Abstract
The structural and antioxidant activity of two flavonols, namely, Fisetin and Robinetin, have been investigated employing the density functional theory (DFT) using B3LYP functional and 6–311++G (d, p) basis set. The calculations were performed in the gas phase and under the solvent effect of water, dimethylsulfoxide (DMSO), methanol, and benzene. The hydrogen-atom transfer (HAT), single electron transfer followed by proton transfer (SET-PT), and sequential proton loss electron transfer (SPLET) mechanisms were investigated to rationalize the radical scavenging capacities and to identify the favored antioxidant mechanism. Hence, the bond dissociation enthalpies (BDE) ionization potential (IP), IE, proton dissociation enthalpy (PDE), proton affinity (PA), and electron transfer enthalpy (ETE) related to each mechanism were reported and discussed in function of the solvent effect. For both flavonols, the results showed that 4′-OH hydroxyl is the preferred active site following the trend 4′-OH > 3′-OH > 3-OH > (5′-OH) > 7-OH. Besides, the HAT mechanism is energetically the most favored pathway. The energetically favored solvents follow the trends water > DMSO > benzene > methanol and benzene > DMSO > methanol > water, for Fisetin and Robinetin, respectively.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flavonoid compounds were discovered in 1936 by the Hungarian Nobel laureate Albert Szent-Györgyi [1]. They are omnipresent in green plant cells and, therefore, expected to take part in the photosynthetic process [2]. Since their discovery, the study of these compounds has drastically increased, mainly because of their health benefits [3,4,5]. Flavonoids are mainly found in fruits, vegetables, propolis, and honey; they represent a common constituent of the human diet [2, 6,7,8,9,10]. Nutritionists estimate the average human intake of flavonoids on a normal diet by 203.0 ± 243.2 mg/day, with mostly quercetin and kaempferol [11,12,13]. The flavonoids’ structure is composed of two aromatic rings denoted A, B connected through three carbons in an oxygenated heterocycle supplement. Multiple groups could be attached to the core structure, commonly hydroxyl, methoxy groups, and sugar. Differences in the structure of the heterocycle (C ring) classify them as flavonols, flavones, flavanols, flavanones, and isoflavones (Fig. 1). Flavonols are characterized by a 2, 3-double bond, a 4-keto group, and a 3-hydroxyl group in the C-ring. The flavonol structures of Fisetin and Robinetin are depicted in Fig. 2.
Flavonoid compounds have various properties including the antiradical activity or free radical scavenging property [14, 15], anti-cancer [16,17,18], the control of cellular growth [19,20,21], the destruction of pathogen organisms [22, 23], and the inhibition of human immunodeficiency viruses [24, 25]. Fisetin has been found in plants like Arbutus unedo L. [26, 27]; it is also abundant in strawberries and in some other fruits and vegetables [28]; and it has several biological activities such anticancer activity [29] anti-inflammatory [30], anti-HIV [31], and enhances memory [32]. Robinetin which has been isolated from Intsia bijuga plant [33] showed a powerful inhibitory action on lipid peroxidation [28].
Flavonoid compounds including Fisetin and Robinetin have various biological activities but their ability to scavenge free radicals is still the most interesting [28, 34, 35]. These free radicals are dangerous and can damage biomolecules such as proteins, membrane lipids, and nucleic acids [36, 37]; thus, they are involved in several diseases [38]. Fisetin [39,40,41,42,43,44,45,46,47] and Robinetin [47,48,49] have been reported for their antiradical activity but the solvent effect on this activity is investigated in details here for the first time.
Therefore, in this study, we report the antiradical activity of Fisetin and Robinetin by the use of density functional theory (DFT) applying the most known mechanisms: hydrogen-atom transfer (HAT), single electron transfer followed by proton transfer (SET-PT), and sequential proton loss electron transfer (SPLET) [50,51,52], together with the solvent effect of polar, non-polar, protic, and aprotic solvents: benzene, DMSO, methanol, and water.
Theoretical and computational methods
The geometries of neutral molecules and the related species were firstly preoptimized by the PM7 semiempirical method as implemented in MOPAC2009 [53]. DFT calculations of optimization and vibrational frequencies were performed using B3LYP functional [54,55,56]; the exchange–correlation functional level was used without constraints, employing the 6–311 + + G(d,p) basis set [57,58,59] using Gaussian09 software [60]. The B3LYP level of theory is commonly used for organic molecule property calculations, especially the antiradical properties of flavonoids and nanostructures derived from them [61,62,63,64,65]. The solvents’ effect was computed by DFT in the framework of the self-consistent reaction field polarizable continuum model (SCRF-PCM) [66,67,68], using the UAHF [69] set of solvation radii to build the cavity for the solute in its gas-phase equilibrium geometry. As implemented in Gaussian09 software to mimic experimental conditions, the following dielectric constants were used; ε = 78.3553 was chosen to perform calculations in water solution, ε = 2.2706 for benzene, ε = 46.826 for dimethylsulfoxide (DMSO), and ε = 32.613 for methanol.
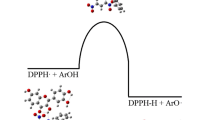
The harmful action of free radicals \(\left({\mathrm{R}}^{\cdot }\right)\) can be avoided by scavenging them with flavonoids (FlOH) as shown in reaction (1):
The product of this reaction is flavonoid phenoxyl radical (Fl–O•). A higher stability of the radical (Fl–O•) corresponds to a better efficiency of the antioxidant (Fl–OH). It is assumed that the resonance makes (Fl–O•) non-reactive (or less harmful) [70].
This reaction could happen through at least three mechanisms [71, 72]:
-
a-
The hydrogen-atom transfer (HAT) as shown in reaction 2: this mechanism is characterized by the homolytic bond dissociation enthalpy (BDE) of OH group calculated as shown in Eq. 3
$$FlOH\rightarrow{FlO}^\bullet+H^\bullet$$(2)$$\mathrm{BDE}=H\left(\mathrm{FlO}^\bullet\right)+H\left(\mathrm H^\bullet\right)-H\left(\mathrm{FlOH}\right)$$(3)
H(Fl-O•) is the enthalpy of the flavonoid phenoxyl radical generated after H abstraction, H(H) is the enthalpy of the hydrogen atom, and H(Fl–OH) is the enthalpy of the parent flavonoid molecule. A lower BDE value, usually related to a greater ability to donate a hydrogen atom from the hydroxyl group, results in an easier free radical scavenging reaction [40].
-
b-
The single electron transfer followed by proton transfer (SET-PT): this mechanism occurs via two steps, starting from a single electron transfer characterized by the ionization potential (IP) as shown in reaction 4 and Eq. 5, respectively.
$$FlOH\rightarrow{FlOH}^{\bullet+}+e^-$$(4)$$\mathrm{IP}=H\left(\mathrm{FlOH}^{+\bullet}\right)+H\left(\mathrm e^-\right)-H\left(\mathrm{FlOH}\right)$$(5)
The second step is characterized by a proton transfer as a proton dissociation enthalpy (PDE) as shown in reaction 6 and equation 7, respectively.
-
c-
The sequential proton loss electron transfer (SPLET): this mechanism has two steps, starting from a deprotonation characterized by the proton affinity (PA) as shown in reaction 8 and Eq. 9, respectively.
$$FlOH\to {FlO}^{-}+{H}^{+}$$(8)$$\mathrm{PA}=H\left({\mathrm{FlO}}^{-}\right)+H\left({\mathrm{H}}^{+}\right)-H\left(\mathrm{FlOH}\right)$$(9)
The second step consists of an electron transfer characterized by the electron transfer enthalpy (ETE) as shown in reaction 10 and equation 10, respectively.
Starting from neutral Fisetin in the gas phase, an H-atom is removed from the 7-OH position, giving rise to the 7-O• radical. Likewise, 3-O•, 4′-O•, and 3′-O• radical species (four radicals) are generated (Fig. 2). Then, each neutral structure and radical species was optimized under B3LYP/6–311++G (d, p) theory level in gas phase, water, benzene, and DMSO. Thus, the generated radical species generated from Robinetin were assigned as 7-O•, 3-O•, 3′-O•, 4′-O•, and 5′-O•. All the calculations were performed at 298.15 K.
DFT-based reactivity descriptors
According to Koopman’s theorem [73], the following DFT-based descriptors are defined as follows:
The first ionization energy (I) and the highest occupied molecular orbital energy (EHOMO)
The electron affinity (A) and the lowest unoccupied molecular orbital energy (ELUMO)
where μ is the chemical potential, and χ is the electro-negativity.
Using a finite difference approximation and Koopmans’s theorem, the above expressions can be written as follows:
Results and discussion
HOMO–LUMO energy gaps
Figure 3 depicts the optimized geometries and the frontier orbitals HOMO and LUMO of Fisetin and Robinetin at B3LYP/6–311++G(d,p) theory level in gas phase.
HOMO and LUMO magnitudes are directly associated with the ability of the molecule to donate electrons and to accept electrons, respectively. Moreover, large HOMO–LUMO gap energy (Egap) indicates a high molecular stability; in contrast, a small gap energy indicates a high chemical reactivity. The values of HOMO, LUMO, and their related energy gaps are shown in Table 1. The Egap changes slightly in the gas phase, Egap of Fisetin is calculated as 3.931 eV, whereas the Egap for Robinetin is 3.932 eV. Robinetin has the lowest Egap values 3.676, 3.701, and 3.648 eV; in contrast, Fisetin has the following Egap values 3.938, 3.950, and 3.912 eV in benzene, DMSO, and water, respectively. In contrast, Fisetin has shown (Fig. 4) in methanol lower Egap value (3.753 eV) compared to Robinetin Egap (3.843 eV). These Egap values are in the same range as previous works which adopted the same level of theory and different basis sets [49, 74,75,76].
To evaluate the antioxidant properties of Fisetin and Robinetin, it is important to analyze the DFT-based reactivity descriptors, especially the chemical hardness, chemical softness, and the ionization potential (Table 2). The chemical hardness is a measure of resistance to charge transfer; it is remarkable that there is a slight reduction in the magnitude of hardness of Robinetin (1.8470 eV) compared to Fisetin (1.8815 eV). Consequently, the magnitude of softness of Robinetin (0.5414 eV) is greater than that of Fisetin (0.5315 eV). Electronegativity is a measure of the tendency to attract electrons. It is observed that the IP value of Robinetin (5.8534 eV) is lesser than Fisetin (6.1805 eV), which indicates that electron donating capability of Robinetin is higher than that of Fisetin. The calculated molecular properties clearly confirm that Robinetin acts as an electron donor rather than electron acceptor better than Fisetin.
BDE, IP, PDE, PA, and ETE energies
Flavonoid compounds have the capability to protect cells from oxidative stress by scavenging the free radicals either by donating an H-atom. It is well known that the easiest homolytic cleavage of an O–H bond (BDEmin, (IP + PDE)min, and (PA + ETE)min) indicates specifically the most favorable radical for the antioxidant activity.
Tables 3 and 4 show the computed thermodynamic parameters BDE, IE, PDE, PA, and ETE associated with the major three radical-scavenging mechanisms: HAT, SET-PT, and SPLET. Therefore, the most active site for the radical-scavenging reaction and the thermodynamically preferable reaction pathway could be predicted. In addition to the gas phase, these parameters were computed in the presence of polar, non-polar, protic, and aprotic solvents. The solvents are organized from non-polar and aprotic to polar and protic starting from benzene, DMSO, methanol, then water.
The different positions of hydroxyls on A, B, and C rings of the flavonoid main structure directly impact on its radical scavenging potency. The most-active OH group of each studied flavonol was determined by the minimal sum of the enthalpies of the specific reaction pathways including BDEmin, (IP + PDE)min, and (PA + ETE)min related to HAT, SET-PT, and SPLET mechanisms, respectively (based on the histograms in Fig. 5b and c and Fig. 6b and c). Robinetin exhibits a higher antioxidant activity than Fisetin and the 4′-OH hydroxyl was found as the preferred active site for both flavonols, independently of the solvent nature and the adopted mechanism.
Robinetin is more active than Fisetin as revealed by BDE (Tables 3 and 4); this is in agreement with the experimental published results for the reduction of DPPH radical (IC50: 11.02 ± 0.56 µM and 14.06 ± 0.21 µM) by Robinetin and Fisetin, respectively [77]. The most stable radical is 4′-O•, which results from a homolytic removal of the hydrogen atom from the OH group attached to the C4’ position; it is mainly due to the hydrogen bond between 3-OH and the 4-oxo group and the keto-enol tautomerism via the 2,3-double bond and the benzyl cycle. For Fisetin, B-ring has the most active antioxidant hydroxyls then A-ring followed by C-ring: B-ring > C-ring > A-ring. It exhibits the following BDE, IP + PDE, and PA + ETE favorable hydroxyls 4′-OH > 3′-OH > 3-OH > 7-OH (Fig. 5a-c). For Robinetin, the most stable radical is also 4′-O• and exhibits the following BDE, IP + PDE, and PA + ETE order for the hydroxyl groups: 4′-OH > 3′-OH > 3-OH > 5′-OH > 7-OH (exceptionally 5′-ArO• in the gas phase) as depicted in Fig. 6a, b and c. Several studies of solvent effects on flavonoids showed that the most favorable hydroxyl associated with the antioxidant activity is 4′-OH as found in quercetin [78], myricetin [79], kaempferol [70], apigenin [80], and in diglycosylated flavonoids such isorhamnetin-3,5′-O-β-D-diglucoside and isorhamnetin-3,7-O-β-D-diglucoside [81].
Compared to SET-PT and SPLET mechanisms, HAT is thermodynamically the most favored pathway [81] for the antioxidant activity of Fisetin and Robinetin (Tables 3 and 4). Fisetin 4′-O• radical has a BDE energy need of 296.46, 302.53, 300.73, 300.72, and 299.13 kJ/mol in gas phase, benzene, DMSO, methanol, and water, respectively. However, IP + PDE and PA + ETE values are higher than 400 kJ/mol as shown in Fig. 5b and c . Robinetin has BDE energy need of 347.21, 282.31, 282.69, 291.06, and 294.25 kJ/mol in gas phase, benzene, DMSO, methanol, and water respectively whereas IP + PDE and PA + ETE values are higher than 400 kJ/mol as shown in Fig. 6b and c.
Water is the most favored solvent (Fig. 5a); the BDE for 4′-O• Fisetin radical species pursues the following trend in solvents: water > DMSO > benzene > methanol; in contrast as shown in Fig. 6a, the favored solvent for 4′-O• Robinetin radical species is benzene: benzene > DMSO > methanol > water.
On the basis of the computed BDE, it has been demonstrated that the 4′-O• radical species is more stable than any other radical species. These results are in agreement with many previous BDE determined by various scientific reports [39, 47, 74, 82,83,84]. Through the analysis of the values shown in Table 5, it is possible to notice that the most BDE values were reported in gas phase; they vary in function of the level of theory: BDE values for Fisetin range from 289.67 [74] to 355.72 kJ/mol [43] calculated at B3LYP/6–31G(d,p) and B3LYP/6–31+G(d,p) levels of theory, respectively. Robinetin BDE values range from 298.41 [47] to 347.21 kJ/mol (this work) computed at PM7 and B3LYP/6–311+ +G(d,p), respectively.
HOMO, LUMO frontiers, spin densities, and the stability of the radical species
As shown in Fig. 7 and Fig. 8, the HOMO and LUMO frontier compositions of all the radical species related to both flavonols reveal clearly the presence of elevated charge density delocalization on the cinnamoyl part (C and B rings). Exceptionally, the 7-O• LUMO shows a charge density delocalization on the A-ring and slightly in C-ring which is probably due to the resonance in just one cycle A-cycle in contrary for the other radicals: 4′-O•, 3′-O•, 3-O•, 5′-O• where the resonance exists over the cinnamoyl part.
The spin densities are an important parameter which provides information about the stability of radicals. According to Parkinson [86], the more delocalized the spin density in the radical, the easier the radical formed, and thus the lower is the BDE. Spin densities for Fisetin and Robinetin species were calculated (Fig. 7 and 8).
On one hand, Fig. 7 shows the spin densities of the Fisetin radical species 3-O•, 3′-O• and 7-O• corresponding to 0.306, 0.306, and 0.298; these values are higher than the spin density of 4′-O• (0.254). On the other hand, Fig. 8 indicates that the spin densities of the Robinetin species 3-O•, 3′-O•, 5-O•, and 7-O• are 0.304, 0.309, 0.324, and 0.301 which are also higher than the spin density of 4′-O• (0.254). This means that the formation of 4′-O• is favorable, taking account that in this species, the spin densities are delocalized over the cinnamoyl part of the molecule, which contributes to the stability of the radical. Furthermore 4′-O• radical corresponds to the lowest BDE in all the studied solvents (Tables 3 and 4).
Conclusions
Quantum-chemical methods are still the reliable theoretical approaches to study flavonoids and their electronic properties, especially the antioxidant activity. In this work, density functional theory (DFT) method B3LYP/6–311++G(d,p) level of theory was utilized to investigate the antioxidant activity of two flavonols, namely, Fisetin and Robinetin, by comparing the following thermodynamic parameters: BDE, (IP + PDE), and (PA + ETE) related to the three fundamental mechanisms: hydrogen-atom transfer (HAT), single electron transfer followed by proton transfer (SET-PT), and sequential proton loss electron transfer (SPLET) mechanisms.
The results showed that 4′-OH hydroxyl is the preferred antioxidant active site for both Fisetin and Robinetin: 4′-OH > 3′-OH > 3-OH > (5′-OH) > 7-OH. Spin densities and orbital frontier analysis are in agreement with the stability of the 4′-O• radical species. HAT mechanism was found to be the most favored pathway besides the energetically preferred solvents, following the trends water > DMSO > benzene > methanol and benzene > DMSO > methanol > water for Fisetin and Robinetin, respectively.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Rusznyák ST, Szent-Györgyi A (1936) Vitamin P: Flavonols as vitamins. Nature 138:27–27. https://doi.org/10.1038/138027a0
Mukohata Y, Nakabayashi S, Higashida M (1978) Quercetin, an energy transfer inhibitor in photophosphorylation. FEBS Lett 85:215–218
Lee E-R, Kang G-H, Cho S-G (2007) Effect of flavonoids on human health: old subjects but new challenges. Recent Pat Biotechnol 1:139–150
Hollman PCH, Katan MB (1999) Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol 37:937–942
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2:270–278. https://doi.org/10.4161/oxim.2.5.9498
Wolfe KL, Liu RH (2008) Structure−activity relationships of flavonoids in the cellular antioxidant activity assay. J Agric Food Chem 56:8404–8411
Wolfe KL, Liu RH (2007) Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 55:8896–8907
Wolfe KL, Kang X, He X et al (2008) Cellular antioxidant activity of common fruits. J Agric Food Chem 56:8418–8426. https://doi.org/10.1021/jf801381y
Liu RH (2004) Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 134:3479S-3485S. https://doi.org/10.1093/jn/134.12.3479S
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504. https://doi.org/10.1016/s0031-9422(00)00235-1
de Vrie JHM, de Vrie JHM, Karin Janssen PLT et al (1997) Consumption of quercetin and kaempferol in free-living subjects eating a variety of diets. Cancer Lett 114:141–144
Mullie P, Clarys P, Deriemaeker P, Hebbelinck M (2007) Estimation of daily human intake of food flavonoids. Plant Foods Hum Nutr 62:93–98
Jun S, Shin S, Joung H (2016) Estimation of dietary flavonoid intake and major food sources of Korean adults. Br J Nutr 115:480–489
Teixeira S, Siquet C, Alves C et al (2005) Structure-property studies on the antioxidant activity of flavonoids present in diet. Free Radic Biol Med 39:1099–1108. https://doi.org/10.1016/j.freeradbiomed.2005.05.028
Bubols GB, da Vianna DR, Medina-Remon A et al (2013) The antioxidant activity of coumarins and flavonoids. Mini Rev Med Chem 13:318–334. https://doi.org/10.2174/138955713804999775
Chahar MK, Sharma N, Dobhal MP, Joshi YC (2011) Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev 5:1–12. https://doi.org/10.4103/0973-7847.79093
Galati G, O’Brien PJ (2004) Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radical Biol Med 37:287–303
Plate AYA (2006) Cruciferous vegetables, secondary metabolites of glucosinolates, and colon cancer risk in rats. University of Minnesota
Mekhancha-Dahel CC (2008) Anthropométrie nutritionnelle et santé des sujets jeunes: données actuelles dans le monde et en Algérie. Editions Dar El Gharb
Kuntz S, Wenzel U, Daniel H (1999) Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr 38:133–142. https://doi.org/10.1007/s003940050054
Huang YT, Hwang JJ, Lee PP et al (1999) Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol 128:999–1010. https://doi.org/10.1038/sj.bjp.0702879
Bais HP, Weir TL, Perry LG et al (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Mierziak J, Kostyn K, Kulma A (2014) Flavonoids as important molecules of plant interactions with the environment. Molecules 19:16240–16265. https://doi.org/10.3390/molecules191016240
Mantas A, Deretey E, Ferretti FH et al (2000) Structural analysis of flavonoids with anti-HIV activity. Theochem 504:171–179. https://doi.org/10.1016/s0166-1280(00)00362-6
Wu J-H, Wang X-H, Yi Y-H, Lee K-H (2003) Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg Med Chem Lett 13:1813–1815. https://doi.org/10.1016/s0960-894x(03)00197-5
Khan N, Syed DN, Ahmad N, Mukhtar H (2013) Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 19:151–162
Bouzid K, Toumi Benali F, Chadli R, Bouzouina M, Bouzid A, Benchohra A, Dif MM (2014) Extraction, identification and quantitative HPLC analysis of flavonoids from fruit extracts of Arbutus unedo L. from Tiaret area (Western Algeria). Eur J Mol Biotechnol 6:160–169
Pahari B, Chakraborty S, Chaudhuri S et al (2012) Binding and antioxidant properties of therapeutically important plant flavonoids in biomembranes: insights from spectroscopic and quantum chemical studies. Chem Phys Lipids 165:488–496. https://doi.org/10.1016/j.chemphyslip.2011.10.006
Kim JY, Jeon YK, Jeon W, Nam MJ (2010) Fisetin induces apoptosis in Huh-7 cells via downregulation of BIRC8 and Bcl2L2. Food Chem Toxicol 48:2259–2264. https://doi.org/10.1016/j.fct.2010.05.058
Park H-H, Lee S, Oh J-M et al (2007) Anti-inflammatory activity of Fisetin in human mast cells (HMC-1). Pharmacol Res 55:31–37. https://doi.org/10.1016/j.phrs.2006.10.002
Brinkworth RI, Stoermer MJ, Fairlie DP (1992) Flavones are inhibitors of HIV-1 proteinase. Biochem Biophys Res Commun 188:631–637. https://doi.org/10.1016/0006-291x(92)91103-w
Maher P, Akaishi T, Abe K (2006) Flavonoid Fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci U S A 103:16568–16573. https://doi.org/10.1073/pnas.0607822103
Hillis WE (1996) Formation of Robinetin crystals in vessels of Intsia species. IAWA J 17:405–419
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584. https://doi.org/10.1016/s0955-2863(02)00208-5
Procházková D, Boušová I, Wilhelmová N (2011) Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82:513–523. https://doi.org/10.1016/j.fitote.2011.01.018
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2:219–236
Fridovich I (1999) Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci 893:13–18. https://doi.org/10.1111/j.1749-6632.1999.tb07814.x
Imbalance in antioxidant defence and human diseases: multiple approach of natural antioxidants therapy (2021) https://paperpile.com/app/p/a6f0664f-d5cb-07a2-ab0c-817300092165. Accessed 17 Nov 2021
Marković ZS, Mentus SV, Dimitrić Marković JM (2009) Electrochemical and density functional theory study on the reactivity of Fisetin and its radicals: implications on in vitro antioxidant activity. J Phys Chem A 113:14170–14179
Maciel EN, Soares IN, da Silva SC, de Souza GLC (2019) A computational study on the reaction between Fisetin and 2,2-diphenyl-1-picrylhydrazyl (DPPH). J Mol Model 25:103. https://doi.org/10.1007/s00894-019-3969-8
Bayat A, Fattahi A (2017) A quantum chemical study on the OH radical quenching by natural antioxidant Fisetin. J Phys Org Chem 30:e3692. https://doi.org/10.1002/poc.3692
Dimitrić Marković JM, Amić D, Lučić B, Marković ZS (2014) Oxidation of kaempferol and its iron(III) complex by DPPH radicals: spectroscopic and theoretical study. Monatsh Chem 145:557–563. https://doi.org/10.1007/s00706-013-1135-z
Spiegel M, Andruniów T, Sroka Z (2020) Flavones’ and flavonols’ antiradical structure-activity relationship-a quantum chemical study. Antioxidants (Basel) 9. https://doi.org/10.3390/antiox9060461
Dimitrić Marković JM, Marković ZS, Brdarić TP, Filipović ND (2011) Comparative spectroscopic and mechanistic study of chelation properties of Fisetin with iron in aqueous buffered solutions. Implications on in vitro antioxidant activity. Dalton Trans 40:4560–4571. https://doi.org/10.1039/c0dt01834a
Kakiuchi T, Mukai K, Ohara K, Nagaoka S-I (2009) Tunneling effect in antioxidant reaction of flavonoid. Bull Chem Soc Jpn 82:216–218
Naeimi AF, Alizadeh M (2017) Antioxidant properties of the flavonoid Fisetin: an updated review of in vivo and in vitro studies. Trends Food Sci Technol 70:34–44. https://doi.org/10.1016/j.tifs.2017.10.003
Lučić B, Stepanić V, Plavšić D et al (2014) Correlation between 13C NMR chemical shifts and antiradical activity of flavonoids. Monatsh Chem 145:457–463. https://doi.org/10.1007/s00706-013-1130-4
Manrique-de-la-Cuba MF, Gamero-Begazo P, Valencia DE et al (2019) Theoretical study of the antioxidant capacity of the flavonoids present in the Annona muricata (Soursop) leaves. J Mol Model 25:200. https://doi.org/10.1007/s00894-019-4083-7
Sadasivam K, Jayaprakasam R, Kumaresan R (2012) A dft study on the role of different oh groups in the radical scavenging process. J Theor Comput Chem 11:871–893. https://doi.org/10.1142/s0219633612500599
Hossen J, Ali MA, Reza S (2021) Theoretical investigations on the antioxidant potential of a non-phenolic compound thymoquinone: a DFT approach. J Mol Model 27:173. https://doi.org/10.1007/s00894-021-04795-0
Mendes RA, E Silva BLS, Takeara R et al (2018) Probing the antioxidant potential of phloretin and phlorizin through a computational investigation. J Mol Model 24:101. https://doi.org/10.1007/s00894-018-3632-9
Zheng Y-Z, Deng G, Liang Q et al (2017) Antioxidant activity of quercetin and its glucosides from propolis: a theoretical study. Sci Rep 7:7543. https://doi.org/10.1038/s41598-017-08024-8
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213. https://doi.org/10.1007/s00894-007-0233-4
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter 37:785–789. https://doi.org/10.1103/physrevb.37.785
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211. https://doi.org/10.1139/p80-159
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry. Acc Chem Res 41:157–167. https://doi.org/10.1021/ar700111a
Hehre WJ, Ditchfield R, Pople JA (1972) Self—consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261. https://doi.org/10.1063/1.1677527
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–728. https://doi.org/10.1063/1.1674902
gaussian 09, Revision d. 01, Gaussian (2021) https://paperpile.com/app/p/0de7953f-eccc-0633-9342-09859bebe6b9. Accessed 17 Nov 2021
Payán-Gómez SA, Flores-Holguín N, Pérez-Hernández A et al (2010) Computational molecular characterization of the flavonoid rutin. Chem Cent J 4:12. https://doi.org/10.1186/1752-153X-4-12
de Souza GLC, Peterson KA (2021) Benchmarking antioxidant-related properties for gallic acid through the use of DFT, MP2, CCSD, and CCSD(T) approaches. J Phys Chem A 125:198–208. https://doi.org/10.1021/acs.jpca.0c09116
Santos JLF, Kauffmann AC, da Silva SC et al (2020) Probing structural properties and antioxidant activity mechanisms for eleocarpanthraquinone. J Mol Model 26:233. https://doi.org/10.1007/s00894-020-04469-3
Mendes RA, Almeida SKC, Soares IN et al (2019) Evaluation of the antioxidant potential of myricetin 3-O-α-L-rhamnopyranoside and myricetin 4-O-α-L-rhamnopyranoside through a computational study. J Mol Model 25:89. https://doi.org/10.1007/s00894-019-3959-x
Maciel EN, Almeida SKC, da Silva SC, de Souza GLC (2018) Examining the reaction between antioxidant compounds and 2,2-diphenyl-1-picrylhydrazyl (DPPH) through a computational investigation. J Mol Model 24:218. https://doi.org/10.1007/s00894-018-3745-1
(1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255:327–335. https://doi.org/10.1016/0009-2614(96)00349-1
Miertus̃ S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65:239–245. https://doi.org/10.1016/0301-0104(82)85072-6
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem Phys 55:117–129. https://doi.org/10.1016/0301-0104(81)85090-2
Barone V, Cossi M, Tomasi J (1997) A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J Chem Phys 107:3210–3221. https://doi.org/10.1063/1.474671
Leopoldini M, Marino T, Russo N, Toscano M (2004) Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A 108:4916–4922
Kreilick RW, Weissman SI (1966) Hydrogen atom transfer between free radicals and their diamagnetic precursors. J Am Chem Soc 88:2645–2652
Buettner GR (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300:535–543. https://doi.org/10.1006/abbi.1993.1074
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113. https://doi.org/10.1016/s0031-8914(34)90011-2
Messaadia L, Bekkar Y, Benamira M, Lahmar H (2020) Predicting the antioxidant activity of some flavonoids of Arbutus plant: a theoretical approach. Chemical Physics Impact 1:100007. https://doi.org/10.1016/j.chphi.2020.100007
Bitew M, Desalegn T, Demissie TB et al (2021) Pharmacokinetics and drug-likeness of antidiabetic flavonoids: molecular docking and DFT study. PLoS ONE 16:e0260853. https://doi.org/10.1371/journal.pone.0260853
Marković S, Tošović J (2015) Application of time-dependent density functional and natural bond orbital theories to the UV-vis absorption spectra of some phenolic compounds. J Phys Chem A 119:9352–9362. https://doi.org/10.1021/acs.jpca.5b05129
Seyoum A, Asres K, El-Fiky FK (2006) Structure-radical scavenging activity relationships of flavonoids. Phytochemistry 67:2058–2070. https://doi.org/10.1016/j.phytochem.2006.07.002
Leopoldini M, Marino T, Russo N, Toscano M (2004) Density functional computations of the energetic and spectroscopic parameters of quercetin and its radicals in the gas phase and in solvent. Theor Chem Acc 111:210–216. https://doi.org/10.1007/s00214-003-0544-1
Sadasivam K, Kumaresan R (2011) Antioxidant behavior of mearnsetin and myricetin flavonoid compounds–a DFT study. Spectrochim Acta A Mol Biomol Spectrosc 79:282–293. https://doi.org/10.1016/j.saa.2011.02.042
Sadasivam K, Kumaresan R (2011) A comparative DFT study on the antioxidant activity of apigenin and scutellarein flavonoid compounds. Mol Phys 109:839–852. https://doi.org/10.1080/00268976.2011.556576
Thong NM, Vo QV, Huyen TL et al (2019) Theoretical Study for exploring the diglycoside substituent effect on the antioxidative capability of isorhamnetin extracted from. ACS Omega 4:14996–15003. https://doi.org/10.1021/acsomega.9b01780
Dias MM, Machado NFL, Marques MPM (2011) Dietary chromones as antioxidant agents–the structural variable. Food Funct 2:595–602. https://doi.org/10.1039/c1fo10098j
Vagánek A, Rimarčík J, Dropková K et al (2014) Reaction enthalpies of OH bonds splitting-off in flavonoids: the role of non-polar and polar solvent. Comput Theor Chem 1050:31–38
Amić D, Lucić B (2010) Reliability of bond dissociation enthalpy calculated by the PM6 method and experimental TEAC values in antiradical QSAR of flavonoids. Bioorg Med Chem 18:28–35. https://doi.org/10.1016/j.bmc.2009.11.015
Alvareda E, Denis PA, Iribarne F, Paulino M (2016) Bond dissociation energies and enthalpies of formation of flavonoids: a G4 and M06–2X investigation. Comput Theor Chem 1091:18–23. https://doi.org/10.1016/j.comptc.2016.06.021
Parkinson CJ, Mayer PM, Radom L (1999) An assessment of theoretical procedures for the calculation of reliable radical stabilization energies †‡. J Chem Soc Perkin Trans 2:2305–2313
Acknowledgements
The authors thank Dr. Ahmed Abousafat and Dr. Durre Khalil for their technical English assistance. The calculations were performed thanks to the HPC resources of “Unité de Calcul intensif” of the University Frères Mentouri Constantine 1.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. The first draft of the manuscript was written by Rafik Menacer and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The credit author statement is as follows:
Rafik Menacer: writing—original draft, conceptualization, methodology, resources, data curation, software, investigation, formal analysis, visualization, validation, writing—review and editing. Rekkab Seifeddine: resources, data curation, software, investigation, visualization. Zahia Kabouche: supervision, validation, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The antioxidant activity of Fisetin and Robinetin compounds was theoretically rationalized under gas phase and solvent effect of water, dimethylsulfoxide (DMSO), methanol, and benzene at B3LYP/6-311++G(d, p) level of theory.
• The fundamental mechanisms related to the antioxidant activity: hydrogen-atom transfer (HAT), single electron transfer followed by proton transfer (SET-PT), and Sequential Proton Loss Electron Transfer (SPLET), were investigated. It was found that the HAT mechanism is thermodynamically the most favored pathway.
• The favorable active hydroxyls associated with the antioxidant activity follow the trend 4′-OH > 3′-OH > 3-OH > (5′-OH) > 7-OH.
• The energetically favored solvents follow the trends water > DMSO > benzene > methanol and benzene > DMSO > methanol > water for Fisetin and Robinetin, respectively.
Rights and permissions
About this article
Cite this article
Menacer, R., Rekkab, S. & Kabouche, Z. Fisetin and Robinetin antiradical activity under solvent effect: density functional theory study. J Mol Model 28, 240 (2022). https://doi.org/10.1007/s00894-022-05223-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05223-7