pagoria PF, Matzger AJ (2012) High power explosive with good sensitivity: a 2:1 cocrystal of CL-20:HMX. Cryst Growth Des 12:4311–4314
Sun T, Liu Q, Xiao JJ, Zhao F, Xiao HM (2014) Molecular dynamics simulation of interface interactions and mechanical properties of CL-20/HMX cocrystal and its based PBXs. Acta Chim Sin 72:1036–1042
Ding X, Gou RJ, Ren FD, Liu F, Zhang SH, Gao HF (2016) Molecular dynamics simulation and density functional theory insight into the cocrystal explosive of hexaazaisowurtzitane/nitroguanidine. Int J Quantum Chem 116:88–96
Xiong SL, Chen SS, Jin SH, Zhang CY (2016) Molecular dynamics simulations on dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate/hexanitrohexaazaisowurtzitane cocrystal. RSC Adv 6:4221–4226
Li HQ, An CW, Guo WJ, Geng XH, Wang JY, Xu WZ (2015) Preparation and performance of nano HMX/TNT cocrystals. Propellants Explos Pyrotech 40:652–658
Lin H, Zhu SG, Li HZ, Peng XH (2013) Structure and detonation performance of a novel HMX/LLM-105 cocrystal explosive. J Phys Org Chem 26:898–907
Yang ZW, Zhang YL, Li HZ, Zhou XQ, Nie FD, Li JS, Huang H (2012) Preparation, structure and properties of CL-20/TNT cocrystal. Chin J Energ Mater 20:674–679
Guo CY, Zhang HB, Wang XC, Liu XF, Sun J (2013) Study on a novel energetic cocrystal of TNT/TNB. J Mater Sci 48:1351–1357
Lin H, Zhu SG, Zhang L, Peng XH, Li HZ (2013) Synthesis and first principles investigation of HMX/NMP cocrystal explosive. J Energ Mater 31:261–272
Zhou JH, Shi LW, Zhang CY, Li HZ, Chen MB, Chen WM (2016) Theoretical analysis of the formation driving force and decreased sensitivity for CL-20 cocrystals. J Mol Struct 1116:93–101
Wei YJ, Ren FD, Shi WJ, Zhao Q (2016) Theoretical insight into the influences of molecular ratios on stabilities and mechanical properties, solvent effect of HMX/FOX-7 cocrystal explosive. J Energ Mater 34:426–439
Urbelis JH, Young VG, Swift JA (2015) Using solvent effects to guide the design of a CL-20 cocrystal. Cryst Eng Comm 17:1564–1568
Chen J, Duan XH, Pei CH (2013) Preparation and characterization of HMX/AP co-crystal. Chin J Energ Mater 21:409–413
Feng RZ, Zhang SH, Ren FD, Gou RJ, Gao L (2016) Theoretical insight into the binding energy and detonation performance of ε-, γ-, β-CL-20 cocrystals with β-HMX, FOX-7, and DMF in different molar ratios, as well as electrostatic potential. J Mol Model 22:123
Li YX, Chen SS, Ren FD (2015) Theoretical insights into the structures and mechanical properties of HMX/NQ cocrystal explosives and their complexes, and the influence of molecular ratios on their bonding energies. J Mol Model 21:245
Agrawal JP (2005) Some new high energy materials and their formulations for specialized applications. Propellants Explos Pyrotech 30:316–328
Foltz MF, Coon CL, Garcia F (1994) The thermal stability of the polymorphs of hexanitrohexaazaisowurtzitane, Part I. Propellants Explos Pyrotech 19:19–25
Chang SC, Henry PB (1970) A study of the crystal structure of β-cyclotetramethylene tetranitramine by neutron diffraction. Acta Crystallogr B 26:1235–1240
Cady HH, Larson AC, Cromer DT (1963) The crystal of α-HMX and a refinement of the structure of β-HMX. Acta Crystallogr 16:617–623
Materials Studio 7.0 (2013) Accelrys Software Inc, San Diego, CA
Zhao XQ, Shi NC (1995) Crystal structure of ε-hexanitrohexaazaisowurtzitane. Chin Sci Bull 40:2158–2160
Sun H, Ren PJ, Fried R (1998) The COMPASS force field: parameterization and validation for phosphazenes. Comput Theor Polym Sci 8:229–246
Bunte SW, Sun H (2000) Molecular modeling of energetic materials: the parameterization and validation of nitrate esters in the COMPASS Forcefield. J Chem Chem B 104:2477–2489
Michael JM, Sun H, Rigby D (2004) Development and validation of COMPASS force field parameters for molecules with aliphatic azide chains. J Comput Chem 25:61–71
Wu JL (1993) Mechanics of Elasticity. Tongji University Press, Shanghai
Weiner JH (1983) Statistical Mechanics of Elasticity. John Wiley, New York
Zhu W, Xiao JJ, Zhu WH, Xiao HM (2009) Molecular dynamics simulations of RDX and RDX-based plastic-bonded explosives. J Hazard Mater 164:1082–1088
Xu XJ, Xiao HM, Xiao JJ, Zhu W, Huang H, Li JS (2006) Molecular dynamics simulations for pure ε-CL-20 and ε-CL-20-based PBXs. J Phys Chem B 110:7203–7207
Qiu L, Xiao HM (2009) Molecular dynamics study of binding energies, mechanical properties, and detonation performances of bicyclo-HMX-based PBXs. J Hazard Mater 164:329–336
Muthurajan H, Sivabalan R, Talawar MB, Asthana SN (2004) Computer simulation for prediction of performance and thermodynamic parameters of high energy materials. J Hazard Mater 112:17–33
Cooper PW (1992) Extending estimation of C-J pressure of explosives to the very low-density region. Proceed 18th Int Pyrotech Symp
Ou YX (2006) Explosives. Beijing Institute of Technology Press, Beijing
Jin SH, Song QC (2010) Explosive Theory. Northwestern Polytechnical University Press, Xi’an
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hang, Gy., Yu, Wl., Wang, T. et al. Theoretical insights into the effects of molar ratios on stabilities, mechanical properties, and detonation performance of CL-20/HMX cocrystal explosives by molecular dynamics simulation. J Mol Model 23, 30 (2017). https://doi.org/10.1007/s00894-016-3193-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3193-8